Abstract
Familial incontinentia pigmenti (IP [MIM 308310]), or Bloch-Sulzberger syndrome, is an X-linked dominant and male-lethal disorder. We recently demonstrated that mutations in NEMO (IKK-γ), which encodes a critical component of the NF-κB signaling pathway, were responsible for IP. Virtually all mutations eliminate the production of NEMO, causing the typical skewing of X inactivation in female individuals and lethality in male individuals, possibly through enhanced sensitivity to apoptosis. Most mutations also give rise to classic signs of IP, but, in this report, we describe two mutations in families with atypical phenotypes. Remarkably, each family included a male individual with unusual signs, including postnatal survival and either immune dysfunction or hematopoietic disturbance. We found two duplication mutations in these families, at a cytosine tract in exon 10 of NEMO, both of which remove the zinc (Zn) finger at the C-terminus of the protein. Two deletion mutations were also identified in the same tract in additional families. However, only the duplication mutations allowed male individuals to survive, and affected female individuals with duplication mutations demonstrated random or slight skewing of X inactivation. Similarly, NF-κB activation was diminished in the presence of duplication mutations and was completely absent in cells with deletion mutations. These results strongly indicate that male individuals can also suffer from IP caused by NEMO mutations, and we therefore urge a reevaluation of the diagnostic criteria.
Familial incontinentia pigmenti (IP [MIM 308310]), or Bloch-Sulzberger syndrome, is a rare genodermatosis that segregates as an X-linked dominant trait with prenatal male lethality (Landy and Donnai 1993). The hallmark sign of IP is the presence of skin lesions in affected female newborns. These lesions progress through various stages and result in hypopigmented patches along lines of X inactivation (Spitz 1996, pp. 66–67). The name of the disorder derives from the observation of incontinence of melanin from the epidermis into the dermis. The most significant medical problem in IP is blindness due to hypervascularization and consequent retinal detachment. Other manifestations include alopecia, hypodontia or anodontia, eosinophilia, nail dystrophy, and neurological complications. These medical problems, together with the skin-pigmentation abnormality, constitute the typical IP phenotype. The IP gene is thought to be essential for viability, since affected hemizygous male individuals die in utero and female patients survive with the mutant X chromosome selectively inactivated (Parrish et al. 1996; Woffendin et al. 1999). On the basis of these characteristics, we screened a gene called “NEMO” and identified mutations in multiple IP patients (International IP Consortium 2000).
NEMO (IKK-γ) encodes a regulatory subunit of the IKK complex, which is central to activation of the NF-κB pathway (DiDonato et al. 1997; Israel 2000). NF-κB is sequestered in the cytoplasm by IκB inhibitory proteins, but, on stimulation by cytokines, IKK phosphorylates IκB and targets it for ubiquitin-mediated degradation. Release from IκB allows NF-κB to translocate into the nucleus and activate transcription of target genes. Knockout models for NF-κB subunits or components of the signaling cascade have shown that NF-κB is responsible for preventing apoptosis, particularly in response to TNF-α (Barkett and Gilmore 1999; Gerondakis et al. 1999). Hence, loss of NEMO in cells of IP patients most likely results in apoptosis, which can explain both the male lethality and the skewing of X inactivation in female individuals. The C-terminus of NEMO is indispensable to activate NF-κB downstream, although the exact residues necessary for this function have yet to be determined (Rothwarf et al. 1998; Yamaoka et al. 1998).
Most cases (∼85%) of IP are due to a recurrent deletion within NEMO that removes exons 4–10 (International IP Consortium 2000). All reported mutations, including the rearrangement type, cause typical IP. However, while its penetrance approaches 100%, this disorder shows variable expressivity, even within the same family. To add to this complexity, although IP has traditionally been classified as a male-lethal disorder, a few cases have been reported in male patients (Kirchman et al. 1995; Scheuerle 1998). The first NEMO mutation was recently found in a surviving male patient with abnormalities of the skin, teeth, and eyes, but also with osteopetrosis and immune dysfunction (International IP Consortium 2000). This patient died of tuberculosis at age 2.5 years. We have characterized two additional male cases, to test the hypothesis that male IP patients survive due to milder (hypomorphic) mutations. Analysis of these two families now reveals the genetic basis for the occurrence of IP in male individuals.
Family XL320 was described elsewhere (Roberts et al. 1998), but the diagnosis of IP was complicated by substantial phenotypic variation among the affected female family members. A male infant (XL320-04), who had been carried to term, died from severe hemorrhage 24 h after birth (table 1). Prenatal testing by linkage analysis, using nearby informative markers, in a subsequent pregnancy, predicted another affected male fetus (XL320-09). This pregnancy was terminated, and the mother (XL320-01) experienced severe bleeding during the procedure, further suggesting that the mutation in this family disrupted hemostasis. A second family, XL344, also included female members with typical signs of IP (table 1). An affected male individual (XL344-04) exhibited skin pigmentation and dental problems but also demonstrated immune dysfunction. He suffered multiple episodes of infection, including meningitis and pneumonia, due to poor lymphocyte function and remarkably low levels of circulating IgG. He also exhibited heat intolerance with hyperthermia, anhidrosis, eczema, and fine sparse hair, which led to a diagnosis of ectodermal dysplasia (ED) (X-linked anhidrotic ectodermal dysplasia [MIM 305100], autosomal recessive hypohidrotic ectodermal dysplasia [MIM 224900], and autosomal dominant hidrotic ectodermal dysplasia [MIM 129500]). Now, at age 3 years, he receives routine supplements of IgG, to prevent recurrent infections. He is currently losing weight, complains of abdominal pains, and tests positive for hepatosplenomegaly. He has also contracted mycobacterium avium intracellulare, an infection common among patients with AIDS.
Table 1.
Exon-10 Cytosine Tract Mutations, Corresponding Phenotypes, X-Inactivation Status, and NEMO Activity
| Family | Phenotypein FemaleSubjects | Phenotype in Male Subjects | Mutation | Predicted Amino Acids | X-InactivationStatus | Average NEMO ActivityRelative to Wild Typea |
| XL213 | Typicalb | 1 spontaneous abortion | ΔC1161 | P389fsX60c | Skewed | 10% = background |
| XL267 | Typical | No male subjects in pedigree | dupC1161 | P389fsX4 | Random | 34% |
| XL287 | Typical | 1 spontaneous abortion | ΔC1161 | P389fsX60c | Skewed | 10% = background |
| XL320 | Typicald | Death after 1 d, from hemorrhaging | dup1166-78 | P393fsX4 | Slight skewing | 19% |
| XL344 | Typical | Immune dysfunction; skinb; teethb | dupC1161 | P389fsX4 | Random | 34% |
| XL345 | Typical | No male subjects in pedigree | ΔC1161 | P389fsX60c | Skewed | 10% = background |
| XL374 | Typical | 3 spontaneous abortions | Δ1163-75 | S387fsX58e | Skewed | Not done |
By genetic complementation assay in a NEMO-mutant cell line. All values relative to wild type (100%).
Typical of IP—skin pigmentation, conical teeth, retinal detachment, alopecia, CNS problems. Not all signs demonstrated in families.
60 novel amino acids: RSHLTSAVPSASIRPLIWTPCRYMSWSALSRAGQCKATACRGRARDRAVCAFLSRLPSPGX.
Female family members show variation in expression of phenotype.
58 novel amino acids: HLTSAVPSASIRPLIWTPCRYMSWSALSRAGQCKATACRGRARDRAVCAFLSRLPSPGX.
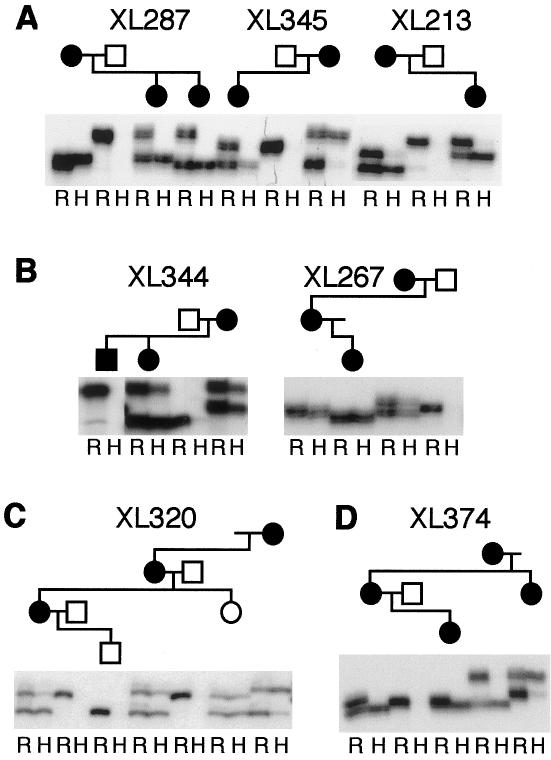
DNA samples from these two families (XL320 and XL344) were examined for all coding exons of NEMO by conformation-sensitive gel electrophoresis (CSGE) (Ganguly et al. 1993). PCR products from exon 10 demonstrated altered band sizes in both families (fig. 1a). Sequencing of exon-10 PCR products from XL320-01 and XL320-04 revealed a 13-base duplication (dup1166-78) (fig. 1b and 1c) at the end of a cytosine tract (the “C7 tract”). This duplication would cause frameshift after amino acid P393 and protein truncation after addition of four novel amino acids (fig. 1d and table 1). Sequence analysis of all NEMO exons in affected male subject XL320-04, including the noncoding exon 1, failed to identify other base changes. Exon-10 PCR products from family XL344 (fig. 1a) contained a duplication of one cytosine (dupC1161), also within the C7 tract (fig. 1b). A frameshift after amino acid P389 causes addition of the same four novel amino acids as are found in the XL320 mutant protein (fig. 1c). Despite the addition of identical novel amino acids, the phenotypes of the male subjects in these two families were entirely different (table 1). One additional family (XL267) demonstrated the same base duplication, dupC1161, as in family XL344, but no male offspring have been reported in this pedigree (table 1). The shorter protein sizes predicted from these mutations were demonstrated by in vitro translation experiments (fig. 2b).
Figure 1.
Mutation analysis of NEMO exon 10. A, CSGE bandshifts in affected members. B, Sequence analysis reveals mixed sequence (arrows indicate start point) due to mutations. C, DNA-sequence alignments between normal and mutant exon-10 sequences, with the cytosine tract at the left. Arrows point to mutations, and blue boxes mark the duplications. D, Protein-sequence alignments show the addition of novel amino acids, enclosed in blue boxes. Orange boxes indicate stop codon positions. E, Mutational mechanism at the cytosine tract, with duplications (1, 3) on top and deletions (2, 4) at the bottom.
Figure 2.
Analysis of NEMO activity by means of a genetic complementation assay in a NEMO-minus cell line. A, Expression analyses with various NEMO cDNA constructs were performed in six trials; the average luciferase-expression values are shown. Specific mutations introduced into the NEMO cDNA are indicated below the chart. The ΔC1161 deletion shows complete absence of complementation activity (same as background). In contrast, the duplication mutations, dupC1161 and dup1166-78, show diminished and residual activities. B, In vitro translation experiment confirms both the expected smaller protein sizes due to the duplication mutations and a larger protein size with the ΔC1161 mutation.
Given the nature of the mutations in families XL320 and XL344, we expected that reciprocal mutations—namely, a 13-base deletion and a single-cytosine deletion—might also be found. A 13-base deletion (Δ1163-75) at the C7 tract was identified in family XL374 (fig. 1a–c). However, this mutation was not the precise reciprocal of the XL320 mutation but was shifted 5' by two bases. This mutation predicts a frameshift after amino acid S387 and protein truncation after addition of 58 novel amino acids to the C-terminus of the protein (fig. 1d and table 1). Three additional families exhibited a deletion of a single cytosine (ΔC1161) within the C7 tract (fig. 1a–c and table 1), which is also expected to cause a frameshift at amino acid P389 and protein truncation after addition of 60 novel amino acids (fig. 1d and table 1). This larger protein size, caused by ΔC1161, was demonstrated by in vitro translation experiments (fig. 2b). Notably, after the first two residues, both deletion mutations add the same novel amino acid sequence to the truncated protein.
We analyzed all available family members for the duplication and deletion mutations, and altered band sizes were detected exclusively in affected members (fig. 1a). Moreover, CSGE analysis of 50 normal female subjects (100 X chromosomes) failed to reveal variations, confirming that the base alterations we found were not common polymorphisms (data not shown). During preparation of this report, another group reported that the mutation in family XL320 was a deletion that removes the first exon (Makris et al. 2000). We have analyzed the entire NEMO gene in XL320 and have found a mutation in exon 10, with complete preservation of exon 1, as described above. Southern blot analysis also failed to identify aberrant bands indicative of potential genomic rearrangements within NEMO.
We hypothesized that some male individuals survive because their mutations are “milder” (hypomorphic) and not cell lethal. Thus, we expected female individuals with the same mutations to show random or only minimal skewing of X inactivation, rather than the complete skewing observed in female patients with classic IP (Parrish et al. 1996). We examined the X-inactivation status in families with the duplication mutations by means of a method established elsewhere, based on digesting genomic DNA with a methylation-sensitive enzyme, HpaII, and amplifying a polymorphism at the HUMARA locus (Allen et al. 1992; Parrish et al. 1996). In support of our hypothesis, blood leukocytes from female subjects carrying either the dup1166-78 or dupC1161 mutations showed slight skewing or random X inactivation, respectively (fig. 3b and 3c). Hence, these NEMO mutations were apparently not deleterious enough to cause lethality in cells with an active mutant X chromosome and are consistent with the survival to term of affected male individuals in these families. In contrast with family XL344, the slight skewing in family XL320 could account for the more-severe phenotype in the XL320-04 male patient. We also examined the X-inactivation status in families with the deletion mutations (Δ1163–75 and Δ1161) and showed that they result in complete skewing of X inactivation (fig. 3a and 3d). Hence, the deletion mutations appear to be cell lethal, similar to other IP mutations (International IP Consortium 2000). Moreover, all families with deletion mutations, except XL345, have reported spontaneous abortions, presumably caused by in utero demise of affected male fetuses (table 1).
Figure 3.
X-inactivation analysis in pedigrees with exon-10 mutations. The methylation-based assay detects the active X chromosome after digestion with a methylation-sensitive enzyme, HpaII (H). Two bands in the “H” lanes indicate random X inactivation, and a single band suggests complete skewing of X inactivation. The RsaI (R) digest serves as control, and both alleles are amplified. A, Affected members with the ΔC1161 mutation show complete skewing of X inactivation. B, Patients with the dupC1161 mutation show random X inactivation. C, Affected female subjects with the dup1166-78 mutation in family XL320 show mild skewing (∼60%) of X inactivation. D, Family XL374 has the Δ1163-75 mutation, which causes complete skewing in affected members.
NEMO is central to activation of the NF-κB pathway, and its absence completely abolishes this signaling pathway (Yamaoka et al. 1998; International IP Consortium 2000; Makris et al. 2000; Schmidt-Supprian et al. 2000). Therefore, concordant with X inactivation, we expected hypomorphic mutations to cause reduction, rather than elimination, of NF-κB activation. The dupC1161, dup1166-78, and ΔC1161 mutations were engineered into an expression vector and, to evaluate their genetic complementation ability by a method described elsewhere, were transiently transfected into NEMO-minus cells (Courtois et al. 1997; Yamaoka et al. 1998; International IP Consortium 2000). The mutant cDNA constructs introduced into these cells produce NEMO proteins that activate NF-κB in varying degrees, depending on the mutations they contain. NF-κB then activates a reporter gene (luciferase) by binding target sites in the promoter, and, hence, luciferase levels reflect NEMO activity and NF-κB activation. In support of the X inactivation and phenotypic data, the deletion mutation completely failed to complement NEMO activity in these cells (fig. 2a). The duplication mutations resulted in residual (19.5%, with XL320 mutation) or reduced (34%, with XL344 mutation) activity relative to wild type (fig. 2a). The greater reduction in XL320 may explain the postnatal lethality of the male subject (XL344-04) carrying this mutation, although the 34% activity in XL344 is apparently sufficient for survival past infancy. These experiments measured activity in vitro, but these mutations may be of a different nature in vivo, with effects on protein structure and stability. Analysis of fibroblast cells from patients would have been useful to validate these results, but the death of the first male subject and difficult access to the second male subject prevented investigations in vivo.
Although IP has been considered a male-lethal disease, this report now describes mutations in male patients that demonstrate atypical forms of IP. However, it is imperative to understand that affected female relatives of these male individuals exhibited classic IP signs. Another group recently reported mutations in male individuals diagnosed with ectodermal dysplasia and immune dysfunction (Zonana et al. 2000). Although these families were described as having a novel syndrome in the form of ED and ID, it is likely that they are simply variants of IP, since they present clinical features associated with IP such as abnormal dentition, skin pigmentation abnormalities, and alopecia. The proband from the fourth pedigree (HED-ID 4), in this report, resembled the XL344 family described here. However, although the carrier female in family HED-ID 4 did not suffer from any medical problems, affected female individuals in family XL344 demonstrated typical IP skin-pigmentation and retinal abnormalities, a contrast that could be explained by variation in expression due to differences in X inactivation between the affected female individuals of these two families. Similarly, while the XL320 phenotype appears to be a novel disorder of IP combined with disrupted hemostasis, this should also be considered a variation of IP in male individuals. These variant phenotypes arise because of the full expression of mutations in male indiviuals, whereas the X inactivation in carrier female individuals prevents the complete phenotypic manifestation of the mutations, resulting in typical IP.
These data provide a basis for understanding the function of the NEMO protein, especially the C-terminus, which is indispensable for function (Rothwarf et al. 1998; Yamaoka et al. 1998). We have suggested elsewhere that removal of a Zn finger at the C-terminus is lethal, since nearly all mutations initially identified removed this domain (International IP Consortium 2000). In support of this interpretation, we have elsewhere described a male patient with IP with a stop codon mutation that resulted in the addition of 21 novel amino acids to the C-terminus, but this mutation preserved the Zn finger and allowed this patient to survive beyond birth (International IP Consortium 2000). However, although all the mutations reported here lead to removal of the Zn finger, only the deletion mutations were lethal in the embryonic period. Thus, male individuals may survive in spite of mutations that remove the Zn finger, indicating that the region within the C-terminus of NEMO that is indispensable for function must lie beyond the Zn finger domain. The specific role of the Zn finger is not known, and these mutations should be helpful in the determination of its function.
With knowledge of NEMO’s role in the NF-κB pathway, how can we explain the unusual phenotypes observed in male subjects from families XL320 and XL344? A few reports have associated IP with immune problems (Dahl et al. 1975; Jessen et al. 1978; Ment et al. 1978; Person 1985; Menni et al. 1990), and knockout-mouse models for NEMO suggest that immune dysfunction in IP results from uncontrolled apoptosis in the thymus and spleen (Makris et al. 2000). The phenotype of the male individual with the stop codon mutation was attributed to the addition of the novel amino acids to the end of the NEMO protein (International IP Consortium 2000). This may affect the stability of the protein or may mask the nearby leucine zipper domain, which is essential for dimerization. The immune dysfunction in this male subject, as well as in the XL344 male subject, may arise from cellular apoptosis in tissues critical to the immune system, including the thymus and spleen. Similarly, the XL320 mutation may affect hematopoietic or vascular development due to a deleterious effect on tissues involved in these processes. Vascular development is also thought to be the basis for the most significant medical problems in IP—namely, blindness caused by retinal detachment and mental retardation due to central nervous system malformations (Goldberg and Custis 1993; Francis and Sybert 1997). In this regard, a recent report demonstrated that primary congenital lymphedema (MIM 153100) results from mutations in vascular endothelial growth-factor receptor 3 (VEGFR-3), which implements its effects via the NF-κB pathway (Karkkainen et al. 2000). Knockout models for VEGFR-3 demonstrate absence of a primary vascular network and embryonic lethality (Dumont et al. 1998). Therefore, the vascular anomalies in IP may be due to disruption, via NEMO, of the signaling between VEGFR-3 and NF-κB.
Since IP has always been considered a male-lethal disease, it is likely that numerous cases of IP in male patients have been misdiagnosed with other human disorders. Our data clearly demonstrate that male individuals can suffer from IP. This observation has implications for clinical care, genetic counseling, and prenatal diagnosis. In the past, linkage analysis was the only method for prenatal diagnosis, but with knowledge of the involvement of NEMO, prenatal DNA samples can now be quickly subjected to molecular analysis. Because of the formerly held belief that affected male individuals undergo spontaneous abortion, some families currently continue a pregnancy when a male fetus has inherited the mutant haplotype. Therefore, if male patients do not have the common rearrangement mutation, it is imperative to rule out exon-10 mutations, because a devastating phenotype is still possible. In this regard, we emphasize that IP male individuals may present additional complications not seen in classic IP, such as immune dysfunction or hemorrhaging. Such variant phenotypes often cause misdiagnosis in any disease, as illustrated by an original diagnosis of ectodermal dysplasia in family XL344. The genes responsible for X-linked and autosomal ectodermal dysplasia have been discovered, and they produce proteins similar to TNF and its receptor, TNFR (Kere et al. 1996; Monreal et al. 1999). These proteins activate NF-κB through NEMO (Kumar et al. 2000) and will be useful in understanding the skin-pigmentation and hair abnormalities that are common to both ED and IP. However, since IP has been associated with immune deficiency but ED has not, and because the XL344-04 male subject was initially diagnosed with ED, we had predicted that, in cases of apparently variant forms of ED with immune deficiency, mutations in NEMO would be present. Indeed, this was demonstrated recently by another group (Zonana et al. 2000). Similarly, variant phenotypes of Partington syndrome II (MIM 301220) and Goltz syndrome (MIM 305600), especially if they occur sporadically, and unexplained cases of skin abnormalities with immunodeficiency (Ment et al. 1978; Ruiz-Maldonado and Orozco-Covarrubias 1997) are good candidates to examine for NEMO mutations.
Acknowledgments
The authors are members of the International IP Consortium, established with the encouragement and help of the National IP Foundation (NIPF), in New York. Susanne Emmerich, Director of NIPF, deserves special mention for arranging collaborative meetings. We thank all involved families for their invaluable and continuing participation in these research investigations. John Bargerstock, Laura Molinari, and Yumei Ying, of the BCM MRRC tissue culture core, provided expert technical assistance with cell lines. We also thank Kerry L. Wright for editing the manuscript. This work was supported, in part, by National Institutes of Health grants 5 R01 HD35617 and 2 P30 HD24064, to D.L.N., and by grants from Ligue Nationale contre le Cancer (“Equipe labellisee”), to A.I. R.A.L. is a Senior Scientific Investigator of The Foundation Fighting Blindness, Hunt Valley, Maryland.
Electronic-Database Information
Accession numbers and URL for data in this article are as follows:
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim (for IP [MIM 308310]; for X-linked anhidrotic ectodermal dysplasia [MIM 305100]; for autosomal recessive hypohidrotic ectodermal dysplasia [MIM 224900]; for autosomal dominant hidrotic ectodermal dysplasia [MIM 129500]; for primary congenital lymphedema [MIM 153100]); for Partington syndrome II [MIM 301220]; and for Goltz syndrome [MIM 305600])
References
- Allen RC, Zoghbi HY, Moseley AB, Rosenblatt HM, Belmont JW (1992) Methylation of Hpall and Hhal sites near the polymorphic CAG repeat in the human androgen-receptor gene correlates with X chromosome inactivation. Am J Hum Genet 51:1229–1239 [PMC free article] [PubMed] [Google Scholar]
- Barkett M, Gilmore TD (1999) Control of apoptosis by Rel/NF-kappaB transcription factors. Oncogene 18:6910–6924 [DOI] [PubMed] [Google Scholar]
- Courtois G, Whiteside ST, Sibley CH, Israel A (1997) Characterization of a mutant cell line that does not activate NF-kappaB in response to multiple stimuli. Mol Cell Biol 17:1441–1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl MV, Matula G, Leonards R, Tuffanelli DL (1975) Incontinentia pigmenti and defective neutrophil chemotaxis. Arch Dermatol 111:1603–1605 [PubMed] [Google Scholar]
- DiDonato JA, Hayakawa M, Rothwarf DM, Zandi E, Karin M (1997) A cytokine-responsive IkappaB kinase that activates the transcription factor NF-kappaB. Nature 388:548–554 [DOI] [PubMed] [Google Scholar]
- Dumont DJ, Jussila L, Taipale J, Lymboussaki A, Mustonen T, Pajusola K, Breitman M, Alitalo K (1998) Cardiovascular failure in mouse embryos deficient in VEGF receptor-3. Science 282:946–949 [DOI] [PubMed] [Google Scholar]
- Francis JS, Sybert VP (1997) Incontinentia pigmenti. Semin Cutan Med Surg 16:54–60 [DOI] [PubMed] [Google Scholar]
- Ganguly A, Rock MJ, Prockop DJ (1993) Conformation-sensitive gel electrophoresis for rapid detection of single-base differences in double-stranded PCR products and DNA fragments: evidence for solvent-induced bends in DNA heteroduplexes. Proc Natl Acad Sci USA 90:10325–10329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerondakis S, Grossmann M, Nakamura Y, Pohl T, Grumont R (1999) Genetic approaches in mice to understand Rel/NF-kappaB and IkappaB function: transgenics and knockouts. Oncogene 18:6888–6895 [DOI] [PubMed] [Google Scholar]
- Goldberg MF, Custis PH (1993) Retinal and other manifestations of incontinentia pigmenti (Bloch-Sulzberger syndrome). Ophthalmology 100:1645–1654 [DOI] [PubMed] [Google Scholar]
- International IP Consortium (2000) Genomic rearrangement in NEMO impairs NF-kappaB activation and is a cause of incontinentia pigmenti. Nature 405:466–472 [DOI] [PubMed] [Google Scholar]
- Israël A (2000) The IKK complex: an integrator of all signals that activate NF-kappaB? Trends Cell Biol 10:129–133 [DOI] [PubMed] [Google Scholar]
- Jessen RT, Van Epps DE, Goodwin JS, Bowerman J (1978) Incontinentia pigmenti. Evidence for both neutrophil and lymphocyte dysfunction. Arch Dermatol 114:1182–1186 [DOI] [PubMed] [Google Scholar]
- Karkkainen MJ, Ferrell RE, Lawrence EC, Kimak MA, Levinson KL, McTigue MA, Alitalo K, Finegold DN (2000) Missense mutations interfere with VEGFR-3 signalling in primary lymphoedema. Nat Genet 25:153–159 [DOI] [PubMed] [Google Scholar]
- Kere J, Srivastava AK, Montonen O, Zonana J, Thomas N, Ferguson B, Munoz F, Morgan D, Clarke A, Baybayan P, Chen EY, Ezer S, Saarialho-Kere U, de la Chapelle A, Schlessinger D (1996) X-linked anhidrotic (hypohidrotic) ectodermal dysplasia is caused by mutation in a novel transmembrane protein. Nat Genet 13:409–416 [DOI] [PubMed] [Google Scholar]
- Kirchman TT, Levy ML, Lewis RA, Kanzler MH, Nelson DL, Scheuerle AE (1995) Gonadal mosaicism for incontinentia pigmenti in a healthy male. J Med Genet 32:887–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Eby MT, Sinha S, Jasmin A, Chaudhary PM (2000) Ectodermal dysplasia receptor activates the nuclear factor kappa B, c-Jun N-terminal kinase and cell death pathways and binds to ectodysplasmin A. J Biol Chem (electronically published) [DOI] [PubMed]
- Landy SJ, Donnai D (1993) Incontinentia pigmenti (Bloch-Sulzberger syndrome). J Med Genet 30:53–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris C, Godfrey VL, Krahn-Senftleben G, Takahashi T, Roberts JL, Schwarz T, Feng L, Johnson RS, Karin M (2000) Female mice heterozygous for IKKγ/NEMO deficiencies develop a dermatopathy similar to the human X-linked disorder incontinentia pigmenti. Mol Cell 5:969–979 [DOI] [PubMed] [Google Scholar]
- Menni S, Piccinno R, Biolchini A, Plebani A (1990) Immunologic investigations in eight patients with incontinentia pigmenti. Pediatr Dermatol 7:275–277 [DOI] [PubMed] [Google Scholar]
- Ment L, Alper J, Sirota RL, Holmes LB (1978) Infant with abnormal pigmentation, malformations, and immune deficiency. Arch Dermatol 114:1043–1044 [PubMed] [Google Scholar]
- Monreal AW, Ferguson BM, Headon DJ, Street SL, Overbeek PA, Zonana J (1999) Mutations in the human homologue of mouse dl cause autosomal recessive and dominant hypohidrotic ectodermal dysplasia. Nat Genet 22:366–369 [DOI] [PubMed] [Google Scholar]
- Parrish JE, Scheuerle AE, Lewis RA, Levy ML, Nelson DL (1996) Selection against mutant alleles in blood leukocytes is a consistent feature in Incontinentia Pigmenti type 2. Hum Mol Genet 5:1777–1783 [DOI] [PubMed] [Google Scholar]
- Person JR (1985) Incontinentia pigmenti: a failure of immune tolerance? J Am Acad Dermatol 13:120–124 [DOI] [PubMed] [Google Scholar]
- Roberts JL, Morrow B, Vega-Rich C, Salafia CM, Nitowsky HM (1998) Incontinentia pigmenti in a newborn male infant with DNA confirmation. Am J Med Genet 75:159–163 [DOI] [PubMed] [Google Scholar]
- Rothwarf DM, Zandi E, Natoli G, Karin M (1998) IKK-γ is an essential regulatory subunit of the IkappaB kinase complex. Nature 395:297–300 [DOI] [PubMed] [Google Scholar]
- Ruiz-Maldonado R, Orozco-Covarrubias ML (1997) Postinflammatory hypopigmentation and hyperpigmentation. Semin Cutan Med Surg 16:36–43 [DOI] [PubMed] [Google Scholar]
- Scheuerle AE (1998) Male cases of incontinentia pigmenti: case report and review. Am J Med Genet 77:201–218 [PubMed] [Google Scholar]
- Schmidt-Supprian M, Bloch W, Courtois G, Addicks K, Israel A, Rajewsky K, Pasparakis M (2000) NEMO/IKK gamma-deficient mice model incontinentia pigmenti. Mol Cell 5:981–992 [DOI] [PubMed] [Google Scholar]
- Spitz JL (1996) Genodermatoses: a full color clinical guide to genetic skin disorders. Williams & Wilkins, New York, pp. 66–67 [Google Scholar]
- Woffendin H, Jakins T, Jouet M, Stewart H, Landy S, Haan E, Harris A, Donnai D, Read A, Kenwrick S (1999) X-inactivation and marker studies in three families with incontinentia pigmenti: implications for counseling and gene localisation. Clin Genet 55:55–60 [DOI] [PubMed] [Google Scholar]
- Yamaoka S, Courtois G, Bessia C, Whiteside ST, Weil R, Agou F, Kirk HE, Kay RJ, Israel A (1998) Complementation cloning of NEMO, a component of the IkappaB kinase complex essential for NF-kappaB activation. Cell 93:1231–1240 [DOI] [PubMed] [Google Scholar]
- Zonana J, Elder ME, Schneider LC, Orlow SJ, Moss C, Golabi M, Shapira SK, Farndon PA, Wara DW, Emmal SA, Ferguson BM (2000) A novel X-linked disorder of immune deficiency and hypohidrotic ectodermal dysplasia is allelic to incontinentia pigmenti and due to mutations in IKK-gamma. Am J Hum Genet 67:1555–1562 [DOI] [PMC free article] [PubMed] [Google Scholar]