Abstract
We compared the disk diffusion and broth microdilution methods for susceptibility testing of caspofungin against Aspergillus (n = 78) and Fusarium (n = 22) isolates. Microdilution testing followed the NCCLS M-38P guidelines but was performed in antibiotic medium 3 supplemented to 2% glucose (AM3). Disk diffusion assays were performed on AM3 agar plates with a 2-μg caspofungin disk. By both methods, caspofungin showed favorable activity against Aspergillus isolates and no activity against Fusarium isolates. In the disk-based format, intrazonal growth that was not influenced by the drug concentration gradient was consistently observed for all of the Aspergillus isolates tested.
The development of novel, water-soluble echinocandins is an exciting development in systemic antifungal therapy. These agents exert antifungal activity via inhibition of (1,3)-β-D-glucan synthesis. Because of the lack of this target in mammalian tissue and the dissimilarity between the mechanism of action of echinocandins and other systemic antifungal drugs (particularly polyenes and azoles), echinocandins appear to be advantageous and promising for the treatment of invasive mycoses (2, 4, 5, 7, 8, 10, 12, 13, 19, 22). Caspofungin (Cancidas; Merck Research Laboratories) is a novel echinocandin that was licensed in January 2001 in the United States.
While caspofungin has proven to be efficacious in the treatment of candidiasis, aspergillosis, and possibly other mycoses (9; J. Hiemenz, I. Raad, M. Boogaerts, J. Maertens, A. Saah, C. A. Sable, J. A. Chodakewitz, M. Severino, P. Saddier, R. Berman, M. J. DiNubile, T. F. Patterson, D. W. Denning, and T. J. Walsh, Focus on Fungal Infections 11, abstr. 21, 2001; J. Maertens, I. Raad, C. A. Sable, A. Ngai, R. Berman, T. F. Patterson, D. Denning, and T. Walsh, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1103, 2000; M. A. Powles, J. Anderson, P. Liberator, and D. M. Schmatz, Abstr. 36th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-42, 1996; C. A. Sable, A. Villanueva, E. Arathon, E. Gotuzzo, G. Turcato, D. Uip, L. Nriega, C. Rivera, E. Rojas, V. Taylor, R. Berman, G. B. Calandra, and J. Chodakewitz, Abstr. 37th Intersci. Conf. Antimicrob. Agents Chemother., abstr. LB-33, 1997), in vitro susceptibility testing method and interpretive test parameters have not been fully established for this novel drug. In addition, there are few data correlating its in vitro activity and the in vivo response to it. Previous studies have focused on broth dilution methods for susceptibility testing of caspofungin against yeasts and molds (6, 17, 20, 21). When testing caspofungin and other echinocandins against molds, a distinctive microscopic interpretive parameter called the minimum effective concentration (MEC) has been found to be more consistent and also appears to be better correlated with clinical outcome than the conventional macroscopic MIC-2 (1, 9, 11, 14; C. M. Douglas, J. C. Bowman, G. K. Abruzzo, A. M. Flattery, C. J. Gill, L. Kong, C. Leighton, J. G. Smith, V. B. Pikounis, K. Bartizal, M. B. Kurtz, and H. Rosen, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1683, 2000). Agar-based testing methods are the other obvious avenue of investigation. Some investigators have explored the use of disk diffusion susceptibility testing for caspofungin against yeasts (specifically, Candida isolates) (16), but data on caspofungin testing against molds by the disk diffusion method are lacking. In this study, we investigated the applicability of a disk diffusion assay in susceptibility testing of caspofungin against Aspergillus and Fusarium isolates and compared the results of this disk diffusion assay with those of the broth microdilution method for these isolates.
(This work was presented at the 41st Interscience Conference on Antimicrobial Agents and Chemotherapy [abstr. J-571].)
Aspergillus strains (n = 78; 27 Aspergillus flavus, 26 Aspergillus fumigatus, 16 Aspergillus niger, and 9 Aspergillus terreus) and Fusarium strains (n = 22; 18 Fusarium solani and 4 Fusarium oxysporum) isolated from clinical samples were included in this study. One of the Aspergillus fumigatus isolates (strain 2-160) was included in each run of susceptibility tests as a reference strain to validate the reproducibility and quality of the test results. The isolates were defined to the species level by standard methods (15) and stored on Sabouraud dextrose agar slants at −70°C until tested. Standard caspofungin powder was provided by Merck Research Laboratories for use in susceptibility tests.
Caspofungin susceptibility tests were done by using the broth microdilution and disk diffusion methods. Except as noted otherwise, broth microdilution tests were done in accordance with the NCCLS guidelines for conidium-forming filamentous fungi (18). Antibiotic medium 3 (lot JD4ZSG; BBL, Becton Dickinson) buffered by addition of 1 g of Na2HPO4 and 1g of NaH2PO4 to each liter and supplemented to 2% glucose (AM3) was used as the test medium. Serial twofold dilutions of caspofungin over a range of 16 to 0.03 μg/ml were prepared in microdilution plates. The results were read after 24, 48, and 72 h of incubation by using two different parameters: the visual MIC-2 (the minimum concentration [micrograms per milliliter] of caspofungin that provides an ∼50% reduction in growth compared to the growth in the control well) and the microscopic MEC (the minimum concentration [micrograms per milliliter] of caspofungin that results in the formation of abnormal hyphal growth with short, abundant branchings [14]). The broth microdilution assay results for the isolates included in this study were published in one of our previous reports (1).
Disk diffusion tests were done by using empirically defined parameters. Caspofungin disks were prepared in house. Blank paper disks (6.3 mm in diameter; Becton Dickinson Microbiology Systems, Cockeysville, Md.) were impregnated with 20 μl of a caspofungin suspension (concentration, 100 μg/ml), resulting in a final concentration of 2 μg/disk. The disks were allowed to dry at room temperature. (This disk concentration was chosen on the basis of the results of preliminary experiments in which blank disks were impregnated with 20 μl from suspensions containing caspofungin at concentrations of 200, 100, 50, and 25 μg/ml. The concentration of 100 μg/ml was chosen because it yielded inhibition zones [IZs] that are wide enough to be measured and sufficiently narrow to be accurately determined.) The strain to be tested was initially suspended in saline, adjusted spectrophotometrically to ∼81 or ∼70% transmittance (for Aspergillus and Fusarium, respectively), and then diluted 1/100 in distilled water to achieve a final concentration of ∼104 CFU/ml. The prepared inoculum was swabbed onto an AM3 agar plate, and the plate was left to dry at room temperature for 20 min. A caspofungin disk was then placed onto the center of the inoculated agar plate. The plates were incubated for 72 h at 35°C, and the IZ diameters (millimeters) were measured at 24, 48, and 72 h of incubation. The edges of the IZs were taken as the points of a marked decrease in fungal density.
For comparative evaluation of the broth microdilution and disk diffusion methods, the geometric mean (GM) and range of the MICs and MECs and the arithmetic mean and range of the IZ diameters were calculated for each genus-species combination. For computation of GM values, high off-scale MICs and MECs were converted to the twofold concentration just above the highest drug concentration tested.
The results obtained by the microdilution and disk diffusion methods at 24, 48, and 72 h of incubation of the test isolates and reference strain 2-160 are shown in Tables 1 and 2, respectively. While broth microdilution test results could be determined at all of the reading time points (24, 48, and 72 h), disk diffusion test results could not be interpreted at 24 h because of poor growth. At 48 and 72 h, IZs with sharply defined edges were observed on agar plates.
TABLE 1.
Microdilution and disk diffusion test results at 24, 48, and 72 h of incubation
| Species (no. of isolates) | Incubation period (h)a | IZ diam (mm)
|
MIC-2 (μg/ml)
|
MEC (μg/ml)
|
|||
|---|---|---|---|---|---|---|---|
| AMb | Range | GMc | Range | GM | Range | ||
| Aspergillus spp. (78) | |||||||
| A. flavus (27) | 24 | 2.27 | 0.125->16 | 0.26 | 0.125-0.5 | ||
| 48 | 21.2 | 16-25 | 3.7 | 0.125->16 | 0.26 | 0.125-0.5 | |
| 72 | 20.1 | 15-23 | >16 | >16 | 0.26 | 0.125-0.5 | |
| A. fumigatus (26) | 24 | 0.43 | 0.25->16 | 0.31 | 0.25-0.5 | ||
| 48 | 16.2 | 12-23 | 2.83 | 0.25->16 | 0.31 | 0.25-0.5 | |
| 72 | 15.3 | 11-19 | 17.4 | 0.25->16 | 0.31 | 0.25-0.5 | |
| A. niger (16) | 24 | 0.23 | 0.125-1 | 0.2 | 0.125-0.25 | ||
| 48 | 18.1 | 15-24 | 0.26 | 0.125-0.5 | 0.2 | 0.125-0.25 | |
| 72 | 17.4 | 15-24 | 0.32 | 0.25-1 | 0.27 | 0.25-0.5 | |
| A. terreus (9) | 24 | 0.25 | 0.25 | 0.25 | 0.25 | ||
| 48 | 19.6 | 12-26 | 0.25 | 0.25 | 0.25 | 0.25 | |
| 72 | 17.4 | 10-23 | 1.26 | 0.25->16 | 0.25 | 0.25 | |
| Fusarium spp. (22) | |||||||
| F. solani (18) | 24 | 19.4 | 16->16 | 16.63 | 4->16 | ||
| 48 | NZd | 26.4 | 16->16 | 26.4 | 16->16 | ||
| 72 | NZ | >16 | >16 | >16 | >16 | ||
| F. oxysporum (4) | 24 | 16 | 16 | 16 | 16 | ||
| 48 | NZ | 26.91 | 16->16 | 26.91 | 16->16 | ||
| 72 | NZ | >16 | >16 | >16 | >16 | ||
Disk diffusion test results could not be evaluated at 24 h because of the poor growth of the isolates.
AM, arithmetic mean.
GM, geometric mean.
NZ, no IZ detected.
TABLE 2.
Microdilution and disk diffusion test results obtained for reference strain 2-160a
| Incubation period (h) | IZ diam range (mm) | MIC-2 range (μg/ml) | MEC range (μg/ml) |
|---|---|---|---|
| 24 | 0.125-0.25 | 0.125-0.25 | |
| 48 | 15-17 | 0.25->16 | 0.25 |
| 72 | 13-17 | 0.25->16 | 0.25 |
The results shown were obtained in four runs and by testing the isolate in duplicate in each run. Disk diffusion test results could not be evaluated at 24 h because of poor growth.
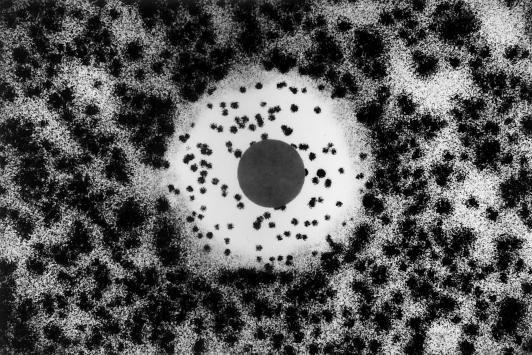
All of the Aspergillus strains generated measurable IZs, and the diameters of these IZs were distributed over a relatively narrow range. Interestingly and in addition, Aspergillus microcolonies were visualized inside the IZs and these colonies were found uniformly distributed right up to and even under the disks without any evident effect of the drug concentration gradient (Fig. 1). These colonies were observed for all of the Aspergillus species and strains tested. It is noteworthy that the number of intrazonal colonies observed for A. niger was lower than the number observed for other Aspergillus species. When examined under a microscope, these intrazonal colonies produced short, stubby hyphal branchings and a star-like morphology. This appearance was similar to that observed at the MEC. The colonies outside the zone, on the other hand, showed a normal, elongated, branching hyphal morphology. When the intrazonal colonies were subcultured and retested, they yielded a pattern identical to that seen with the original isolate. On the other hand, confluent growth and absence of an IZ were consistently observed for all of the Fusarium isolates. Comparison of the results obtained by the broth microdilution and disk diffusion assays showed that, as for the Fusarium isolates, the high MECs obtained by the microdilution method correlated very well with absence of IZs on disk diffusion agar plates. Relatedly and as for Aspergillus isolates, relatively lower MECs correlated with the production of measurable IZs around caspofungin disks.
FIG. 1.
Aspergillus growth pattern obtained by the disk diffusion test method. Note the clear zone edges and the microcolonies inside the IZ. The disk contained 2 μg of caspofungin.
In the present study, our major goal was to investigate the relevance of the disk diffusion assay for caspofungin susceptibility testing against Aspergillus and Fusarium strains. We thus compared the disk diffusion assay results with our MECs previously determined by the broth microdilution method. Since MICs tended to increase inconsistently, particularly with extended incubation (1), we focused more on the correlation of MECs with IZ diameters. The comparative evaluation of the two methods showed that while lower MECs corresponded to the generation of IZs, higher MECs were in absolute correlation with the absence of IZs. Relatively lower MECs and measurable IZs were obtained with Aspergillus isolates, while Fusarium spp. consistently generated very high MECs and no IZs. Being less time-consuming and less labor-intensive, the disk diffusion method is preferable to the microdilution method. However, the inability to determine the susceptibility test result at 24 h for an individual isolate appears to be a notable limitation of the disk diffusion assay. Further investigation is required to determine whether higher inoculum concentrations yield satisfactory growth on disk diffusion agar plates at 24 h.
Our observation of the growth of Aspergillus microcolonies inside the IZs was noteworthy. Since the intrazonal colonies produced the same growth pattern when retested, the possibility of heterogeneous resistance was ruled out. Thus, this finding appeared to be a special growth pattern. This might originate from the partial inhibitory nature of caspofungin and other echinocandins and correspond to the lack of complete inhibition of growth observed both in microdilution plate wells with high caspofungin concentrations and by examination of the intrazonal colonies under a microscope. The mechanism, meaning, and clinical significance of this observation remain unclear and merit investigation.
In this study, we used the disk diffusion assay for susceptibility testing of caspofungin against Aspergillus and Fusarium isolates and compared the results with those obtained by the microdilution method. A preliminary report has been previously published on the use of the disk diffusion assay for candin derivatives L-733560, L-705589, and L-731373 against Aspergillus spp. In that study, potato dextrose agar was seeded with 106 CFU of A. fumigatus spores and poured into petri dishes. Disks impregnated with each of the candins (at concentrations of 128 to 0.06 μg/ml) were placed on the agar plates. IZs were observed after 24 h of incubation, demonstrating the favorable in vitro activity of the compounds against Aspergillus strains. No observation was noted regarding intrazonal growth (3).
We conclude that the disk diffusion and microdilution methods appear to be correlated in the susceptibility testing of caspofungin against Aspergillus and Fusarium isolates. The intrazonal growth of colonies of Aspergillus spp. is of uncertain relevance.
REFERENCES
- 1.Arikan, S., M. Lozano-Chiu, V. Paetznick, and J. H. Rex. 2001. In vitro susceptibility testing methods for caspofungin against Aspergillus and Fusarium isolates. Antimicrob. Agents Chemother. 45:327-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arikan, S., and J. H. Rex. 2000. New agents for treatment of systemic fungal infections. Emerg. Drugs 5:135-160. [DOI] [PubMed] [Google Scholar]
- 3.Bartizal, K., T. Scott, G. K. Abruzzo, C. J. Gill, C. Pacholok, L. Lynch, and H. Kropp. 1995. In vitro evaluation of the pneumocandin antifungal agent L-733560, a new water-soluble hybrid of L-705589 and L-731373. Antimicrob. Agents Chemother. 39:1070-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beauvais, A., and J. P. Latge. 2001. Membrane and cell wall targets in Aspergillus fumigatus. Drug Resist. Update 4:38-49. [DOI] [PubMed] [Google Scholar]
- 5.Cuenca-Estrella, M., E. Mellado, T. M. Diaz-Guerra, A. Monzon, and J. L. Rodriguez-Tudela. 2000. Susceptibility of fluconazole-resistant clinical isolates of Candida spp. to echinocandin LY303366, itraconazole and amphotericin B. J. Antimicrob. Chemother. 46:475-477. [DOI] [PubMed] [Google Scholar]
- 6.Del Poeta, M., W. A. Schell, and J. R. Perfect. 1997. In vitro antifungal activity of pneumocandin L-743,872 against a variety of clinically important molds. Antimicrob. Agents Chemother. 41:1835-1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Denning, D. W. 1997. Echinocandins and pneumocandins—a new antifungal class with a novel mode of action. J. Antimicrob. Chemother. 40:611-614. [DOI] [PubMed] [Google Scholar]
- 8.Georgopapadakou, N. H. 2001. Update on antifungals targeted to the cell wall: focus on beta-1,3-glucan synthase inhibitors. Expert Opin. Investig. Drugs 10:269-280. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez, G. M., R. Tijerina, L. K. Najvar, R. Bocanegra, M. Luther, M. G. Rinaldi, and J. R. Graybill. 2001. Correlation between antifungal susceptibilities of Coccidioides immitis in vitro and antifungal treatment with caspofungin in a mouse model. Antimicrob. Agents Chemother. 45:1854-1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graybill, J. R. 2001. Hitting a new target with echinocandins. Why chase something else? Curr. Opin. Investig. Drugs 2:468-471. [PubMed] [Google Scholar]
- 11.Hawser, S. P., C. Jessup, J. Vitullo, and M. A. Ghannoum. 2001. Utility of2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenyl-amino)carbonyl]-2H-tetrazolium hydroxide (XTT) and minimum effective concentration assays in the determination of antifungal susceptibility of Aspergillus fumigatus to the lipopeptide class of compounds. J. Clin. Microbiol. 39:2738-2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hossain, M. A., and M. A. Ghannoum. 2000. New investigational antifungal agents for treating invasive fungal infections. Expert Opin. Investig. Drugs 9:1797-1813. [DOI] [PubMed] [Google Scholar]
- 13.Kurtz, M. B., and C. M. Douglas. 1997. Lipopeptide inhibitors of fungal glucan synthase. J. Med. Vet. Mycol. 35:79-86. [DOI] [PubMed] [Google Scholar]
- 14.Kurtz, M. B., I. B. Heath, J. Marrinan, S. Dreikorn, J. Onishi, and C. Douglas. 1994. Morphological effects of lipopeptides against Aspergillus fumigatus correlate with activities against (1,3)-β-D-glucan synthase. Antimicrob. Agents Chemother. 38:1480-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larone, D. H. 1995. Medically important fungi: a guide to identification, 3rd ed. ASM Press, Washington, D.C.
- 16.Lozano-Chiu, M., P. W. Nelson, V. L. Paetznick, and J. H. Rex. 1999. Disk diffusion method for determining susceptibilities of Candida spp. to MK-0991. J. Clin. Microbiol. 37:1625-1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marco, F., M. A. Pfaller, S. A. Messer, and R. N. Jones. 1998. Activity of MK-0991 (L-743,872), a new echinocandin, compared with those of LY303366 and four other antifungal agents tested against blood stream isolates of Candida spp. Diagn. Microbiol. Infect. Dis. 32:33-37. [DOI] [PubMed] [Google Scholar]
- 18.National Committee for Clinical Laboratory Standards. 1998. Reference method for broth dilution antifungal susceptibility testing of conidium-forming filamentous fungi; proposed standard. NCCLS document M38-P. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 19.Onishi, J., M. Meinz, J. Thompson, J. Curotto, S. Dreikorn, M. Rosenbach, C. Douglas, G. Abruzzo, A. Flattery, L. Kong, A. Cabello, F. Vicente, F. Pelaez, M. T. Diez, I. Martin, G. Bills, R. Giacobbe, A. Dombrowski, R. Schwartz, S. Morris, G. Harris, A. Tsipouras, K. Wilson, and M. B. Kurtz. 2000. Discovery of novel antifungal (1,3)-β-D-glucan synthase inhibitors. Antimicrob. Agents Chemother. 44:368-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pfaller, M. A., F. Marco, S. A. Messer, and R. N. Jones. 1998. In vitro activity of two echinocandin derivatives, LY303366 and MK-0991 (L-743,792), against clinical isolates of Aspergillus, Fusarium, Rhizopus, and other filamentous fungi. Diagn. Microbiol. Infect. Dis. 30:251-255. [DOI] [PubMed] [Google Scholar]
- 21.Vazquez, J. A., M. Lynch, D. Boikov, and J. D. Sobel. 1997. In vitro activity of a new pneumocandin antifungal, L-743,872, against azole-susceptible and -resistant Candida species. Antimicrob. Agents Chemother. 41:1612-1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Warnock, D. W., B. A. Arthington-Skaggs, and R. K. Li. 1999. Antifungal drug susceptibility testing and resistance in Aspergillus. Drug Resist. Update 2:326-334. [DOI] [PubMed] [Google Scholar]