Abstract
Familial hemiplegic migraine, episodic ataxia type 2 (EA2), and spinocerebellar ataxia type 6 are allelic disorders of the CACNA1A gene (coding for the α1A subunit of P/Q calcium channels), usually associated with different types of mutations (missense, protein truncating, and expansion, respectively). However, the finding of expansion and missense mutations in patients with EA2 has blurred this genotype-phenotype correlation. We report the first functional analysis of a new missense mutation, associated with an EA2 phenotype—that is, T→C transition of nt 4747 in exon 28, predicted to change a highly conserved phenylalanine residue to a serine at codon 1491, located in the putative transmembrane segment S6 of domain III. Patch-clamp recording in HEK 293 cells, coexpressing the mutagenized human α1A-2 subunit, together with human β4 and α2δ subunits, showed that channel activity was completely abolished, although the mutated protein is expressed in the cell. These results indicate that a complete loss of P/Q channel function is the mechanism underlying EA2, whether due to truncating or to missense mutations.
Mutations of the CACNA1A gene, coding for the pore-forming α1A subunit of voltage-gated P/Q Ca2+ channels, were found to be responsible for three allelic disorders with autosomal dominant inheritance: familial hemiplegic migraine (FHM [MIM 141500]), episodic ataxia type 2 (EA2 [MIM 108500]) (Ophoff et al. 1996), and spinocerebellar ataxia type 6 (SCA6 [MIM 183086]) (Zhuchenko et al. 1997). The gene is expressed throughout the brain and particularly in the cerebellar Purkinje and granule cells (Westenbroek et al. 1995; Volsen et al. 1995). P/Q-type Ca2+ channels play a prominent role in controlling neurotransmitter release in many synapses (Dunlap et al. 1995). Furthermore, their localization in dendrites and cell bodies suggests additional postsynaptic roles. FHM, characterized by hemiplegic migraine attacks with or without interictal cerebellar signs, is associated with missense mutations in all families so far reported (Ophoff et al. 1996; Battistini et al. 1999; Ducros et al. 1999). EA2, with ataxia/vertigo episodes and interictal cerebellar signs of variable severity, is most often due to mutations disrupting the reading frame and/or leading to premature stop (Ophoff et al. 1996; Denier et al. 1999; Jen et al. 1999). SCA6, whose clinical features widely overlap with those of EA2 (Jodice et al. 1997; Frontali et al. 1999; Frontali and Jodice, in press), is caused by small expansions of the CAG-repeat sequence at the 3′ end of the gene (Zhuchenko et al. 1997). However, patients with an EA2 phenotype were also found to carry either a CAG expansion (Jodice et al. 1997) or missense mutations (Yue et al. 1997; Denier et al. 1999; Friend et al. 1999), thus blurring the picture of the above genotype/phenotype correlation.
The functional analysis of missense mutations causing FHM showed different patterns of altered channel activity (Kraus et al. 1998; Hans et al. 1999). It has been shown that the changes in single-channel function and channel expression produced by different FHM mutations can lead to opposite effects on Ca2+ influx (Hans et al. 1999). It is worth noting that only mutations that are predicted to cause a reduction of Ca2+ influx are associated with a phenotype that includes cerebellar ataxia in addition to FHM (Hans et al. 1999). Moreover, a reduced channel availability has been reported for the channels formed by the α1A subunit containing the SCA6 mutation—that is, an expanded polyglutamine repeat (Matsuyama et al. 1999; Toru et al. 2000).
No functional analysis has as yet been performed on mutations responsible for EA2. Most EA2 mutations are predicted to produce a truncated protein and are highly likely to be associated with a loss of channel function, either because of difficulties in channel assembly or loss of truncated polypeptides. Nothing is known about the channel function in the presence of EA2 nontruncating mutations such as the gly293arg mutation, located in the putative P region of the I domain (Yue et al. 1997), del Y1594/A1593D, in the putative S2 segment of domain IV (Denier et al. 1999), and Arg1666His, in the IV S4 domain (Friend et al. 1999).
We report a family with EA2 (fig. 1) carrying a de novo missense mutation in the transmembrane S6 segment of the III protein domain. The functional analysis of the mutated α1A subunit showed that no P/Q type Ca2+ channel activity was detected, although protein expression is similar to that of wild type (wt), thus differentiating this missense mutation from those associated with FHM (Hans et al. 1999).
Figure 1.
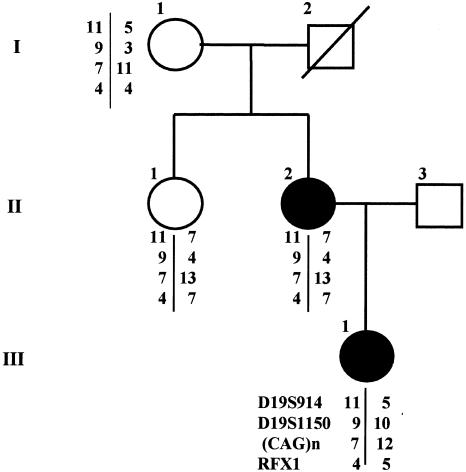
Pedigree of the family with EA2 carrying the missense CACNA1A mutation. Blackened circles indicate patients with EA2. Chromosome-19–marker haplotypes, typed as previously reported (Kern et al. 1994), are also shown. The proband (III-1), with a congenital strabismus surgically corrected, has had vertigo episodes since age 5 years. At age 12 years, a progressive gait imbalance began with subcontinuous vertigo exacerbated by rapid head movements and emotional stress. Neurological examination at age 17 years showed generalized hypotonia, nystagmus on lateral gaze, impaired smooth pursuit, gait ataxia, and mild dysmetria. More recently, neurological examination showed a complex dyskinetic movement of the right leg, most likely of a dystonic type. The proband’s mother (II-2) had a strabismus surgically corrected in infancy; her episodes, which began at age 7 years, were more severe and prolonged in the past (with vertigo, trunk ataxia, rotatory nystagmus on lateral and vertical gaze, nausea, and vomiting) and milder and shorter recently. She has had migraine attacks without aura since age 13 years. Neurological examination, at age 49 years, revealed interictal rotatory nystagmus on lateral gaze, with impairment of smooth-eye pursuit, mild truncal ataxia and dysmetria, and a writer’s cramp of the right hand. Appropriate informed consent was obtained by all family members analyzed.
The mutation has been detected by SSCP analysis and sequencing in two members of the family reported in figure 1. SSCP analysis of patients’ DNA was performed, according to published protocols (Orita et al. 1989; Ravnik-Glavac et al. 1994), on all 47 exons and intron-exon junctions of CACNA1A gene (Ophoff et al. 1996) and on the alternative exon 37 (Trettel et al. 2000). Analysis of exon 28 showed an aberrantly migrating fragment in the proband (III-1) and in her mother (II-2). Its sequencing showed a T→C transition at position 4747 in the CACNA1A sequence, reported by Ophoff et al. (1996) (GenBank). The aberrant migration was not found in 50 random Italian subjects, whose DNA was analyzed by the Amplification Refractory Mutation System. The mutation predicts a substitution of a highly conserved phenylalanine, a nonpolar hydrophobic amino acid, with serine, a polar noncharged amino acid, at codon 1491 (GenBank)—that is, in the putative segment 6 of the III protein domain (fig. 2). The only other detected migration anomaly was in the intron between exons 23 and 24, and it consisted of an already described T/C polymorphism at nt 4142 (Ophoff et al. 1996). Sequencing of exon 28 in the other two members of the family (I-1 and II-1) showed a wt sequence. Typing the whole family for 19p13 markers D19S914, D19S1150, CAGn, and RFX1 showed that the same haplotype was shared by the two affected subjects and the two healthy ones, indicating that a de novo mutation arose in the proband’s mother. No CAG expansion was detected in any of the family members.
Figure 2.
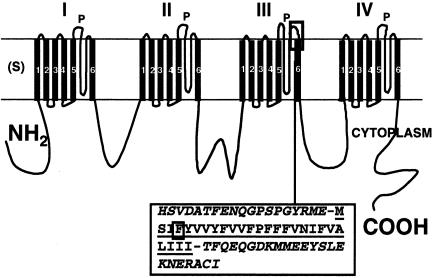
Linear view of the α1A subunit of calcium channel, coded by CACNA1A gene. Each of four domains (I-IV) has six transmembrane segments (S), and one pore-forming segment (P) between S5 and S6. The F1491S mutation (squared F in the sequence) involves the fourth amino acid in the sequence of segment III S6 (underlined), immediately downstream from the end of the P-segment sequence (italics).
The phenotype (see also fig. 1 legend) associated with this mutation includes vertigo episodes, sometimes so prolonged as to require admission to a hospital, interictal cerebellar signs of variable severity including a permanent ataxia, cerebellar vermis atrophy at MRI scan, and sensitivity to acetazolamide treatment, as in most EA2 patients (von Brederlow et al. 1995; Denier et al. 1999; Jen et al. 1999). In addition, a strabismus and a dystonic dyskinesia were present in both patients. Given the lack of clinical details about the strabismus before its surgical correction, no hypotheses can be proposed about its being purely coincidental with EA2 or part of the phenotype due to the mutation. It is worth noting, however, that strabismus was part of the clinical picture of a family, reported by Harris et al. (1993), in association with a dominant vestibulocerebellar pattern of oculomotor anomalies very similar to that found in EA2, but without extraocular cerebellar signs. The presence of dystonic features, instead, might be part of the mutation-associated phenotype, since a severe dystonia is reported in mice homozygotes for the null CACNA1A mutation (Jun et al. 1999; Fletcher et al., in press). Migraine with visual aura was present in the proband’s grandmother (I-1) and in the maternal aunt (II-1), who does not carry the mutation and who had a normal neurological examination. Migraine without aura was affecting the proband’s mother (II-2) but not the proband herself. Migraine, therefore, appears to segregate independently of the mutation. On the whole, the phenotype, despite exhibiting some peculiar features, is markedly different from that of FHM associated with other missense mutations (Battistini et al. 1999; Ducros et al. 1999) and is fully compatible with that of EA2 (von Brederlow et al. 1995; Denier et al. 1999; Jen et al. 1999).
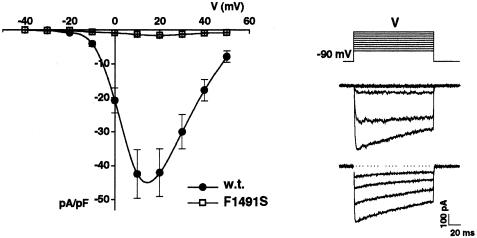
To investigate its functional consequences, the mutation F1491S was inserted, by standard site-directed mutagenesis, into the human cDNA of the α1A-2 subunit (Hans et al. 1999), which is 99.5% identical to the human BI-1 isoform (Zhuchenko et al. 1997). The wt or the mutant α1A-2 subunits were expressed in human embryonic kidney (HEK) 293 cells, together with human α2bδ and human β4a subunits. Figure 3 shows the average current-voltage relationships measured for wt and F1491S mutant channels. The maximal current density measured at +10 mV in cells expressing wt human recombinant P/Q-type Ca2+ channels was 42.8 ± 6 pA/pF (n=15; 5 mM Ba2+), whereas in cells expressing the mutant P/Q channels, the current density at +10 mV was 1.31±0.14 pA/pF (n=28; 10 mM Ba2+) or .99±.09 pA/pF (n=6; 5 mM Ba2+). The small current measured in cells expressing the mutant channels is due to endogenous Ca2+ channels of HEK293 cells, since a similar current was measured in untransfected HEK293 cells (1.10±.16 pA/pF at +10 mV, n=13; 10 mM Ba2+). The endogenous Ba2+ current density, measured after incubation of the cells with both ω-CTx-MVIIC (5 μM) and ω-CgTxGVIA (1 μM), was not significantly different (.95±.28 pA/pF, n=6), thus excluding that P/Q and N-type Ca2+ channels significantly contribute to this current. These results show that human α1A subunits containing mutation F1491S do not form functional P/Q-type Ca2+ channels.
Figure 3.
Whole-cell current density of human recombinant wt and F1491S calcium channels. HEK293 cells were grown in culture and transfected with human pcDNA1α1A-2 or human pcDNA1_α 1A-2F1491S together with cDNA1_α 2bδ,_pCMVβ4a and pCMVCD4, as described by Hans et al. (1999). Cells were placed into a recording chamber with Tyrode’s solution and, after attainment of the whole-cell configuration, were perfused with the external recording solution containing 5 (for wt channel) or 10 (for mutant channel, to allow for more accurate measurement of very small current) mM BaCl2, 148 mM TEA-Cl, and 10 mM HEPES. Internal solution contained 100 mM Cs-methanesulfonate, 5 mM MgCl2, 30 mM HEPES, 10 mM EGTA, 4 mM ATP, 0.5 mM GTP, and 1 mM c-AMP. Patch-clamp recordings were performed at room temperature (21°–25°C), following standard techniques (Hamill et al. 1981), using an Axopatch-200A amplifier, were low-pass filtered at 1 kHz and were digitized at 5 kHz. Step depolarizations, lasting 136 ms, were delivered from a holding potential of −90 mV. Compensation (typically 70%–80%) for series resistance was generally used, and only data from cells with a voltage error of <5 mV were analyzed. Current-voltage relationships were pooled from 11 cells transfected with either wt or mutant channels. For each cell, the peak current was divided by the cell capacitance to obtain the current density. Representative current traces at increasing test depolarizations, from a cell expressing wt channels, are shown on the right (top: Vt = −50 to +10 mV; bottom: Vt = +20 to +50 mV).
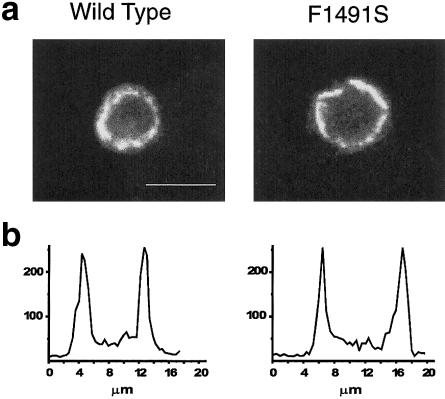
To assess whether the absence of functional mutant channels was due to lack of channel activity or to lack of channel expression, we used a specific polyclonal antihuman α1A antibody and confocal immunofluorescence analysis to localize the subunit in HEK293 cells transfected either with wt or with mutant cDNA, together with that of auxiliary subunits. Figure 4 shows that the level of protein expression appeared to be very similar for wt and for mutant α1A subunits. The fluorescence measured along a line drawn on each cell shows two rather broad peaks, whose values (in arbitrary units) were, on average, 171±13 and 183±15, respectively, in 19 and 16 cells transfected with wt and mutant α1A subunits. Thus, the absence of functional mutant P/Q-type calcium channels is not due to lack of channel expression. It remains unclear whether the lack of functional mutant channels is due to lack of membrane targeting or to complete loss of function of channels localized in the membrane.
Figure 4.
Immunofluorescence localization of recombinant human wt and mutant α1A subunits. Top, laser scanning confocal images of representative HEK293 cells transfected with either wt human α1A-2 or human α1A-2 F1491S subunits, together with β4a and α2bδ subunits, and labeled with a specific rabbit antihuman α1A antibody prepared against unique amino acid sequences residing in the cytoplasmic intracellular loop between IIS6 and IIIS1, as described by Volsen et al. (1995). Cell samples were mounted on 90% (v/v) glycerol, 0.2% (w/v) N-propylgallate in PBS, and cells were viewed using the MRC-1024 Laser Scanning Confocal Imaging System (Bio-Rad). Quantification of the fluorescence along selected lines drawn on cells was obtained using the LaserSharp software. The transfected cells were identified using GFP. Scale bar: 10 μm. Untransfected GFP-negative cells do not show any labeling by the human α1A antibody (data not shown). Bottom, fluorescence (in arbitrary units) measured along a line drawn on each cell. The immunofluorescence shows two rather broad peaks of similar amplitude in cells transfected with wt and mutant α1A subunits.
The mutated amino acid F1491 lies in the putative transmembrane segment IIIS6, very near the outer channel mouth, and is highly conserved across all the different classes of voltage-gated calcium channel α1 subunits. S6 segments are thought to contribute to the pore lining of voltage-dependent ion channels (Armstrong and Hille 1998). Consistent with this idea, mutations in S6 segments of Ca2+ channels have been shown to change single-channel conductance (Hans et al. 1999) and channel selectivity (Hockerman et al. 1997). Moreover, several lines of evidence show that S6 segments contain key elements for the voltage-dependent inactivation of Ca2+ channels (Hering et al. 1998). In a chimeric rabbit-carp α1A subunit containing the IVS6 segment of the α1C subunit, the inactivation time course was slowed down by the substitution of the phenylalanine corresponding to human F1491 with an alanine (Hering et al. 1997). However, it appears very unlikely that the lack of functional human F1491S P/Q channels, reported here, may be due to permanent inactivation of the channels, since we did not measure any Ca2+ current in the entire voltage range, even with highly negative holding potentials. Moreover, it should be assumed that the effects on inactivation go in opposite directions, depending on whether phenylalanine is changed to alanine or serine. Instead, it is more likely that the presence of a serine residue, a small hydrophilic amino acid, instead of a phenylalanine, a larger hydrophobic amino acid, could impair the proper folding of the protein segment, with the consequence that it will not assume a correct position within the membrane, grossly altering the pore structure. According to the latter hypothesis, it is unlikely that other protein isoforms, not tested in the present study, would yield results different from those obtained here.
Assuming that the mutation would display similar effects in physiologically different HEK293 and neuronal cells, a heterozygous condition for the mutation, in vivo, would reduce by 50% the number of functional P/Q channels and could act through a haploinsufficiency mechanism or, alternatively, a dominant-negative effect. In the latter case, a competition between functional and nonfunctional channels for regulatory-associated proteins might be hypothesized. This would also provide a possible explanation for the presence of a neurological phenotype in heterozygous humans, in contrast with its absence in heterozygous Cacna1a null mice—in which protein expression is reduced by 50%, but in which only functional P/Q channels are present in the membrane (Fletcher et al., in press).
The present study indicates that a complete loss of P/Q channel function is most likely the mechanism underlying EA2, whether due to truncating or to missense mutations, thus differentiating the present EA2 missense mutation from those causing pure FHM, shown to exhibit a gain rather than a loss of function (Hans et al. 1999). Whether other nontruncating mutations that cause EA2 are also leading to a loss of function remains to be determined. A partial loss of channel function was also displayed by missense mutations, causing FHM with additional cerebellar signs (Hans et al. 1999), and by CAGn expansions (Matsuyama et al. 1999; Toru et al. 2000), suggesting, as a working hypothesis for future research, the possibility that a common basis underlies the cerebellar involvement in all three allelic disorders.
Acknowledgment
Telethon Italia (grant E847) supported this research.
Electronic-Database Information
Accession numbers and URLs for data in this article are as follows:
- GenBank: http://www3.ncbi.nlm.nih.gov/ (for CACNA1A nucleotide sequence, accession number X99897, and for amino acid sequence, accession number CAA68172)
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for FHM [MIM 141500], EA2 [MIM 108500]), and SCA6 [MIM 183086])
References
- Armstrong CM, Hille B (1998) Voltage-gated ion channels and electrical excitability. Neuron 20:371–380 [DOI] [PubMed] [Google Scholar]
- Battistini S, Stenirri S, Piatti M, Gelfi C, Righetti PG, Rocchi R, Giannini F, Battistini N, Guazzi GC, Ferrari M, Carrera P (1999) A new CACNA1A gene mutation in acetazolamide-responsive familial hemiplegic migraine and ataxia. Neurology 53:38–43 [DOI] [PubMed] [Google Scholar]
- Denier C, Ducros A, Vahedi K, Joutel A, Thierry P, Ritz A, Castelnovo G, Deonna T, Gerard P, Devoize JL, Gayou A, Perrouty B, Soisson T, Autret A, Warter JM, Vighetto A, Van Bogaert P, Alamowitch S, Roullet E, Tournier-Lasserve E (1999) High prevalence of CACNA1A truncations and broader clinical spectrum in episodic ataxia type 2. Neurology 52:1816–1821 [DOI] [PubMed] [Google Scholar]
- Ducros A, Denier C, Joutel A, Vahedi K, Michel A, Darcel F, Madigand M, Guerouaou D, Tison F, Julien J, Hirsch E, Chedru F, Bisgard C, Lucotte G, Després P, Billard C, Barthez MA, Ponsot G, Bousser MG, Tournier-Lasserve E (1999) Recurrence of T666M calcium channel CACNA1A gene mutation in familial hemiplegic migraine with progressive cerebellar ataxia. Am J Hum Genet 64:89–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap K, Luebke JI, Turner TJ (1995) Exocytotic Ca2+ channels in mammalian central neurons. Trends Neurosci 18:89–98 [PubMed] [Google Scholar]
- Fletcher C, Tottene A, Lennon V, Wilson S, Dubel S, Paylor R, Hosford D, Tessarollo L, McEnery M, Pietrobon D, Copeland N, Jenkins N. Dystonia and cerebellar atrophy in Cacna1a null mice lacking P/Q calcium channel activity. FASEB J, in press [DOI] [PubMed] [Google Scholar]
- Friend KL, Crimmins D, Phan TG, Sue CM, Colley A, Fung VS, Morris JG, Sutherland GR, Richards RI (1999) Detection of a novel missense mutation and second recurrent mutation in the CACNA1A gene in individuals with EA-2 and FHM. Hum Genet 105:261–265 [DOI] [PubMed] [Google Scholar]
- Frontali M, Jodice C. Spino-cerebellar ataxia type 6. In: Manto M, Pandolfo M (eds) Cerebellum. Cambridge University Press, Cambridge, United Kingdom, in press. [Google Scholar]
- Frontali M, Novelletto A, Annesi G, Jodice C (1999) CAG repeat instability, cryptic sequence variation and pathogeneticity: evidence from different loci. Philos Trans R Soc Lond B Biol Sci 354:1089–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ (1981) Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch 391:85–100 [DOI] [PubMed] [Google Scholar]
- Hans M, Luvisetto S, Williams ME, Spagnolo M, Urrutia A, Tottene A, Brust PF, Johnson EC, Harpold MM, Stauderman KA, Pietrobon D (1999) Functional consequences of mutations in the human alpha1A calcium channel subunit linked to familial hemiplegic migraine. J Neurosci 19:1610–1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris CM, Walker J, Shawkat F, Wilson J, Russell-Eggitt I (1993) Eye movements in a familial vestibulocerebellar disorder. Neuropediatrics 24:117–122 [DOI] [PubMed] [Google Scholar]
- Hering S, Aczel S, Kraus RL, Berjukow S, Striessnig J, Timin EN (1997) Molecular mechanism of use-dependent calcium channel block by phenylalkylamines: role of inactivation. Proc Natl Acad Sci USA 94:13323–13328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hering S, Berjukow S, Aczel S, Timin EN (1998) Ca2+ channel block and inactivation: common molecular determinants. Trends Pharmacol Sci 19:439–443 [DOI] [PubMed] [Google Scholar]
- Hockerman GH, Johnson BD, Abbott M, Scheuer T, Catterall WA (1997) Molecular determinants of high affinity phenilalkylamine block of L-type calcium channels in transmembrane segment IIIS6 and the pore region of the alpha-1 subunit. J Biol Chem 272:18759–18765 [DOI] [PubMed] [Google Scholar]
- Jen J, Yue Q, Nelson SF, Yu H, Litt M, Nutt J, Baloh RW (1999) A novel nonsense mutation in CACNA1A causes episodic ataxia and hemiplegia. Neurology 53:34–37 [DOI] [PubMed] [Google Scholar]
- Jodice C, Mantuano E, Veneziano L, Trettel F, Sabbadini G, Calandriello L, Francia A, Spadaro M, Pierelli F, Salvi F, Ophoff RA, Frants RR, Frontali M (1997) Episodic ataxia type 2 (EA2) and spinocerebellar ataxia type 6 (SCA6) due to CAG repeat expansion in the CACNA1A gene on chromosome 19p. Hum Mol Genet 6:1973–1978 [DOI] [PubMed] [Google Scholar]
- Jun K, Piedras-Renteria ES, Smith SM, Wheeler DB, Lee SB, Lee TG, Chin H, Adams ME, Scheller RH, Tsien RW, Shin HS (1999) Ablation of P/Q-type Ca(2+) channel currents, altered synaptic transmission, and progressive ataxia in mice lacking the alpha(1A)-subunit. Proc Natl Acad Sci USA 96:15245–15250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern I, Ucla C, Mach B (1994) Dinucleotide repeat polymorphism in the human RFX1 gene. Hum Mol Genet 3:216 [DOI] [PubMed] [Google Scholar]
- Kraus RL, Sinnegger MJ, Glossmann H, Hering S, Striessnig J (1998) Familial hemiplegic migraine mutations change alpha1A Ca2+ channel kinetics. J Biol Chem 273:5586–5590 [DOI] [PubMed] [Google Scholar]
- Matsuyama Z, Wakamori M, Mori Y, Kawakami H, Nakamura S, Imoto K (1999) Direct alteration of the P/Q-type Ca2+ channel property by polyglutamine expansion in spinocerebellar ataxia 6. J Neurosci 19:RC14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ophoff RA, Terwindt GM, Vergouwe MN, van Eljik R, Oefner PJ, Hoffman SMG, Laerdin JE, Mohrenwiser HW, Bulman DE, Ferrari M, Haan J, Lindhout D, van Ommen GB, Hafker MH, Ferrari MD, Frants RR (1996) Familial hemiplegic migraine and episodic ataxia type-2 are caused by mutations in the Ca2+ channel gene CACNL1A4. Cell 87:543–552 [DOI] [PubMed] [Google Scholar]
- Orita M, Suzuki Y, Sekiya T, Hayashi K (1989) Rapid and sensitive detection of point mutations and DNA polymorphisms using the polymerase chain reaction. Genomics 5:874–879 [DOI] [PubMed] [Google Scholar]
- Ravnik-Glavac M, Glavac D, Dean M (1994) Sensitivity of single strand conformation polymorphism and heteroduplex method for mutation detection in the cystic fibrosis gene. Hum Mol Genet 3:801–807 [DOI] [PubMed] [Google Scholar]
- Toru S, Murakoshi T, Ishikawa K, Saegusa H, Fujigasaki H, Uchihara T, Nagayama S, Osanai M, Mizusawa H, Tanabe T (2000) Spinocerebellar ataxia type 6 mutation alters P-type calcium channel function. J Biol Chem 275:10893–10898 [DOI] [PubMed] [Google Scholar]
- Trettel F, Mantuano E, Calabresi V, Veneziano L, Olsen AS, Georgescu A, Gordon L, Sabbadini G, Frontali M, Jodice C (2000) A fine physical map of the CACNA1A gene region on 19p13.1-p13.2 chromosome. Gene 241:45–50 [DOI] [PubMed] [Google Scholar]
- Volsen SG, Day NC, McCormack AL, Smith W, Craig PJ, Beattie R, Ince PG, Shaw PJ, Ellis SB, Gillespie A, Harpold MM, Lodge D (1995) The expression of neuronal voltage-dependent calcium channels in human cerebellum. Mol Brain Res 34:271–282 [DOI] [PubMed] [Google Scholar]
- von Brederlow B, Hahn AF, Koopman WJ, Ebers GC, Bulman DE (1995) Mapping the gene for acetazolamide responsive hereditary paroxysmal cerebellar ataxia to chromosome 19p. Hum Mol Genet 4:279–284 [DOI] [PubMed] [Google Scholar]
- Westenbroek RE, Sakurai T, Elliott EM, Hell JW, Starr TV, Snutch TP, Catterall WA (1995) Immunochemical identification and subcellular distribution of the alpha 1A subunits of brain calcium channels. J Neurosci 15:6403–6418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Q, Jen JC, Nelson SF, Baloh RW (1997) Progressive ataxia due to a missense mutation in a calcium-channel gene. Am J Hum Genet 61:1078–1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuchenko O, Bailey J, Bonnen P, Ashizawa T, Stockton DW, Amos C, Dobyns WB, Subramony SH, Zoghbi HY, Lee CC (1997) Autosomal dominant cerebellar ataxia (SCA6) associated with small polyglutamine expansions in the alpha 1A-voltage-dependent calcium channel. Nat Genet 15:62–69 [DOI] [PubMed] [Google Scholar]