Connexins are the major proteins of gap junctions and are important in the key process of intercellular communication in most metazoan cell types. Distinct dominant mutations in the same connexin molecules have been demonstrated to underlie either skin disease or deafness or, indeed, both disorders. Connexin mutations also underlie other disorders, including peripheral neuropathy and cataract formation (Francis et al. 1999; Scherer et al. 1999). This review will focus on recent genetic studies that have demonstrated the importance of gap junctions in epidermal disease and hearing loss.
Gap Junctions and Connexins
Gap-junction intercellular communication allows a mechanism of synchronized cellular response to a variety of intercellular signals by regulating the direct passage of low-molecular-weight metabolites (<1,000 daltons) and ions between the cytoplasm of adjacent cells (Pitts 1998). The skin and inner ear have numerous gap junctions. In the epidermis, gap junctions appear to play a role in the coordination of keratinocyte growth and differentiation (Choudhry et al. 1997), whereas in the sensory epithelia of the inner ear they are proposed to regulate the recycling of potassium ions during auditory transduction (Kikuchi et al. 1995). The major proteins of gap junctions are the connexins (Simon and Goodenough 1998). These are proteins that have four transmembrane domains and that are encoded by a large gene family, of which 15 human genes have been identified to date. Connexins form hexameric hemichannels (termed “connexons”) in the endoplasmic reticulum, which are then translocated into the plasma membrane. The connexon then “docks” with a connexon of an adjacent cell to form a functional channel termed a “gap junction.” Connexons can form either homotypic, heterotypic, or heteromeric channels.
Two types of nomenclature are used to classify the human connexins, either by molecular mass (in the range of 26–59 kD) or by sequence similarities, into three groups: gap junction α (GJA), gap junction β (GJB), or gap junction γ (GJC). Mutations in the gap-junction genes encoding the β connexins have been shown to cause epidermal disease, peripheral neuropathy, and sensorineural hearing loss (White and Paul 1999; Rabionet et al. 2000a) (The Connexin-Deafness Homepage). In the human genome, the majority of β-connexin genes map to two gene clusters—at either 1p34-p35 or 13q11-q12. The following is a summary of the disorders associated with mutations in β connexins (with the exception of GJB1).
Dominant Connexin Disorders of Keratoderma and/or Hearing Loss
The inherited keratodermas, a clinically diverse branch of the genodermatoses, are characterized by thickened or hyperkeratotic skin on the palms and soles (Kelsell and Stevens 1999). These epidermal disorders are further subclassified clinically on the basis of the specific pattern of palmoplantar thickening and on whether they associate with generalized epidermal lesions plus abnormalities of the hair, teeth, nails, and/or sweat glands (abnormalities such as the ectodermal dysplasias). In addition, keratodermas may occur in syndromes with abnormalities of other organs, such as cardiomyopathy (McKoy et al. 2000; Norgett et al. 2000) and hearing impairment (Fitzgerald and Verbov 1996; Sevior et al. 1998). Autosomal dominant mutations in four β connexins have been demonstrated in epidermal disorders. In three of these connexins, certain mutations may also result in syndromic or nonsyndromic sensorineural hearing loss.
GJB2 Encoding Connexin 26 (Cx26)
Vohwinkel syndrome (MIM 124500) is an autosomal dominant condition classified as a “mutilating” diffuse keratoderma in which hyperkeratosis may develop around the circumference of the digits at points of flexion, such as the knuckle. These may form constrictions (or pseudoainhum) sometimes leading to autoamputation of the digit. Other classical epidermal features include a honeycomb pattern of keratoderma with starfishlike keratoses on the knuckles. Mild- to-moderate sensorineural hearing loss is often associated with the skin disease. Two genetic studies have demonstrated that a specific mutation, D66H, in the Cx26 gene (GJB2), underlies Vohwinkel syndrome (Maestrini et al. 1999; Kelsell et al. 2000). In one of these families (Fitzgerald and Verbov 1996), the Vohwinkel pattern of keratoderma was of a mild form and associated with varying types of hearing impairment. Two D66H-heterozygous individuals with the keratoderma in this family were also profoundly deaf and had previously been shown to be heterozygous for another Cx26 variant, M34T (Kelsell et al. 1997). The association between M34T and profound hearing loss in this family led to the subsequent discovery of recessive GJB2 mutations in nonsyndromic hearing loss (NSHL) (discussed in the “Mutation M34T” subsection, below) (Kelsell et al. 1997). It should be noted that a clinical variant of Vohwinkel syndrome, which is associated with ichthyosis (dry rough skin with persistent scaling) but not with hearing loss, is genetically distinct from GJB2-associated Vohwinkel syndrome. Instead, germline mutations in the gene encoding loricrin, a major protein component of the cornified cell envelope of the epidermis, have been described (Maestrini et al. 1996; Korge et al. 1997).
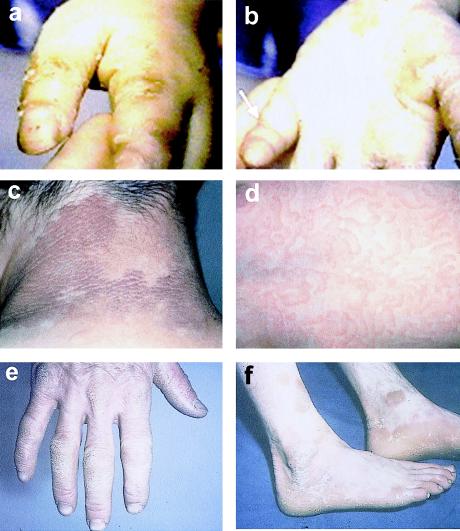
Other epidermis-associated GJB2 mutations have been described. In a family in which an autosomal dominant palmoplantar keratoderma and high-frequency hearing loss (MIM 148350) were cosegregating, the mutation G59A was identified (Heathcote et al. 2000). A heterozygous 3-bp deletion of the residue E42 (ΔE42) has also been associated with deafness and palmoplantar keratoderma (Bale et al. 1999). The GJB2 mutation, R75W, has been described in a father and daughter from an Egyptian family with a skin disease similar to Vohwinkel syndrome (Richard et al. 1998b). The skin disease was described as a diffuse hyperkeratosis, with peeling of the palmoplantar and pseudoainhum (see fig. 1A and 1B). Both individuals also had profound prelingual hearing impairment. The authors also identified an individual out of their “control” cohort who was heterozygous for R75W but who had no skin disease. The hearing status of the individual was unknown. An additional individual heterozygous for R75W has been documented; this individual had profound hearing loss but only a mild form of diffuse palmoplantar keratoderma, with no evidence of pseudoainhum or other classical epidermal features of Vohwinkel syndrome (Loffeld et al. 2000). The variability in severity of the palmoplantar keratoderma in R75W heterozygotes (also noted between D66H heterozygotes) suggests that other factors—either genetic or environmental—may modify the penetrance of epidermal disease–associated GJB2 alleles.
Figure 1.
Examples of epidermal phenotypes associated with connexin mutations. A and B, R75W mutation in GJB2 in a patient with palmoplantar keratoderma and profound hearing loss (clinical pictures courtesy of G. Richard, S. Bale, and the Ain-Shams Medical Genetics Clinic in Cairo, Egypt). C and D, F137L mutation in GJB4 in a patient with erythrokeratoderma variabilis (clinical pictures courtesy of D. Hohl and B. Mevorah). E and F, R42P mutation in GJB3 in a patient with erythrokeratoderma variabilis (clinical pictures courtesy of C. Kennedy).
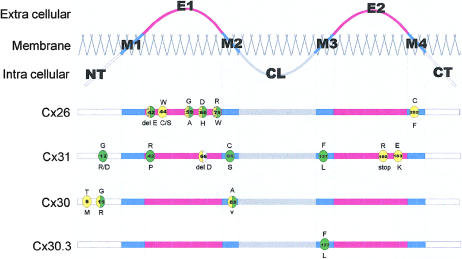
Specific GJB2 mutations have also been demonstrated to underlie dominant NSHL mapping to 13q11-q12 (DFNA3 [MIM 601544]). The first mutation proposed to be a dominant deafness allele, M34T (Kelsell et al. 1997), has now been shown to be a recessive deafness-associated GJB2 allele (discussed in the “Mutation M34T” subsection, below). However, other dominant NSHL GJB2 mutations have been described (Denoyelle et al. 1998; Morle et al. 2000). The position of these mutations in the Cx26 protein is shown in figure 2, in relation to other β-connexin mutations associated with skin disease and hearing loss.
Figure 2.
Schematic of protein domains of Cx26, Cx31, Cx30, and Cx30.3, with localization of disease-associated mutations. “M1”–“M4” denote transmembrane-spanning domains; “E1” and “E2” denote extracellular domains; “CL” denotes cytoplasmic loop; “NT” denotes cytoplasmic amino terminus; “CT” denotes cytoplasmic carboxy terminus. Green circles denote mutations associated with epidermal disease; yellow circles denote mutations associated with hearing loss; yellow circles with black dots denote mutations associated with profound hearing loss; yellow circles with horizontal lines denote mutations associated with mild hearing loss; white circles denote mutation associated with peripheral neuropathy.
GJB3 Encoding Connexin 31 (Cx31)
The erythrokeratodermas represent a group of disorders characterized by the presence of fixed or slowly moving erythematous hyperkeratotic plaques (Rook et al. 1998). Erythrokeratoderma variabilis (EKV [MIM 133200]) is an autosomal dominant disorder presenting with diffuse palmoplantar keratoderma and transient red figurata at other epidermal sites (fig. 1B, 1C, 1D, and 1E). The erythematous patches affect the whole body but are more often found on the face, buttocks, and extensor surfaces of the limbs. With increasing age, the areas of the body affected by EKV become more restricted to the palmoplantar epidermis. EKV in a number of families is linked to the chromosomal region 1p34-p35, where a gene cluster of β connexins map (van der Schroeff et al. 1984, 1988; Richard et al. 1997). Subsequently, mutations in affected members from a number of these pedigrees were identified in the gap-junction β-3 gene (GJB3 [MIM 603324]) encoding connexin-31 (Richard et al. 1998a). Five GJB3 mutations causing EKV have been described throughout the Cx31 protein (fig. 2), occurring in the intracellular, extracellular, and transmembrane domains (Wilgoss et al. 1999; Richard et al. 2000). A family with a disease with phenotypic similarities to EKV—that is, progressive symmetric erythrokeratoderma (PSEK)—has been described in which the disease is associated with a mutation in loricrin (Ishida-Yamamoto et al. 1997). It is of interest that loricrin mutations have also been described in individuals affected with the variant form of Vohwinkel syndrome (Maestrini et al. 1996).
Unlike GJB2 encoding Cx26, none of the GJB3 mutations associated with skin disease are associated with genetic hearing loss. However, two proposed dominant GJB3 mutations (R180X and E183K), which are associated with progressive hearing loss but not with epidermal manifestations, have also been described (Xia et al. 1998). Adding complexity is the recent identification of another dominant GJB3 mutation, 66delD, in a family with peripheral neuropathy and sensorineural hearing loss (Lopez-Bigas et al. 2000), thereby raising to three the number of disorders resulting from GJB3 mutations. The D66 residue is conserved and functionally important in other connexins as well. A D66H substitution in GJB2 causes Vohwinkel syndrome (see the preceding subsection, “GJB2 Encoding Connexin 26 (Cx26)”), whereas 66delD in the gene for another β connexin, GJB1 encoding Cx32, results in the peripheral neuropathy disorder X-linked Charcot-Marie-Tooth (Haites et al. 1998). It should be noted that a number of Cx32 mutations are also associated with hearing loss in combination with peripheral neuropathy.
GJB4 Encoding Connexin 30.3 (Cx30.3)
Although EKV in all families described to date is linked to the chromosomal region 1p34-1p35, not all have mutations in GJB3. Recently, an F137L mutation in GJB4, which also maps to 1p34-35, was identified in the affected members of a family with EKV (Macari et al. 2000). The mutated residue in Cx30.3, the epidermally expressed β connexin encoded by GJB4, lies in the third transmembrane domain. The same missense mutation has also been demonstrated in GJB3 in another individual with EKV (Richard et al. 2000). Further families with EKV that have Cx30.3 mutations have now been identified (G. Richard, personal communication). However, there are a number of patients with EKV who do not harbor either GJB3 mutations or GJB4 mutations (authors' unpublished data). It is not yet known whether, like the other epidermal disease–associated connexins, there are GJB4 mutations resulting in hearing impairment.
GJB6 Encoding Connexin 30 (Cx30)
Hidrotic ectodermal dysplasia (HED), or Clouston syndrome [MIM 129500]), is inherited as an autosomal dominant disorder and has characteristic changes in the epidermis and the appendages, including diffuse palmoplantar keratoderma, nail dystrophy, and sparse scalp and body hair. In addition, hearing impairment is observed in some individuals with HED. Disease segregating within a large kindred of French Canadian origin was mapped to the chromosomal region 13q11-q12 (Kibar et al. 1996) and, on the basis of linkage studies of families with different ethnic origins, subsequently was shown to be a genetically homogeneous disease (Radhakrishna et al. 1997; Taylor et al. 1998; Stevens et al. 1999). In the chromosomal region harboring the HED locus map two β connexins, Cx26 and Cx30, encoded by GJB2 and GJB6 (MIM 604418), respectively. Previously, GJB2 has been excluded as the genetic basis of HED (Kelsell et al. 1997). A recent study demonstrated that all families with HED that were available for genetic analysis had inherited one of two missense mutations—either G11R or A88V—in GJB6 encoding Cx30 (Lamartine et al. 2000). Prior to this study, the mutation T5M in GJB6 was associated with high-frequency NSHL segregating in a small family (Grifa et al. 1999). Therefore, like GJB2 and GJB3, different dominant mutations in GJB6 result in skin disease, hearing loss, or both disorders.
Connexin Mutations and Autosomal Recessive NSHL
A genetic etiology is associated with the majority (70%) of NSHL cases in developed countries. During the past few years, mapping studies have led to the identification of >50 autosomal recessive (assigned locus DFNB) and autosomal dominant (assigned locus DFNA) NSHL loci. More recently, positional cloning or positional candidate-gene screening has isolated 19 genes involved in NSHL (see Hereditary Hearing Loss Homepage). The identification of recessive mutations in two β connexin–family members—GJB2 and, to a lesser extent, GJB3—has had a large impact on the genetic understanding of NSHL. This interest is due largely to the fact that a significant proportion of NSHL is caused by GJB2 mutations.
GJB2 and Autosomal Recessive NSHL
The first autosomal recessive NSHL locus, DFNB1 (MIM 220290), was mapped to 13q12 in two consanguineous Tunisian families (Guilford et al. 1994). Further mapping studies, of families from different ethnic populations, confirmed linkage and illustrated the potential contribution of the gene at this locus to NSHL (Maw et al. 1995; Gasparini et al. 1997). In 1997, two nonsense mutations of GJB2—W24X and W77X—were identified in three unrelated Pakistani families that have linkage to DFNB1 (Kelsell et al. 1997). An independent study of 35 Mediterranean families with autosomal recessive NSHL refined the DFNB1 interval to ∼5 cM between markers D13S175 and D13S232. Analysis of the GJB2 coding region in these families indicated that, in 19 of the 35 families, hearing-impaired individuals were homozygous for a specific mutation, 35delG (Zelante et al. 1997). Also, in the same study, hearing-impaired individuals from another family were found to be compound heterozygotes for 35delG and 167delT. Both sequence variants produce a frameshift in the GJB2 mRNA, leading to predicted truncation of the protein and, hence, loss of function. Subsequent studies, in a range of different populations, have revealed a multiplicity of mutations that include missense and nonsense base substitutions, deletions, insertions, and a splice-site modification (see The Connexin-Deafness Homepage). Interestingly, the frequency of particular mutations seems to be dependent on the ethnic origin of the population (see table 1), and these mutations are discussed in the following subsections.
Table 1.
Frequency of Common GJB2 Recessive Mutations in Different NSHL Population Groups
|
No. (%) of GJB2-Mutation Alleles Screeneda |
||||||
| PopulationEthnicity | Total No. of NSHL Alleles Screened | 35delG | 167delT | 235delC | M34T | Reference(s) |
| Spanish and Italian | 1,546b | 533 (34.48) | 11 (.71) | ND | 1 (.07) | Zelante et al. (1997); Estivill et al. (1998); Murgia et al. (1999); Rabionet et al. (2000b) |
| United Kingdom | 142b | 21 (14.79) | ND | ND | ND | Denoyelle et al. (1997); Lench et al. (1998) |
| United States | 116c | 33 (28.45) | 9 (7.76) | ND | 2 (1.72) | Kelley et al. (1998) |
| Japanese | 308b | ND | ND | 24 (7.80) | ND | Fuse et al. (1999); Abe et al. (2000); Kudo et al. (2000) |
| Ashkenazi Jewish | 60c | 13 (21.67) | 34 (56.67) | ND | ND | Morell et al. (1998); Lerer et al. (2000) |
| French | 204b | 78 (38.24) | ND | ND | ND | Denoyelle et al. (1997); (1999) |
ND = not detected.
Sib-pair cases and sporadic cases of prelingual NSHL.
Autosomal recessive prelingual cases of NSHL.
Mutation 35delG (or 30delG)
The 35delG mutation appears to be more prevalent in individuals with NSHL who are of European origin (Denoyelle et al. 1997; Estivill et al. 1998; Kelley et al. 1998; Lench et al. 1998; Scott et al. 1998a). In some populations, the 35delG allele may account for ⩽85% of all GJB2 mutant alleles detected (Estivill et al. 1998). Conversely, in 154 individuals with NSHL and in 349 controls of Japanese origin, the 35delG mutation has not been detected (Fuse et al. 1999; Abe et al. 2000; Kudo et al. 2000). Five guanine bases precede the nucleotide deleted in 35delG; it has been suggested that the increased prevalence observed may be due to a mutational hotspot. However, the fluctuation in carrier status of 35delG in normal hearing populations from different ethnic groups (1 in 35 in southern Europe and 1 in 79 in northern Europe) would suggest a possible founder effect and positive selection for 35delG heterozygote status (Gasparini et al. 2000).
Mutation 167delT
The 167delT frameshift mutation was originally reported in (2.3%)1 of 43 GJB2 mutant alleles of Mediterranean origin (Zelante et al. 1997). Both its high prevalence in Ashkenazi Jewish individuals with NSHL and the identification of 167delT on a conserved haplotype at flanking loci suggest a founder event for the origin of the mutation in this population (Morell et al. 1998; Lerer et al. 2000; Sobe et al. 2000).
Mutation 235delC
The 235delC frameshift mutation has been detected only in individuals of Japanese origin, at a frequency of 7.8% in cohorts with NSHL and at a frequency of 0%–1.0% in control cohorts (Fuse et al. 1999; Abe et al. 2000; Kudo et al. 2000). Analysis of four individuals with 235delC who had GJB2 polymorphisms identified a conserved haplotype in all individuals (Kudo et al. 2000). However, this was also the most common GJB2 haplotype observed in the control population.
Mutation M34T
The M34T mutation was originally postulated as causing autosomal dominant NSHL, at DFNA3 (Kelsell et al. 1997). This was substantiated by the functional observations, in the paired-Xenopus-oocyte model, of M34T Cx26 protein as a dominant disrupter of intercellular conductance (White et al. 1998) and having a deleterious effect on Cx26 channels in a mammalian-cell assay system (Martin et al. 1999). In addition, two other dominant GJB2 mutations associated with NSHL have been identified in pedigrees that have linkage to DFNA3 (discussed in the “GJB2 Encoding Connexin 26 (Cx26)” subsection, above; also see Denoyelle et al. 1998; Morle et al. 2000). However, detection of the M34T allele in a heterozygous state in individuals with normal hearing suggested either an autosomal recessive mode of action or a neutral polymorphism (Kelley et al. 1998; Scott et al. 1998b). The association between M34T in trans with alleles V95M, R184W, 35delG, and 167delT, in individuals with NSHL, also supports a recessive mode of action (Kelley et al. 1998; Griffith et al. 2000; Wilcox et al. 2000). Further evidence for the recessive nature of the M34T variant has been gained from the identification of the first individuals homozygous for M34T. Both M34T homozygotes have mid-to-high–frequency hearing loss (Houseman et al. 2001).
Clinical Variability of GJB2 Mutations That Cause Autosomal Recessive NSHL
The audiological phenotype observed in individuals with GJB2 mutations is variable, even in those from the same ethnic population who have the same homozygous or compound-heterozygous GJB2 mutations. Most biallelic GJB2 mutations affect both ears to a similar extent, but asymmetric NSHL has been reported (Denoyelle et al. 1999; Wilcox et al. 2000). For homozygous 35delG individuals, the degree of hearing loss observed among probands and siblings can fluctuate between mild and profound. Whereas the majority of individuals present with nonprogressive severe-to-profound NSHL (Kelley et al. 1998; Denoyelle et al. 1999; Murgia et al. 1999), progression of the sensory deficit in some 35delG homozygotes has also been observed (Cohn et al. 1999). For 167delT homozygotes of Ashkenazi Jewish ancestry, a trend in the variability of the degree of hearing loss is also evident among probands and siblings (Lerer et al. 2000). However, a common characteristic of the sensory deficit in individuals identified as having biallelic GJB2 mutations appears to be onset during early childhood. The association between the variability in the degree of hearing impairment and mutations in GJB2 supports a role for external modifying environmental and genetic factors.
GJB3 and Autosomal Recessive NSHL
Mutations in GJB3 were identified in families with autosomal dominant NSHL and EKV (see the “GJB3 Encoding Connexin 31 (Cx31)” subsection; also see Richard et al. 1998a; Xia et al. 1998). After GJB3 had been found to be associated with autosomal dominant NSHL, 25 Chinese families with autosomal recessive NSHL were screened for GJB3 mutations. Two unrelated families were identified with compound heterozygous GJB3 mutations, indicating that, like GJB2, recessive GJB3 mutations are also associated with NSHL (Liu et al. 2000). No other GJB3 mutations have been associated with autosomal recessive NSHL.
Concluding Remarks
The identification, in the connexins, of specific mutations that are involved in skin disease and hearing impairment has revealed intriguing genotype-phenotype relationships, in addition to supporting the importance of gap-junction intercellular communication in both the epidermis and the epithelial cells of the inner ear. Although mutations in two connexins—GJB3 and GJB4—underlie clinically similar epidermal disease, it has also been observed that, among individuals carrying the same mutation, some dominant mutations in GJB2 have variable penetrance with respect to hearing loss and/or severity of the skin disease, raising the possibility that other genetic and environmental factors modify the penetrance of the mutations. One of the most surprising genetic findings is the association between recessive GJB2 mutations and a significant proportion of nonsyndromic sensorineural hearing loss. The role of connexins in NSHL will facilitate an increased understanding of inner-ear processes and will benefit individuals seeking genetic counseling for autosomal recessive NSHL.
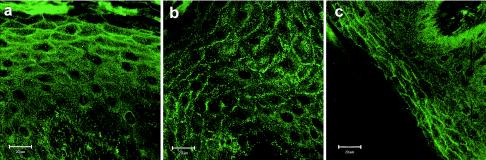
The use of classical model systems to assay connexin function, such as by the measurement of intercellular conductance in the paired-Xenopus-oocyte-model assay and the mammalian cell-culture systems, has produced data on dominant inhibition of intercellular conductance and on defects in gap-junction assembly, respectively (Richard et al. 1998b; White et al. 1998; Martin et al. 1999). With relevance to the β connexins discussed in this review, the only published connexin knockout mice are those for Cx26, which, because of placental failure, result in embryonic lethality at day 9.5 (Gabriel et al. 1998). This differs from the phenotype observed in humans, in which recessive protein-truncating GJB2 mutations are associated with hearing impairment. Better models of human connexin disease may be generated, by means of gene-targeting technology and tissue-specific promoters, through replication, in mice, of the human dominant β-connexin mutations. However, it appears likely that, to really understand why some mutations in the same connexin protein cause skin disease whereas others cause hearing impairment will require study of these mutant connexins in the context of other connexins expressed in the affected tissues and in the unaffected tissues; for example, although Cx26 has a wide tissue distribution, Cx26 recessive mutations cause only NSHL, suggesting that, in other tissues, other connexins can compensate for loss of the Cx26 protein. This is particularly relevant to the epidermis, in which ⩾10 connexins are expressed (examples shown in figure 3; authors' unpublished data). Recent data from the paired-Xenopus-oocyte–model–expression assay have shown that ΔE42 (as well as other dominant Cx26 mutants that result in a skin phenotype) inhibit the channel activity of coexpressed Cx43 (GJA1), another connexin that can colocalize with Cx26 in epidermal keratinocytes (G. Richard and M. Hodgins, personal communication). Although it is not physiologically understood why so many connexins are expressed in the epidermis (or in the inner ear), multiple connexins result in channels with characteristic permeabilities for ion selectivity and cellular metabolites (Cao et al. 1998; Goldberg et al. 1999; Niessen et al. 2000). Some studies have suggested that connexins, because of their association with specific tight junction proteins, may have functions in addition to that of gap-junctional communication (Giepmans and Moolenaar 1998; Kojima et al. 1999).
Figure 3.
Expression patterns of three connexins—Cx26 (a), Cx30 (b), and Cx31 (c)—in human epidermis of the sole. Immunofluorescence staining with connexin antibodies, performed on frozen sections of the sole, shows the distribution and the characteristic punctate membrane staining of connexins.
Because of the link between β-connexin mutations and disease, the study of the functional mechanisms by which these mutations exert their effects will increase our understanding of connexin and gap-junction biology; specifically, the distinct autosomal dominant mutations found in four of the β connexins that underlie hearing loss and/or epidermal disease plus, in one case, peripheral neuropathy will require further functional characterization as to why, in different tissues, they have different effects on channel function. As well as disease-associated mutations, numerous coding single-nucleotide polymorphisms (SNPs) in the connexin genes have been identified (see The Connexin-Deafness Homepage), and these will also aid in the dissection of the structurally important residues and domains in the connexin molecule. It is possible that these SNPs may also modify the severity of disease.
Acknowledgments
We would like to thank Dr. Elizabeth Rugg and Prof. Irene Leigh for their critical reading of the manuscript.
Electronic-Database Information
Accession numbers and URLs for data in this article are as follows:
- Connexin-Deafness Homepage, The, http://www.iro.es/cx26deaf.html
- Hereditary Hearing Loss Homepage, http://dnalab-www.uia.ac.be/dnalab/hhh/
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for Vohwinkel syndrome [MIM 124500], DFNA3 [MIM 601544], EKV [MIM 133200], GJB2 [MIM 121011], DFNB1 [MIM 220290], HED [MIM 129500], GJB3 [MIM 603324https://omim.org/entry/], GJB6 [MIM 604418], and palmoplantar keratoderma with deafness [MIM 148350])
References
- Abe S, Usami S, Shinkawa H, Kelley PM, Kimberling WJ (2000) Prevalent connexin 26 gene (GJB2) mutations in Japanese. J Med Genet 37:41–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale SJ, White TW, Munro C, Taylor AEM, Richard G (1999) Functional defects of Cx26 due to mutations in two families with dominant palmoplantar keratoderma and deafness. J Invest Dermatol 112:A550 [Google Scholar]
- Cao F, Eckert R, Elfgang C, Nitsche JM, Snyder SA, DF Hu, Willecke K, Nicholson BJ (1998) A quantitative analysis of connexin-specific permeability differences of gap junctions expressed in HeLa transfectants and Xenopus oocytes. J Cell Sci 111:31–43 [DOI] [PubMed] [Google Scholar]
- Choudhry R, Pitts JD, Hodgins MB (1997) Changing patterns of gap junctional intercellular communication and connexin distribution in mouse epidermis and hair follicles during embryonic development. Dev Dyn 210:417–430 [DOI] [PubMed] [Google Scholar]
- Cohn ES, Kelley PM, Fowler TW, Gorga MP, Lefkowitz DM, Kuehn HJ, Schaefer GB, Gobar LS, Hahn FJ, Harris DJ, Kimberling WJ (1999) Clinical studies of families with hearing loss attributable to mutations in the connexin 26 gene. Pediatrics 103:546–550 [DOI] [PubMed]
- Denoyelle F, Lina-Granade G, Plauchu H, Bruzzone R, Chaib H, Levi-Acobas F, Weil D, Petit C (1998) Connexin 26 gene linked to a dominant deafness. Nature 393:319–320 [DOI] [PubMed] [Google Scholar]
- Denoyelle F, Marlin S, Weil D, Moatti L, Chauvin P, Garabedian EN, Petit C (1999) Clinical features of the prevalent form of childhood deafness, DFNB1, due to a connexin-26 gene defect: implications for genetic counselling. Lancet 353:1298–1303 [DOI] [PubMed] [Google Scholar]
- Denoyelle F, Weil D, Maw MA, Wilcox SA, Lench NJ, Allen-Powell DR, Osborn AH, Dahl HH, Middleton A, Houseman MJ, Dode C, Marlin S, Boulila-ElGaied A, Grati M, Ayadi H, BenArab S, Bitoun P, Lina-Granade G, Godet J, Mustapha M, Loiselet J, El-Zir E, Aubois A, Joannard A, Petit C (1997) Prelingual deafness: high prevalence of a 30delG mutation in the connexin 26 gene. Hum Mol Genet 6:2173–2177 [DOI] [PubMed] [Google Scholar]
- Estivill X, Fortina P, Surrey S, Rabionet R, Melchionda S, D'Agruma L, Mansfield E, Rappaport E, Govea N, Mila M, Zelante L, Gasparini P (1998) Connexin-26 mutations in sporadic and inherited sensorineural deafness. Lancet 351:394–398 [DOI] [PubMed] [Google Scholar]
- Fitzgerald DA, Verbov JL (1996) Hereditary palmoplantar keratoderma with deafness. Br J Dermatol 134:939–942 [PubMed] [Google Scholar]
- Francis P, Berry V, Moore A, Bhattacharya S (1999) Lens biology: development and human cataractogenesis. Trends Genet 15:191–196 [DOI] [PubMed] [Google Scholar]
- Fuse Y, Doi K, Hasegawa T, Sugii A, Hibino H, Kubo T (1999) Three novel connexin26 gene mutations in autosomal recessive non-syndromic deafness. Neuroreport 10:1853–1857 [DOI] [PubMed] [Google Scholar]
- Gabriel HD, Jung D, Butzler C, Temme A, Traub O, Winterhager E, Willecke K (1998) Transplacental uptake of glucose is decreased in embryonic lethal connexin26-deficient mice. J Cell Biol 140:1453–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparini P, Estivill X, Volpini V, Totaro A, Castellvi-Bel S, Govea, N, Mila M, Della Monica M, Ventruto V, De Benedetto M, Stanziale P, Zelante L, Mansfield ES, Sandkuijl L, Surrey S, Fortina P (1997) Linkage of DFNB1 to non-syndromic neurosensory autosomal-recessive deafness in Mediterranean families. Eur J Hum Genet 5:83–88 [PubMed] [Google Scholar]
- Gasparini P, Rabionet R, Barbujani G, Melchionda S, Petersen M, Brondum-Nielsen K, Metspalu A, Oitmaa E, Pisano M, Fortina P, Zelante L, Estivill X (2000) High carrier frequency of the 35delG deafness mutation in European populations. Genetic Analysis Consortium of GJB2 35delG. Eur J Hum Genet 8:19–23 [DOI] [PubMed] [Google Scholar]
- Giepmans BN, Moolenaar WH (1998) The gap junction protein connexin43 interacts with the second PDZ domain of the zona occludens-1 protein. Curr Biol 8:931–934 [DOI] [PubMed] [Google Scholar]
- Goldberg GS, Lampe PD, Nicholson BJ (1999) Selective transfer of endogenous metabolites through gap junctions composed of different connexins. Nat Cell Biol 1:457–459 [DOI] [PubMed] [Google Scholar]
- Grifa A, Wagner CA, D'Ambrosio L, Melchionda S, Bernardi F, Lopez-Bigas N, Rabionet R, Arbones M, Monica MD, Estivill X, Zelante L, Lang F, Gasparini P (1999) Mutations in GJB6 cause nonsyndromic autosomal dominant deafness at DFNA3 locus. Nat Genet 23:16–18 [DOI] [PubMed] [Google Scholar]
- Griffith AJ, Chowdhry AA, Kurima K, Hood LJ, Keats B, Berlin CI, Morell RJ, Friedman TB (2000) Autosomal recessive nonsyndromic neurosensory deafness at DFNB1 not associated with the compound-heterozygous GJB2 (connexin 26) genotype M34T/167delT. Am J Hum Genet 67:745–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilford P, Ayadi H, Blanchard S, Chaïb H, Le Paslier D, Weissenbach J, Petit C (1994) A human gene responsible for neurosensory, non-syndromic recessive deafness is a candidate homologue of the mouse sh-1 gene. Hum Mol Genet 3:989–993 [DOI] [PubMed] [Google Scholar]
- Haites NE, Nelis E, Van Broeckhoven C (1998) 3rd workshop of the European CMT consortium: 54th ENMC International Workshop on genotype/phenotype correlations in Charcot-Marie-Tooth type 1 and hereditary neuropathy with liability to pressure palsies 28–30 November 1997, Naarden, The Netherlands. Neuromuscul Disord 8:591–603 [DOI] [PubMed] [Google Scholar]
- Heathcote K, Syrris P, Carter ND, Patton MA (2000) A connexin 26 mutation causes a syndrome of sensorineural hearing loss and palmoplantar hyperkeratosis (MIM 148350). J Med Genet 37:50–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houseman MJ, Ellis LA, Pagnamenta A, Di W-L, Rickard S, Osborn A, Dahl H-H, Taylor GR, Bitner-Glindzicz M, Reardon W, Mueller RF, Kelsell DP (2001) Genetic analysis of the connexin-26 M34T variant: identification of genotype M34T/M34T segregating with mild-moderate non-syndromic sensorineural hearing loss. J Med Genet 38:19–24 [DOI] [PMC free article] [PubMed]
- Ishida-Yamamoto A, McGrath JA, Lam H, Iizuka H, Friedman RA, Christiano AM (1997) The molecular pathology of progressive symmetric erythrokeratoderma: a frameshift mutation in the loricrin gene and perturbations in the cornified cell envelope. Am J Hum Genet 61:581–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley PM, Harris DJ, Comer BC, Askew JW, Fowler T, Smith SD, Kimberling WJ (1998) Novel mutations in the connexin 26 gene (GJB2) that cause autosomal recessive (DFNB1) hearing loss. Am J Hum Genet 62:792–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelsell DP, Dunlop J, Stevens HP, Lench NJ, Liang JN, Parry G, Mueller RF, Leigh IM (1997) Connexin 26 mutations in hereditary non-syndromic sensorineural deafness. Nature 387:80–83 [DOI] [PubMed] [Google Scholar]
- Kelsell DP, Stevens HP (1999) The palmoplantar keratodermas: much more than palms and soles. Mol Med Today 5:107–113 [DOI] [PubMed] [Google Scholar]
- Kelsell DP, Wilgoss AL, Richard G, Stevens HP, Munro CS, Leigh IM (2000) Connexin mutations associated with palmoplantar keratoderma and profound deafness in a single family. Eur J Hum Genet 8:141–144 [DOI] [PubMed] [Google Scholar]
- Kibar Z, Der Kaloustian VM, Brais B, Hani V, Fraser FC, Rouleau GA (1996) The gene responsible for Clouston hidrotic ectodermal dysplasia maps to the pericentromeric region of chromosome 13q. Hum Mol Genet 5:543–547 [DOI] [PubMed] [Google Scholar]
- Kikuchi T, Kimura RS, Paul DL, Adams JC (1995) Gap junctions in the rat cochlea: immunohistochemical and ultrastructural analysis. Anat Embryol 191:101–118 [DOI] [PubMed] [Google Scholar]
- Kojima T, Sawada N, Chiba H, Kokai Y, Yamamoto M, Urban M, Lee GH, Hertzberg EL, Mochizuki Y, Spray DC (1999) Induction of tight junctions in human connexin 32 (hCx32)-transfected mouse hepatocytes: connexin 32 interacts with occludin. Biochem Biophys Res Commun 266:222–229 [DOI] [PubMed] [Google Scholar]
- Korge BP, Ishida-Yamamoto A, Punter C, Dopping-Hepenstal PJ, Iizuka H, Stephenson A, Eady RA, Munro CS (1997) Loricrin mutation in Vohwinkel's keratoderma is unique to the variant with ichthyosis. J Invest Dermatol 109:606–610 [DOI] [PubMed]
- Kudo T, Ikeda K, Kure S, Matsubara Y, Oshima T, Watanabe K, Kawase T, Narisawa K, Takasaka T (2000) Novel mutations in the connexin 26 gene (GJB2) responsible for childhood deafness in the Japanese population. Am J Med Genet 90:141–145 [DOI] [PubMed] [Google Scholar]
- Lamartine J, Munhoz Essenfelder G, Kibar Z, Lanneluc I, Callouet E, Laoudj D, Lemaitre G, Hand C, Hayflick SJ, Zonana J, Antonarakis S, Radhakrishna U, Kelsell DP, Christianson AL, Pitaval A, Der Kaloustian V, Fraser C, Blanchet-Bardon C, Rouleau GA, Waksman G (2000) Mutations in GJB6 cause hidrotic ectodermal dysplasia. Nat Genet 26:142–144 [DOI] [PubMed] [Google Scholar]
- Lench N, Houseman M, Newton V, Van Camp G, Mueller R (1998) Connexin-26 mutations in sporadic non-syndromal sensorineural deafness. Lancet 351:415 [DOI] [PubMed] [Google Scholar]
- Lerer I, Sagi M, Malamud E, Levi H, Raas-Rothschild A, Abeliovich D (2000) Contribution of connexin 26 mutations to nonsyndromic deafness in ashkenazi patients and the variable phenotypic effect of the mutation 167delT. Am J Med Genet 95:53–56 [DOI] [PubMed] [Google Scholar]
- Liu XZ, Xia XJ, Xu LR, Pandya A, Liang CY, Blanton SH, Brown SD, Steel KP, Nance WE (2000) Mutations in connexin31 underlie recessive as well as dominant non-syndromic hearing loss. Hum Mol Genet 9:63–67 [DOI] [PubMed] [Google Scholar]
- Loffeld A, Kelsell DP, Moss C (2000) Palmoplantar keratoderma and sensorineural deafness in an 8-year-old boy: a case report. Br J Dermatol 143:38 [Google Scholar]
- Lopez-Bigas N, Olive M, Rabionet R, Bravo O, Banchs I, Volpini V, Ferrer I, Arbones ML, Estivill X (2000) An amino acid deletion in the human connexin 31 gene (GJB3) is associated with sensorineural deafness and hereditary sensory neuropathy. Am J Hum Genet 67 Suppl 2:413 [Google Scholar]
- Macari F, Landau M, Cousin P, Mevorah B, Brenner S, Panizzon R, Schorderet DF, Hohl D, Huber M (2000) Mutation in the gene for connexin 30.3 in a family with erythrokeratodermia variabilis. Am J Hum Genet 67:1296–1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestrini E, Korge BP, Ocaña-Sierra J, Calzolari E, Cambiaghi S, Scudder P, Hovnanian A, Monaco A, Munro C (1999) A missense mutation in connexin26, D66H, causes mutilating keratoderma with sensorineural deafness (Vohwinkel's syndrome) in three unrelated families. Hum Mol Genet 8:1237–1243 [DOI] [PubMed] [Google Scholar]
- Maestrini E, Monaco AP, McGrath JA, Ishida-Yamamoto A, Camisa C, Hovnanian A, Weeks DE, Lathrop M, Uitto J, Christiano AM (1996) A molecular defect in loricrin, the major component of the cornified cell envelope, underlies Vohwinkel's syndrome. Nat Genet 13:70–77 [DOI] [PubMed] [Google Scholar]
- Martin PE, Coleman L, Casalotti SO, Forge A, Evans WH (1999) Properties of connexin26 gap junctional proteins derived from mutations associated with non-syndromal hereditary deafness. Hum Mol Genet 8:2369–2376 [DOI] [PubMed] [Google Scholar]
- Maw MA, Allen-Powell DR, Goodey RJ, Stewart IA, Nancarrow DJ, Hayward NK, Gardner RJ (1995) The contribution of the DFNB1 locus to neurosensory deafness in a Caucasian population. Am J Hum Genet 57:629–635 [PMC free article] [PubMed] [Google Scholar]
- McKoy G, Protonotarios N, Crosby A, Tsatsopoulou A, Anastasakis A, Coonar A, Norman M, Baboonian C, Jeffrey S, McKenna WJ (2000) Identification of a deletion in plakoglobin in arrhythmogenic right ventricular cardiomyopathy with palmoplantar keratoderma and woolly hair (Naxos disease). Lancet 355:2119–2124 [DOI] [PubMed] [Google Scholar]
- Morell RJ, Kim HJ, Hood LJ, Goforth L, Friderici K, Fisher R, Van Camp G, Berlin CI, Oddoux C, Ostrer H, Keats B, Friedman TB (1998) Mutations in the connexin 26 gene (GJB2) among Ashkenazi Jews with nonsyndromic recessive deafness. N Engl J Med 339:1500–1555 [DOI] [PubMed] [Google Scholar]
- Morle L, Bozon M, Alloisio N, Latour P, Vandenberghe A, Plauchu H, Collet L, Edery P, Godet J, Lina-Granade G (2000) A novel C202F mutation in the connexin26 gene (GJB2) associated with autosomal dominant isolated hearing loss. J Med Genet 37:368–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgia A, Orzan E, Polli R, Martella M, Vinanzi C, Leonardi E, Arslan E, Zacchello F (1999) Cx26 deafness: mutation analysis and clinical variability. J Med Genet 36:829–832 [PMC free article] [PubMed] [Google Scholar]
- Niessen H, Harz H, Bedner P, Kramer K, Willecke K (2000) Selective permeability of different connexin channels to the second messenger inositol 1,4,5-trisphosphate. J Cell Sci 113:1365–1372 [DOI] [PubMed] [Google Scholar]
- Norgett EE, Hatsell SJ, Carvajal-Huerta L, Ruiz Cabezas JC, Common J, Purkis PE, Whittock N, Leigh IM, Stevens HP, Kelsell DP (2000) Recessive mutation in desmoplakin disrupts desmoplakin-intermediate filament interactions and causes dilated cardiomyopathy, woolly hair and keratoderma. Hum Mol Genet 9:2761–2766 [DOI] [PubMed] [Google Scholar]
- Pitts JD (1998) The discovery of metabolic co-operation. Bioessays 20:1047–1051 [DOI] [PubMed] [Google Scholar]
- Rabionet R, Gasparini P, Estivill X (2000a) Molecular genetics of hearing impairment due to mutations in gap junction genes encoding beta connexins. Hum Mutat 16:190–202 [DOI] [PubMed] [Google Scholar]
- Rabionet R, Zelante L, Lopez-Bigas N, D'Agruma L, Melchionda S, Restagno G, Arbones ML, Gasparini P, Estivill X (2000b) Molecular basis of childhood deafness resulting from mutations in the GJB2 (connexin 26) gene. Hum Genet 106:40–44 [DOI] [PubMed] [Google Scholar]
- Radhakrishna U, Blouin JL, Mehenni H, Mehta TY, Sheth FJ, Sheth JJ, Solanki JV, Antonarakis SE (1997) The gene for autosomal dominant hidrotic ectodermal dysplasia (Clouston syndrome) in a large Indian family maps to the 13q11-q12.1 pericentromeric region. Am J Med Genet 71:80–86 [DOI] [PubMed] [Google Scholar]
- Richard G, Brown N, Smith LE, Terrinoni A, Melino G, Mackie RM, Bale SJ, Uitto J (2000) The spectrum of mutations in erythrokeratodermias-novel and de novo mutations in GJB3. Hum Genet 106:321–329 [DOI] [PubMed] [Google Scholar]
- Richard G, Lin JP, Smith L, Ehyte YM, Itin P, Wollina U, Epstein EJ, Hohl D, Giroux JM, Charnas L, Bale SJ, DiGiovanna JJ (1997) Linkage studies in erythrokeratodermias; fine mapping, genetic heterogeneity and analysis of candidate genes. J Invest Dermatol 109:666–671 [DOI] [PubMed] [Google Scholar]
- Richard G, Smith LE, Bailey RA, Itin P, Hohl D, Epstein EH, DiGiovanna JJ, Compton JG, Bale SJ (1998a) Mutations in the human connexin gene GJB3 cause erythrokeratodermia variabilis. Nat Genet 20:366–369 [DOI] [PubMed] [Google Scholar]
- Richard G, White TW, Smith LE, Bailey RA, Compton JG, Paul DL, Bale SJ (1998b) Functional defects of Cx26 resulting from a heterozygous missense mutation in a family with dominant deaf-mutism and palmoplantar keratoderma. Hum Genet 103:393–399 [DOI] [PubMed] [Google Scholar]
- Rook A, Wilkinson D, Ebling F, Champion R, Burton J (1998) Textbook of dermatology, 6th ed. Blackwell Scientific, London [Google Scholar]
- Scherer SS, Bone LJ, Deschenes SM, Abel A, Balice-Gordon RJ, Fischbeck KH (1999) The role of the gap junction protein connexin32 in the pathogenesis of X-linked Charcot-Marie-Tooth disease. Novartis Found Symp 219:175–185 [DOI] [PubMed] [Google Scholar]
- Scott DA, Kraft ML, Carmi R, Ramesh A, Elbedour K, Yairi Y, Srisailapathy CR, Rosengren SS, Markham AF, Mueller RF, Lench NJ, Van Camp G, Smith RJ, Sheffield VC (1998a) Identification of mutations in the connexin 26 gene that cause autosomal recessive nonsyndromic hearing loss. Hum Mutat 11:387–394 [DOI] [PubMed] [Google Scholar]
- Scott DA, Kraft ML, Stone EM, Sheffield VC, Smith RJ (1998b) Connexin mutations and hearing loss. Nature 391:32 [DOI] [PubMed] [Google Scholar]
- Sevior KB, Hatamochi A, Stewart IA, Bykhovskaya Y, Allen-Powell DR, Fischel-Ghodsian N, Maw MA (1998) Mitochondrial A7445G mutation in two pedigrees with palmoplantar keratoderma and deafness. Am J Med Genet 75:179–185 [PubMed] [Google Scholar]
- Simon AM, Goodenough DA (1998) Diverse functions of vertebrate gap junctions. Trends Cell Biol 8:477–483 [DOI] [PubMed] [Google Scholar]
- Sobe T, Vreugde S, Shahin H, Berlin M, Davis N, Kanaan M, Yaron Y, Orr-Urtreger A, Frydman M, Shohat M, Avraham KB (2000) The prevalence and expression of inherited connexin 26 mutations associated with nonsyndromic hearing loss in the Israeli population. Hum Genet 106:50–57 [DOI] [PubMed] [Google Scholar]
- Stevens HP, Choon SE, Hennies HC, Kelsell DP (1999) Evidence for a single genetic locus in Clouston's hidrotic ectodermal dysplasia. Br J Dermatol 140:963–964 [DOI] [PubMed] [Google Scholar]
- Taylor TD, Hayflick SJ, McKinnon W, Guttmacher AE, Hovnanian A, Litt M, Zonana J (1998) Confirmation of linkage of Clouston syndrome (hidrotic ectodermal dysplasia) to 13q11-q12.1 with evidence for multiple independent mutations. J Invest Dermatol 111:83–85 [DOI] [PubMed] [Google Scholar]
- van der Schroeff JG, Nijenhuis LE, Meera Khan P, Bernini LF, Schreuder GMT, van Loghem E Volkers WS, Went LN (1984) Genetic linkage between erythrokeratrodermia variabilis and Rh locus. Hum Genet 68:165–168 [DOI] [PubMed] [Google Scholar]
- van der Schroeff JG, van Leeuwen-Cornelisse I, van Haeringen A, Went LN (1988) Further evidence for localisation of the gene of erythrokeratodermia variabilis. Hum Genet 80:97–98 [DOI] [PubMed] [Google Scholar]
- White TW, Deans MR, Kelsell DP, Paul DL (1998) Connexin mutations in deafness. Nature 394:630–631 [DOI] [PubMed] [Google Scholar]
- White TW, Paul DL (1999) Genetic diseases and gene knockouts reveal diverse connexin functions. Annu Rev Physiol 61:283–310 [DOI] [PubMed] [Google Scholar]
- Wilcox SA, Saunders K, Osborn AH, Arnold A, Wunderlich J, Kelly T, Collins V, Wilcox LJ, McKinlay Gardner RJ, Kamarinos M, Cone-Wesson B, Williamson R, Dahl HH (2000) High frequency hearing loss correlated with mutations in the GJB2 gene. Hum Genet 106:399–405 [DOI] [PubMed] [Google Scholar]
- Wilgoss A, Leigh IM, Barnes MR, Dopping-Hepenstal P, Eady RA, Walter JM, Kennedy CT, Kelsell DP (1999) Identification of a novel mutation R42P in the gap junction protein beta-3 associated with autosomal dominant erythrokeratoderma variabilis. J Invest Dermatol 113: 1119–1122 [DOI] [PubMed] [Google Scholar]
- Xia JH, Liu CY, Tang BS, Pan Q, Huang L, Dai HP, Zhang BR, Xie W, Hu DX, Zheng D, Shi XL, Wang DA, Xia K, Yu KP, Liao XD, Feng Y, Yang YF, Xiao JY, Xie DH, Huang JZ (1998) Mutations in the gene encoding gap junction protein beta-3 associated with autosomal dominant hearing impairment. Nat Genet 20: 370–373 [DOI] [PubMed] [Google Scholar]
- Zelante L, Gasparini P, Estivill X, Melchionda S, D'Agruma L, Govea N, Mila M, Monica MD, Lutfi J, Shohat M, Mansfield E, Delgrosso K, Rappaport E, Surrey S, Fortina P (1997) Connexin26 mutations associated with the most common form of non-syndromic neurosensory autosomal recessive deafness (DFNB1) in Mediterraneans. Hum Mol Genet 6:1605–1609 [DOI] [PubMed] [Google Scholar]