Abstract
In a previous study, zidovudine (ZDV) was shown to cause a concentration-dependent inhibition of antigen-specific cytotoxic T-lymphocyte (CTL) clonal expansion (S. Francke, C. G. Orosz, K. A. Hayes, and L. E. Mathes, Antimicrob. Agents Chemother. 44:1900-1905, 2000). However, this suppressive effect was lost if exposure to ZDV was delayed for 24 to 48 h during the antigen sensitization period, suggesting that antigen-primed CTL may be less susceptible than naive T lymphocytes to the suppressive effects of ZDV. The present study was undertaken to determine if naive T lymphocytes were more sensitive to the suppressive effects of ZDV than T lymphocytes previously exposed to antigen. The 50% inhibitory concentration (IC50) values of ZDV were determined on naive and antigen-primed T-cell responses in an alloantigen system. Lymphocyte cultures with continuous antigen exposure (double prime) were more resistant to ZDV suppression (IC50 = 316 μM) than were naive lymphocytes (IC50 = 87.5 μM). Interestingly, lymphocytes that were antigen primed but deprived of antigen during the final 7 days of culture (prime/hold) were exquisitely sensitive to ZDV suppression (IC50 = 29.3 μM). The addition of 80 μM ZDV during the initial priming of the single-prime (prime/hold) and double-prime cultures did not select for a more drug-resistant cell population. The differences in ZDV sensitivities are likely a reflection of the physiological properties of the lymphocytes related to their activation state.
Zidovudine (azidothymidine; ZDV), in single or combination therapy, is one of the major drugs used to treat AIDS. However, its positive therapeutic value is countered by clinical side effects and the development of drug resistance (4, 16, 17, 27, 37). ZDV also has potentially troubling properties affecting immune cell functions, such as suppression of antigen-driven T-cell proliferation (18), prolongation of the cell cycle (10, 49), and inhibition of a number of other immunologic responses including lectin- and antigen-induced mitosis, mixed lymphocyte culture reactions, and induction of the cytotoxic T-lymphocyte (CTL) response (18, 26, 36, 45). Mechanisms by which ZDV influences cell, and specifically immune cell, physiology are largely unknown, and the biologic relevance of these effects in vivo is speculative. However, the potential impact of ZDV on CTL-mediated cytolysis is of particular concern given the importance of these cells in combating human immunodeficiency virus (HIV) infection (25, 30, 46, 50).
In a previous study that used limiting dilution analysis, we reported that ZDV caused concentration-dependent inhibition of clonal expansion of antigen-specific CTL, suggesting that the basis for ZDV-related immunosuppression is stasis of T-cell expansion (18). In this study the estimated frequency of alloantigen-specific CTLs was profoundly lower in in vitro sensitization assays, where ZDV was present during primary antigen exposure (18). However, this suppressive effect was lost if exposure to ZDV was delayed for 24 to 48 h during the antigen sensitization period. The results suggested that antigen-primed CTL may be less susceptible to the suppressive effects of ZDV than naive T lymphocytes.
The objectives of the present study were (i) to measure the sensitivity of naive T lymphocytes to the suppressive effects of ZDV, and (ii) to determine if T cells sensitized to antigen in the presence of ZDV generated CTL with greater resistance to ZDV suppression. In order to explore this possibility, we used a 50% inhibitory concentration (IC50) assay to measure the relative suppressive effect of ZDV on naive and primed CTL. The results suggest that naive cytotoxic T cells are two to five times more sensitive to the inhibitory effects of ZDV than are activated antigen-primed cells. However, previously antigen-primed T cells that were cultured without antigen but given interleukin-2 (IL-2) were shown to have increased sensitivity to ZDV over that determined for naive CTL. Antigen priming in the presence of ZDV did not generate a cytotoxic T-cell population with greater resistance to ZDV suppression.
MATERIALS AND METHODS
Mice.
Animal studies were performed in accordance with the University Laboratory Animal Care and Use Committee and DHEW publication no. NIH 74-23, Guide for the Care and Use of Laboratory Animals. Six- to 8-week-old female DBA/2 (H-2d, Mlsa) and C57BL/6 (B6; H-2b, Mlsb) mice were purchased from Harlan Sprague Dawley Inc. (Indianapolis, Ind.). The mice were housed in a laminar flow cabinet (animal storage isolator; Nu Aire Inc., Plymouth, Minn.) in groups of 5 to 10 animals per cage. Upon arrival, all mice were allowed a 7-day period of acclimation before use. Animals were sacrificed and spleens were collected within 2 weeks following the acclimation period.
ZDV.
ZDV was obtained from the AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH), as a lyophilized powder and stored light-protected at room temperature. ZDV was dissolved in cell culture medium for in vitro studies.
Cell harvest, separation, and culture.
Intact spleens from mice euthanized in a standard CO2 chamber were dispersed into single-cell suspensions of splenocytes and washed three times in sterile phosphate-buffered saline. Tissue culture medium was Dulbecco's modified Eagle's medium supplemented with 1.6 mM l-glutamine, 0.27 mM folic acid, 0.27 mM l-asparagine, 0.55 mM l-arginine, 10 mM HEPES buffer, 1.0 mM sodium pyruvate, 100 U of penicillin-streptomycin/ml (Gibco, Grand Island, N.Y.), 5 × 10−5 M β-mercaptoethanol (Sigma Chemical, St. Louis, Mo.), and 10% heat-inactivated fetal calf serum, with 10 IU of IL-2/ml (Boehringer Mannheim) freshly added.
Study design. (i) Animal model.
The experimental model for testing T-cell sensitivity to ZDV is based on the one-way mixed lymphocyte response (MLR) between the major histocompatibility complex-distinct mouse strains C57BL6 (responder) and DBA (stimulator). The product of the MLR is a C57BL6 T-cell culture with a high frequency of DBA-specific cytotoxic T cells (18, 43). These single-primed cells can be expanded by restimulation with a second round of antigen treatment (cocultivation with irradiated DBA splenocytes [irrDBA]) to generate what we have termed double-prime cells. The single-prime cells from the MLR can be maintained without a second round of antigen exposure by cocultivation with irradiated autologous C57BL6 splenocytes. We refer to these cell preparations as prime-and-hold (prime/hold) T lymphocytes. Thus, depending on the stage of cell stimulation, ZDV sensitivity can be evaluated in three populations of T cells: naive (fresh splenocytes from C57BL6 mice), single primed (prime/hold), or double primed.
The rationale for using prime/hold and double-prime CTLs is as follows: alloantigen-activated CTL, in contrast to nonactivated CTL precursors, no longer require contact with specific alloantigens to allow lymphokine-mediated clonal expansion and subsequent detection in microcultures (43). Exposure to a single round of allogeneic antigen stimulation, however, leads to activation of only a fraction of all antigen-specific cells. Subsequent reexposure to the same antigen (double priming) increases the percentage of antigen-reactive cells by two mechanisms: (i) clonal expansion of already antigen sensitized cells, and (ii) first-time activation of cells which did not respond to the antigen during the first encounter.
(ii) Experimental design.
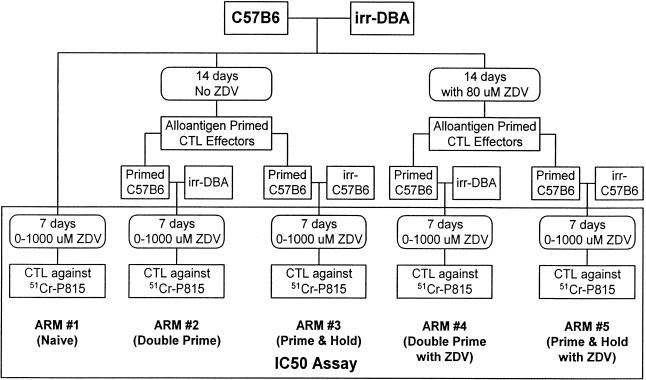
The experimental design had five arms (Fig. 1).
FIG. 1.
CTL culturing protocol for preparing alloantigen-reactive CTL for analysis of their ZDV sensitivity in the IC50 assay.
(a) Arm 1 (naive CTL).
Naive CTL were fresh splenocytes from C57BL6 mice placed directly into the IC50 assay. Briefly, 3 × 104 C57BL6 splenocytes/well (responder cells) were incubated for 7 days with 3 × 104 irrDBA-2 splenocytes/well (sensitizing cells) in U-bottom 96-well plates in the presence of ZDV concentrations ranging between 0 and 1,000 μM. Each ZDV concentration was tested in replicates of six.
(b) Arm 2 and Arm 4 (double prime).
For the double-primed CTL cultures, 105 C57BL6 splenocytes were incubated with 105 irrDBA-2 splenocytes for a period of 14 days at 37°C in 10% CO2 in the presence (Arm 4) or absence (Arm 2) of 80 μM ZDV (Fig. 1). This concentration of ZDV was selected based on previous studies showing suppression of T-cell responses (18). The cultures were given 25 μl of fresh IL-2 containing growth medium after 9 days of culture. For Arm 4, the growth medium contained 80 μM ZDV. After the 14-day culture period, effector cells were harvested, pooled, and then distributed in U-bottom microculture plates to achieve a final concentration of 3 × 103 effector cells/well. In preparation for the IC50 assay, each well received 105 irrDBA-2 splenocytes (second round of antigen stimulation). The IC50 assay is described below.
(c) Arm 3 and Arm 5 (prime and hold).
The prime/hold cultures were the same as the double-prime cultures during the initial 14-day culture period (Fig. 1). At that point, however, instead of adding irradiated DBA cells, these cultures received 105 irradiated C57BL6 splenocytes/well as feeder cells in preparation for the IC50 assay. Arm 5 contained 80 μM ZDV during the initial 14-day culture, while Arm 3 was grown without ZDV (Fig. 1).
IC50 assay.
The IC50 assay followed the procedure outlined in Fig. 1. As described above, the C57BL6/irrDBA-2 allogeneic mouse system was used as a source of CTL to measure the in vitro effect of ZDV on CTL effector cells. The assay procedure was adapted from a previously described protocol for determining precursor frequency by limiting dilution analysis (42, 43). The assay measures drug-mediated inhibition of in vitro sensitization and clonal expansion of C57BL6 splenocytes (effector cells) in response to irrDBA-2 splenocytes (sensitizing cells). By calculating an IC50 for this drug effect, it is possible to compare the relative sensitivities of different subsets of CTL to ZDV and other drugs.
51Cr release assay.
A 100-μl volume of cell suspension from each well was transferred to V-bottom microculture plates containing 3 × 103 51Cr-labeled P815 target cells. The assay mixture was incubated for 5 h at 37°C in 5% CO2 and then assayed for 51Cr release. P815 cells (DBA-2 origin, H-2d, Mlsa), the target for the CTL assay, were propagated in culture medium consisting of equal parts of Leibovitz L-15 and RPMI 1640, 10% heat-inactivated fetal bovine serum, 100 IU of penicillin/ml, and 100 μg of streptomycin/ml at 5% CO2 and 37°C. For 51Cr labeling, a cell pellet of 3 × 106 P815 cells collected from log-phase cultures was resuspended in 650 μCi of Na51CrO4 (New England Nuclear, Boston, Mass.) in a total volume of approximately 100 μl, incubated at 37°C, 5% CO2 for 2 h, washed at least three times in phosphate-buffered saline, and adjusted to the required cell concentration.
Drug IC50 determination.
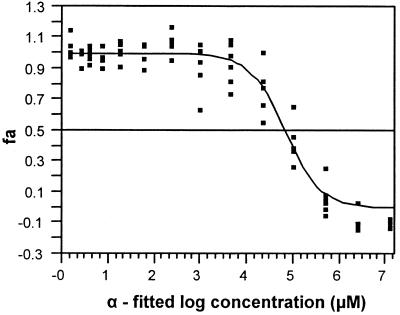
To define the IC50 of ZDV, data points from the IC50 assay were fitted to the logistic model (8) by nonlinear regression using the computer program JUMP-IN (SAS Institute Inc.). The logistic model uses the formula α = θ1/[1 + θ2 × e(θ3 × log dose)], where α was the fitted log dose, and θ1, θ2, and θ3 were adjustable parameters set initially to equal 1 before the fitting iterations, and log dose was the log10 of the ZDV concentration (in micromolar) used in the assay. A fitted curve was plotted on an x-y axis where α was the x value and fa was the y value (see Fig. 3). fa was defined as the fraction affected by drug, using the formula fa = 1 − [(ZDV-treated CTL count − spontaneous release count)/(untreated CTL count − spontaneous release count)].
FIG. 3.
Drug inhibition concentration (IC50) determination. To define the IC50 of ZDV, data points were fitted to a logistic model by nonlinear regression (see Materials and Methods). The log dose is the log10 of the ZDV concentration (micromolar) used in the assay. A fitted line was plotted on an x-y axis. fa was defined as the fraction of cytolysis affected by drug. The median-effect log concentration was determined from the fa value of 0.5 on the y axis (50% effect dose) to the x axis. The median-effect concentration and 95% confidence limits were then calculated from the anti-log of the extrapolated value.
The median-effect log concentration and its corresponding 95% confidence limits were determined by plot extrapolation from the fa value of 0.5 on the y axis (50% effect dose) to the x axis (see Fig. 3). The IC50 and its 95% confidence limits were then calculated from the anti-log of the values extrapolated from the fitted curve and the confidence band for fa.
Determination of cell viability and total cellularity.
Cell viability and cell death were assessed using the trypan blue dye exclusion technique.
RESULTS
The IC50 assay can be used to determine the relative inhibitory activity of antiviral drugs on CTL sensitization.
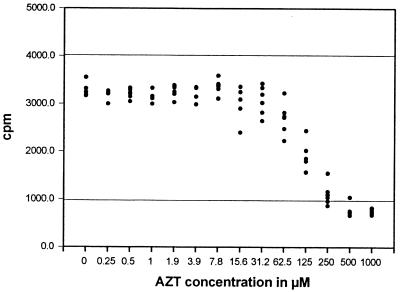
An in vitro system to generate CTL was used to titrate the effects of ZDV on the CTL response. Figure 2 illustrates the results of such an assay. A constant number of effector and stimulator cells in replicates of six were incubated with various concentrations of ZDV during antigen priming. As the ZDV concentration increased, the killing capacity of CTL cultures was reduced in a concentration-dependent sigmoid pattern. From these data, the IC50 value for ZDV inhibition of CTL activity was computed by determining the median effective concentration. Figure 3 is an example showing a best-fit plot for determining the IC50 value.
FIG. 2.
IC50 assay. The influence of ZDV on generation of alloreactive CTL is shown in a representative assay using naive splenocytes. All wells in 96-well culture dishes received the same number of responder cells (C57BL6) and stimulator cells (irrDBA-2). ZDV was present in replicates of six at the indicated concentrations during the 7-day culture period. CTL were measured by 51Cr release. The upper horizontal line represents the mean total release, while the lower horizontal line represents mean spontaneous release from control cultures.
Naive CTL are more sensitive to ZDV inhibition than antigen-primed CTL given a second antigen exposure, but less sensitive than antigen-primed CTL held in culture without a second antigen stimulation.
In these experiments we compared the inhibitory effects of ZDV on naive versus double-prime or prime/hold effector cell cultures. Results of five independent experiments are shown in Table 1. Individual rows indicate experiments performed at the same time. The IC50 values from four independent trials using naive CTL ranged between 79 and 99 μM (mean, 87.5 μM) ZDV (Table 1, Arm 1). By comparison, the IC50 values for the four trials with double-prime CTL ranged between 62 and 638 μM (mean, 316 μM) ZDV (Table 1, Arm 2). However, when the antigen-primed C57BL6 CTL were cultured with syngeneic irradiated C57BL6 feeder cells (prime/hold) instead of the double-prime exposure, they were found to be profoundly sensitive to ZDV inhibition, with IC50 values ranging from 29 to 30 μM (mean, 29.6 μM) ZDV (Table 1, Arm 3).
TABLE 1.
Effect of alloantigen priming on the IC50 of ZDV-mediated reduction of CTL function
| Trial no. | IC50 (95% confidence limit)a
|
||||
|---|---|---|---|---|---|
| Arm 1 | Arm 2 | Arm 3 | Arm 4 | Arm 5 | |
| Naive | No ZDV during first antigen exposure
|
80 μM ZDV during first antigen exposure
|
|||
| Double prime | Prime/hold | Double prime | Prime/hold | ||
| 1 | 79.3 (48-91) | 638 (481-880) | ND | 324 (267-387) | ND |
| 2 | 91 (61-134) | 353 (304-389) | ND | ND | ND |
| 3 | 99 (81-115) | 211 (155-225) | 29 (12-43) | 29 (21-37) | 31 (26-35) |
| 4 | ND | 62 (46-74) | 30 (26-32) | 70 (66-78) | 33 (28-37) |
| 5 | 81 (47-115) | ND | 30 (23-36) | ND | 24 (18-30) |
| Mean | 87.5 | 316 | 29.6 | 141 | 29.3 |
Shown are results from five independent trials. Individual rows indicate experiments performed at the same time.
ZDV exposure of CTL during primary antigen stimulation does not select for a more ZDV-resistant CTL population.
The IC50 values of alloantigen-primed effector cells propagated in the presence of 80 μM ZDV were calculated to determine if ZDV treatment during antigen priming selected for a CTL population that was more resistant to the suppressive effects of ZDV. As previously observed (18), the number of cells collected at the end of the 14-day incubation period in the cultures incubated with ZDV were substantially less than those collected from cultures without ZDV (data not shown). The addition of ZDV to the culture system during the original antigen priming period did not select for a cell population with increased resistance to ZDV suppression. In each of the six paired trials where lymphocytes were primed in the presence of 80 μM ZDV (three in Arm 4 and three in Arm 5), the IC50 values were the same or less than the IC50 values for the lymphocytes primed in the absence of ZDV. It is clear from these assays that preincubation of T cells during alloantigen priming did not produce a more drug-resistant T-cell population.
In summary, effector cells which were alloantigen primed for 14 days but not restimulated with DBA-2 alloantigen during the IC50 assay (incubated with the irradiated C57BL6 feeder cell layer) were most sensitive to ZDV, independent of the concentration of ZDV they encountered during the priming period. Unprimed lymphocytes ranked second in sensitivity, followed by alloantigen-primed and restimulated effector cells when treated with ZDV during the priming phase. Alloantigen-primed and restimulated effector cells not treated with ZDV during the priming phase were significantly less sensitive to ZDV suppression than unprimed or primed cells that did not reencounter the antigen.
DISCUSSION
Recognized limitations of ZDV antiviral therapy are the clinical side effects and the development of drug resistance (4, 37, 51). In addition, both in vitro and in vivo studies have documented that cells of the immune system are sensitive to therapeutic concentrations of ZDV (5, 14, 32, 36, 45, 52). Evaluation of immunomodulation by antiviral drugs on lymphocyte effector cell functions may be crucial because drug-induced interference of immune function, already impaired by virus infection, may potentially reduce even further the capacity to respond to the infection (29, 54).
We previously demonstrated a dose-dependent inhibition of CTL clonal expansion by ZDV in an in vitro alloantigenic murine system (18). In the work described here, using the same alloantigen system, we have introduced a statistical method to determine the IC50 of ZDV on antigen-specific cytotoxic T-cell clonal expansion. Based on previous observations by ourselves and others, we hypothesized that the sensitivity of T lymphocytes to the suppressive effects of ZDV depends on the activation status of those cells (naive versus antigen primed).
Our results revealed that 80 to 100 μM ZDV reduced the capacity of naive splenocytes to become CTL effector cells by one-half (mean IC50 = 87.5 μM). By contrast, antigen-primed lymphocytes with continuous exposure to allogeneic cells (double prime) were substantially more resistant to the suppressive effects of ZDV (mean IC50, 316 μM). Antigen-primed CTLs differ from their naive precursors in many regards, including morphology, surface antigen expression, and responsiveness to antigen reexposure and cytokine expression, e.g., IL-2 (33, 39). It is known that antigen-activated murine CTL do not require additional contact with stimulatory antigen for continued clonal expansion but respond to mitogenic lymphokines for a defined time period after antigen contact (1). Therefore, substituting syngeneic irradiated C57BL6 splenocytes as feeder cells for alloantigenic DBA-2 splenocytes during the IC50 assay following antigen priming permitted evaluation of cytolytic precursors which became activated during the primary antigen encounter (first 14 days of culture). By contrast, cultures which were primed during the first 14 days and were reexposed to alloantigen during the inhibition assay allowed detection of both primed effector cells and antigen-specific precursors which were not stimulated during the first antigen exposure (43). With this in mind, we found that alloantigen-primed lymphocytes, reexposed to the same antigen (double prime), were less sensitive to the suppressive effects of ZDV (higher IC50) than primed cells not reexposed to antigen (prime/hold).
Since the maintenance of antigen-primed but nonrestimulated effector cells is mainly dependent on IL-2 utilization (28, 44), ZDV interference with either receptor expression or receptor-ligand interaction would be potential mechanisms explaining the observed difference in sensitivity. Exogenous IL-2 reverses or reduces the in vitro and in vivo suppressive effects of ZDV, consistent with this hypothesis (36, 40, 45, 47). However, a separate study, which specifically evaluated cytokine production by lymphocytes from HIV-positive patients during ZDV treatment, did not reveal reduced IL-2 production (31), and another study reported a significant increase in IL-2 receptor (CD25) expression by mitogen-stimulated T lymphocytes from ZDV-treated AIDS patients (38). Therefore, even though excess exogenous IL-2 was provided in the tissue culture medium of our cultures during the antigen priming period and in the IC50 assay, we cannot exclude the possibility that ZDV interfered with cytokine-dependent T-cell-mediated helper function.
We further found that 80 μM ZDV treatment of CTL during antigen priming had a drug concentration-related effect on the number of cells harvested after the 14-day priming period (data not shown) but did not affect the ZDV IC50 values of progeny cells compared to non-ZDV-treated cells. This observation was independent of the type of antigen (syngeneic or allogeneic) used during the culture period. We had previously shown that the concentration-response-related reduction of effector cells harvested following priming in the presence of ZDV was most likely mediated by reduced clonal expansion of alloantigen-stimulated effector cells (18). However, based on the present study, it appears that previous antigen exposure rendered the effector cell more sensitive to the suppressive effects of ZDV (prime/hold study). This sensitivity was altered by a second antigen encounter. Both in vitro and in vivo studies suggest that the initial antigen-priming period is the most sensitive to interference by ZDV and other nucleoside analogues (34, 36, 42). This later finding, however, suggests that the activational status of the cell exposed to ZDV is crucially important in terms of drug sensitivity and is independent of previous drug exposure.
The double-primed cultures which were antigen primed in the presence of 80 μM ZDV (Table 1, Arm 4) did not develop greater resistance to ZDV than paired double-primed cultures without ZDV (Table 1, Arm 2). However, IC50 values from the double-prime ZDV data set (Table 1, Arm 4) were variable. It is likely that many of the precursor T cells capable of responding to DBA-2 antigen were suppressed by ZDV during the first antigen exposure period, such that they failed to respond and remained in an unprimed state equivalent to naive CTLs. Thus, a second round of antigen exposure may have stimulated a disproportionate number of naive T cells (Table 1, Arm 4) compared to the cultures which did not have ZDV during antigen priming (Table 1, Arm 2). A relatively larger number of naive compared to primed T cells responding in these cultures could reduce the IC50 value, since naive cells are more sensitive to ZDV than primed cells, as shown in Table 1, Arm 1. In addition, because the extent of primary antigen stimulation (percentage of precursors capable of responding) may vary from trial to trial, one might expect variability in IC50 values for double-prime cultures (Table 1, Arms 2 and 4). In the single-prime cultures (prime/hold), there was no opportunity to recruit additional naive cells, resulting in a more consistent response between either ZDV-treated or untreated cultures (Table 1, Arms 3 and 5). Further, because the cell concentrations were adjusted after primary antigen stimulation (with or without ZDV), both cultures were presumably the same in terms of the number of potential effector cells. In the subsequent 7-day culture period, both prime/hold and double-prime cultures received IL-2-containing medium, which promoted proliferation of primed cells but not naive cells. Because the single-primed effector cells (prime/hold) from both the ZDV-treated and untreated cultures gave essentially identical IC5o values (Table 1, Arms 3 and 5), it appeared that ZDV treatment during priming had no propensity to increase T-cell resistance to ZDV suppression.
The mechanism by which ZDV suppresses CTL leading to variable IC50 values for naive, prime/hold, and double-prime CTL is not known. Based on previous studies, it is assumed that ZDV suppresses clonal expansion of antigen-stimulated CTL (18). ZDV has been shown to prolong mitosis (10, 49), inhibit mitochondrial DNA polymerase (9, 13, 35, 41), and deplete the TTP pool (19), all of which affect cell proliferation. In our previous studies (18), we found ZDV to be cytostatic at concentrations ranging between 15 and 250 μM and cytotoxic at concentrations of 500 μM or greater when tested on naive lymphocyte cultures. These results were gathered using the same C57BL6/DBA-2 alloantigen model (18). We also observed that a delay in the addition of ZDV reduced its suppressive effect in vitro (18) and in vivo (34), allowing more effector cells to be produced.
Tissue culture cell lines as well as peripheral blood mononuclear cells (PBMC) grown long-term in the presence of ZDV become resistant to ZDV antiviral activity by decreasing the concentration of thymidine kinase (TK), a crucial enzyme necessary for the phosphorylation of ZDV to ZDV-monophosphate (ZDV-MP). The failure of the phosphorylation cascade necessary to convert ZDV to its active anabolite, ZDV-triphosphate (ZDV-TP), renders the cells less sensitive to the antiviral activity of ZDV and less sensitive to the toxic effects of ZDV caused by the ZDV-MP intermediate (20, 48). Reduced TK expression in ZDV-resistant Jurkat T cells has been linked to methylation of the human TK gene (52). The observation that PBMC from HIV-infected patients on long-term ZDV have reduced TK is suggestive that ZDV modulates TK expression (22). However, a more likely explanation is that ZDV therapy selects for a T-cell population of naturally low expressors of TK. Phytohemagglutinin-stimulated human PBMC have higher TK expression and greater sensitivity to ZDV inhibition of HIV-1 infection than nonstimulated controls (21). These cells are likely to be more sensitive to the cytostatic and cytotoxic effects of ZDV (low IC50). This latter observation is counter to our work, where highly activated T cells (double prime) had greater resistance to ZDV toxicity.
An alternative explanation for increased resistance to ZDV by activated T cells is increased expression of the multidrug-resistant (MDR) transmembrane P-glycoprotein (p170), which acts as an efflux pump to remove intracellular ions, toxins, and drugs (15), rendering the cell resistant to the cytostatic and cytotoxic effects of ZDV (2, 3, 12, 53). In normal human PBMC, the majority of CD8+ T cells, but less than half of the CD4+ T-cell population, express p170 (11). Interestingly, phytohemagglutinin stimulation of human PBMC causes an increase in p170 by the CD8+ T-cell subset, and anti-p170 blocks the cytolytic activity of alloantigen-specific cytotoxic T cells (23). A natural function of p170 may be in aiding the secretion of certain cytokines (24). Taken together, it appears that p170 expression is easily modulated by immune stimulation and may play an important role in cytotoxic T-cell effector cell function. The upregulation of p170 by antigen-stimulated T cells, as a mechanism for increased drug resistance, would be compatible with our double-prime stimulation results. Further studies will be needed to document the full range of p170 expression in the different activation stages of cytotoxic T cells and to correlate those results with drug sensitivity.
Determining the relevance of this work to the plasma ZDV concentrations of humans on ZDV therapy is made difficult by the species differences in ZDV processing. Mouse lymphoid cells form ZDV-TP at a level 16 times higher than that of human lymphoid cells (6). Human lymphoid cells were reportedly 15 times more sensitive to the cytostatic effects of ZDV and dideoxycytosine than were mouse lymphoid cells (6). The high level of ZDV-MP formed in human lymphoid cells, which is known to inhibit thymidylate kinase and adenylate kinase (7), may account for the cytostatic effect of ZDV. Therefore, studies in mouse systems may underestimate the true cytostatic effect of ZDV treatment in human cells by a factor of 15. The concentration of ZDV found to suppress the CTL response of mouse splenocytes by 50% ranged between approximately 30 and >600 μM (Table 1), while the peak ZDV concentration in patients receiving the recommended ZDV dosage has been calculated to be in the range of 3 to 7 μM. Assuming the 15-fold difference between mice and human cells, one might predict that human naive T cells would be suppressed by ZDV concentrations as low as 2 μM, well within the human peak plasma drug concentration. These assumptions, however, are based on predicted behavior of human lymphocytes and not actual T-cell response data. The IC50 assay used in a mouse alloantigen model will be a useful tool for testing the drug sensitivity of human T-cell responses to HIV antigens.
Taken together, our studies show that ZDV reduced cytolytic effector cell function in a concentration-dependent manner and suggest that alloantigen-primed effector cells, when not given continuous exposure or rescued by a second antigen encounter, are more sensitive to ZDV suppression than naive cells. This observation may have relevance in persons with HIV, where a strong CTL immune response is critical for the prevention of disease progression (25, 30, 46). Administration of ZDV and possibly other nucleoside analogues during the time of initial antigen priming of T cells may reduce the peak CTL response to virus. Possible drug interference with immune function should be considered when determining drug dosage and the time point of ZDV treatment initiation.
Acknowledgments
We acknowledge the support of the Center for Retrovirus Research, the Comprehensive Cancer Center, Arthur G. James Cancer Hospital, and Solove Research Institute, The Ohio State University.
The project was funded in part by Public Health Service grants RO1 AI40855 from the National Institute of Allergy and Infectious Diseases and P30 CA16058 from the National Cancer Institute. ZDV was obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH.
REFERENCES
- 1.Andrew, M. E., V. L. Braciale, and T. J. Braciale. 1984. Regulation of interleukin 2 receptor expression on murine cytotoxic T lymphocyte clones. J. Immunol. 132:839-844. [PubMed] [Google Scholar]
- 2.Antonelli, G., O. Turriziani, M. Cianfriglia, E. Riva, G. Dong, A. Fattorossi, and F. Dianzani. 1992. Resistance of HIV-1 to AZT might also involve the cellular expression of multidrug resistance P-glycoprotein. AIDS Res. Hum. Retrovir. 8:1839-1844. [DOI] [PubMed] [Google Scholar]
- 3.Antonelli, G., O. Turriziani, A. Verri, P. Narciso, F. Ferri, G. D'Offizi, and F. Dianzani. 1996. Long-term exposure to zidovudine affects in vitro and in vivo the efficiency of phosphorylation of thymidine kinase. AIDS Res. Hum. Retrovir. 12:223-228. [DOI] [PubMed] [Google Scholar]
- 4.Arts, E. J., and M. A. Wainberg. 1996. Human immunodeficiency virus type 1 reverse transcriptase and early events in reverse transcription. Adv. Virus Res. 46:97-163. [DOI] [PubMed] [Google Scholar]
- 5.Avramis, V. I., R. Kwock, M. M. Solorzano, and E. Gomperts. 1993. Evidence of in vitro development of drug resistance to azidothymidine in T-lymphocytic leukemia cell lines (Jurkat E6-1/AZT-100) and in pediatric patients with HIV-1 infection. J. Acquir. Immune Defic. Syndr. 6:1287-1296. [PubMed] [Google Scholar]
- 6.Balzarini, J., R. Pauwels, M. Baba, P. Herdewijn, E. De Clercq, S. Broder, and D. G. Johns. 1988. The in vitro and in vivo anti-retrovirus activity, and intracellular metabolism of 3′-azido-2′,3′-dideoxythymidine and 2′,3′-dideoxycytidine are highly dependent on the cell species. Biochem. Pharmacol. 37:897-903. [DOI] [PubMed] [Google Scholar]
- 7.Barile, M., D. Valenti, G. A. Hobbs, M. F. Abruzzese, S. A. Keilbaugh, S. Passarella, E. Quagliariello, and M. V. Simpson. 1994. Mechanisms of toxicity of 3′-azido-3′-deoxythymidine. Its interaction with adenylate kinase. Biochem. Pharmacol. 48:1405-1412. [DOI] [PubMed] [Google Scholar]
- 8.Bates, D. M., and D. G. Watts. 1988. Nonlinear regression analysis and its applications. Wiley, New York, N.Y.
- 9.Benbrik, E., P. Chariot, S. Bonavaud, M. Ammi-Said, E. Frisdal, C. Rey, R. Gherardi, and G. Barlovatz-Meimon. 1997. Cellular and mitochondrial toxicity of zidovudine (AZT), didanosine (ddI) and zalcitabine (ddC) on cultured human muscle cells. J. Neurol. Sci. 149:19-25. [DOI] [PubMed] [Google Scholar]
- 10.Chandrasekaran, B., T. E. Kute, and D. S. Duch. 1995. Synchronization of cells in the S phase of the cell cycle by 3′-azido-3′-deoxythymidine: implications for cell cytotoxicity. Cancer Chemother. Pharmacol. 35:489-495. [DOI] [PubMed] [Google Scholar]
- 11.Chaudhary, P. M., E. B. Mechetner, and I. B. Roninson. 1992. Expression and activity of the multidrug resistance P-glycoprotein in human peripheral blood lymphocytes. Blood 80:2735-2739. [PubMed] [Google Scholar]
- 12.Cinatl, J., Jr., J. Cinatl, H. Rabenau, H. W. Doerr, and B. Weber. 1994. Failure of antiretroviral therapy: role of viral and cellular factors. Intervirology 37:307-314. [DOI] [PubMed] [Google Scholar]
- 13.de la Asuncion, J. G., M. L. del Olmo, J. Sastre, A. Millan, A. Pellin, F. V. Pallardo, and J. Vina. 1998. AZT treatment induces molecular and ultrastructural oxidative damage to muscle mitochondria. Prevention by antioxidant vitamins. J. Clin. Investig. 102:4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dianzani, F., G. Antonelli, O. Turriziani, E. Riva, E. Simeoni, C. Signoretti, S. Strosselli, and M. Cianfriglia. 1994. Zidovudine induces the expression of cellular resistance affecting its antiviral activity. AIDS Res. Hum. Retrovir. 10:1471-1478. [DOI] [PubMed] [Google Scholar]
- 15.Endicott, J. A., and V. Ling. 1989. The biochemistry of P-glycoprotein-mediated multidrug resistance. Annu. Rev. Biochem. 58:137-171. [DOI] [PubMed] [Google Scholar]
- 16.Fischl, M. A., D. D. Richman, D. M. Causey, M. H. Grieco, Y. Bryson, D. Mildvan, O. L. Laskin, J. E. Groopman, P. A. Volberding, R. T. Schooley, et al. 1989. Prolonged zidovudine therapy in patients with AIDS and advanced AIDS-related complex. JAMA 262:2405-2410. [PubMed] [Google Scholar]
- 17.Fischl, M. A., D. D. Richman, N. Hansen, A. C. Collier, J. T. Carey, M. F. Para, W. D. Hardy, R. Dolin, W. G. Powderly, J. D. Allan, et al. 1990. The safety and efficacy of zidovudine (AZT) in the treatment of subjects with mildly symptomatic human immunodeficiency virus type 1 (HIV) infection. A double-blind, placebo-controlled trial. Ann. Intern. Med 112:727-737. [DOI] [PubMed] [Google Scholar]
- 18.Francke, S., C. G. Orosz, K. A. Hayes, and L. E. Mathes. 2000. Effect of zidovudine on the primary cytolytic T-lymphocyte response and T-cell effector function. Antimicrob. Agents Chemother. 44:1900-1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frick, L. W., and D. J. Nelson. 1989. Effects of 3′-azido-3′-deoxythymidine on the deoxynucleoside triphosphate pools of cultured human cells. Adv. Exp. Med. Biol. 253B:389-394. [DOI] [PubMed] [Google Scholar]
- 20.Furman, P. A., J. A. Fyfe, M. H. St. Clair, K. Weinhold, J. L. Rideout, G. A. Freeman, S. N. Lehrman, D. P. Bolognesi, S. Broder, H. Mitsuya, and D. W. Barry. 1986. Phosphorylation of 3′-azido-3′-deoxythymidine and selective interaction of the 5′-triphosphate with human immunodeficiency virus reverse transcriptase. Proc. Natl. Acad. Sci. USA 83:8333-8337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao, W. Y., T. Shirasaka, D. G. Johns, S. Broder, and H. Mitsuya. 1993. Differential phosphorylation of azidothymidine, dideoxycytidine, and dideoxyinosine in resting and activated peripheral blood mononuclear cells. J. Clin. Investig. 91:2326-2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Groschel, B., J. Cinatl, and J. Cinatl, Jr. 1997. Viral and cellular factors for resistance against antiretroviral agents. Intervirology 40:400-407. [DOI] [PubMed] [Google Scholar]
- 23.Gupta, S., and S. Gollapudi. 1993. P-glycoprotein (MDR 1 gene product) in cells of the immune system: its possible physiologic role and alteration in aging and human immunodeficiency virus-1 (HIV-1) infection. J. Clin. Immunol. 13:289-301. [DOI] [PubMed] [Google Scholar]
- 24.Gupta, S., C. H. Kim, T. Tsuruo, and S. Gollapudi. 1992. Preferential expression and activity of multidrug resistance gene 1 product (P-glycoprotein), a functionally active efflux pump, in human CD8+ T cells: a role in cytotoxic effector function. J. Clin. Immunol. 12:451-458. [DOI] [PubMed] [Google Scholar]
- 25.Haynes, B. F., G. Pantaleo, and A. S. Fauci. 1996. Toward an understanding of the correlates of protective immunity to HIV infection. Science 271:324-328. [DOI] [PubMed] [Google Scholar]
- 26.Heagy, W., C. Crumpacker, P. A. Lopez, and R. W. Finberg. 1991. Inhibition of immune functions by antiviral drugs. J. Clin. Investig. 87:1916-1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Langtry, H. D., and D. M. Campoli-Richards. 1989. Zidovudine. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy. Drugs 37:408-450. [DOI] [PubMed] [Google Scholar]
- 28.Larsson, E. L. 1981. Mechanism of T cell activation. II. Antigen- and lectin-dependent acquisition of responsiveness to TCGF is a nonmitogenic, active response of resting T cells. J. Immunol. 126:1323-1326. [PubMed] [Google Scholar]
- 29.Lenderking, W. R., R. D. Gelber, D. J. Cotton, B. F. Cole, A. Goldhirsch, P. A. Volberding, M. A. Testa, et al. 1994. Evaluation of the quality of life associated with zidovudine treatment in asymptomatic human immunodeficiency virus infection. N. Engl. J. Med. 330:738-743. [DOI] [PubMed] [Google Scholar]
- 30.Levy, J. A., B. Ramachandran, E. Barker, J. Guthrie, and T. Elbeik. 1996. Plasma viral load, CD4+ cell counts, and HIV-1 production by cells. Science 271:670-671. [DOI] [PubMed] [Google Scholar]
- 31.Lisignoli, G., M. C. Monaco, A. Degrassi, S. Toneguzzi, E. Ricchi, P. Costigliola, and A. Facchini. 1993. In vitro immunotoxicity of +/− 2′-deoxy-3′-thiacytidine, a new anti-HIV agent. Clin. Exp. Immunol. 92:455-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luster, M. I., D. R. Germolec, K. L. White, Jr., B. A. Fuchs, M. M. Fort, J. E. Tomaszewski, M. Thompson, P. C. Blair, J. A. McCay, A. E. Munson, et al. 1989. A comparison of three nucleoside analogs with anti-retroviral activity on immune and hematopoietic functions in mice: in vitro toxicity to precursor cells and microstromal environment. Toxicol. Appl. Pharmacol. 101:328-339. [DOI] [PubMed] [Google Scholar]
- 33.Mackay, C. R. 1993. Immunological memory. Adv. Immunol. 53:217-265. [DOI] [PubMed] [Google Scholar]
- 34.Mathes, L. E., K. A. Hayes, and G. Kociba. 1996. Evidence that high-dosage zidovudine at time of retrovirus exposure reduces antiviral efficacy. Antimicrob. Agents Chemother. 40:2183-2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCurdy, D. T., III, and J. M. Kennedy. 1998. AZT decreases rat myocardial cytochrome oxidase activity and increases beta-myosin heavy chain content. J. Mol. Cell Cardiol. 30:1979-1989. [DOI] [PubMed] [Google Scholar]
- 36.McKallip, R. J., M. Nagarkatti, and P. S. Nagarkatti. 1995. Immunotoxicity of AZT: inhibitory effect on thymocyte differentiation and peripheral T cell responsiveness to gp120 of human immunodeficiency virus. Toxicol. Appl. Pharmacol. 131:53-62. [DOI] [PubMed] [Google Scholar]
- 37.McLeod, G. X., and S. M. Hammer. 1992. Zidovudine: five years later. Ann. Intern. Med 117:487-501. [DOI] [PubMed] [Google Scholar]
- 38.McMahon, D. K., A. Winkelstein, J. A. Armstrong, G. J. Pazin, H. Hawk, M. Ho, et al. 1991. Zidovudine therapy is associated with an increased capacity of phytohemagglutinin-stimulated cells to express interleukin-2 receptors. AIDS 5:491-496. [DOI] [PubMed] [Google Scholar]
- 39.Mescher, M. F. 1995. Molecular interactions in the activation of effector and precursor cytotoxic T lymphocytes. Immunol. Rev. 146:177-210. [DOI] [PubMed] [Google Scholar]
- 40.Nokta, M. A., and R. B. Pollard. 1989. Differential reconstitution of zidovudine-induced inhibition of mitogenic responses by interleukin-2 in peripheral blood mononuclear cells from patients with human immunodeficiency virus infection. Antivir. Res. 11:191-202. [DOI] [PubMed] [Google Scholar]
- 41.Nusbaum, N. J., and P. E. Joseph. 1996. AZT incorporation into mitochondria: study in a human myeloid cell line. DNA Cell Biol. 15:363-366. [DOI] [PubMed] [Google Scholar]
- 42.Orosz, C. G., P. W. Adams, and R. M. Ferguson. 1988. Frequency of human alloantigen-reactive T lymphocytes. III. Evidence that cyclosporine has an inhibitory effect on human CTL and CTL precursors, independent of CsA-mediated helper T cell dysfunction. Transplantation 46:73S-79S. [PubMed] [Google Scholar]
- 43.Orosz, C. G., B. Horstemeyer, N. E. Zinn, and D. K. Bishop. 1989. Development and evaluation of a limiting dilution analysis technique that can discriminate in vivo alloactivated cytotoxic T lymphocytes from their naive CTL precursors. Transplantation 47:189-194. [DOI] [PubMed] [Google Scholar]
- 44.Sekaly, R. P., H. R. MacDonald, P. Zaech, and M. Nabholz. 1982. Cell cycle regulation of cloned cytolytic T cells by T cell growth factor: analysis by flow microfluorometry. J. Immunol. 129:1407-1414. [PubMed] [Google Scholar]
- 45.Shaw, D. R., D. R. Knight, A. L. Waterman, and J. P. Sommadossi. 1991. 3′-Azido-3′-deoxythymidine inhibition of human lymphocyte cytolytic function in vitro. Biochem. Pharmacol. 41:287-291. [DOI] [PubMed] [Google Scholar]
- 46.Shearer, G. M., and M. Clerici. 1996. Protective immunity against HIV infection: has nature done the experiment for us? Immunol. Today 17:21-24. [DOI] [PubMed] [Google Scholar]
- 47.Stine, K. C., D. S. Tyler, S. D. Stanley, J. A. Bartlett, D. P. Bolognesi, and K. J. Weinhold. 1991. The effect of AZT on in vitro lymphokine-activated killer (LAK) activity in human immunodeficiency virus type-1 (HIV-1) infected individuals. Cell Immunol. 136:165-172. [DOI] [PubMed] [Google Scholar]
- 48.Tornevik, Y., B. Ullman, J. Balzarini, B. Wahren, and S. Eriksson. 1995. Cytotoxicity of 3′-azido-3′-deoxythymidine correlates with 3′-azidothymidine-5′-monophosphate (AZTMP) levels, whereas anti-human immunodeficiency virus (HIV) activity correlates with 3′-azidothymidine-5′-triphosphate (AZTTP) levels in cultured CEM T-lymphoblastoid cells. Biochem. Pharmacol. 49:829-837. [DOI] [PubMed] [Google Scholar]
- 49.Viora, M., G. Di Genova, R. Rivabene, W. Malorni, and A. Fattorossi. 1997. Interference with cell cycle progression and induction of apoptosis by dideoxynucleoside analogs. Int. J. Immunopharmacol. 19:311-321. [DOI] [PubMed] [Google Scholar]
- 50.Westby, M., F. Manca, and A. G. Dalgleish. 1996. The role of host immune responses in determining the outcome of HIV infection. Immunol. Today 17:120-126. [DOI] [PubMed] [Google Scholar]
- 51.Wilde, M. I., and H. D. Langtry. 1993. Zidovudine. An update of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy. Drugs 46:515-578. [DOI] [PubMed] [Google Scholar]
- 52.Wu, S., X. Liu, M. M. Solorzano, R. Kwock, and V. I. Avramis. 1995. Development of zidovudine (AZT) resistance in Jurkat T cells is associated with decreased expression of the thymidine kinase (TK) gene and hypermethylation of the 5′ end of human TK gene. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 8:1-9. [PubMed] [Google Scholar]
- 53.Yusa, K., T. Oh-hara, A. Yamazaki, S. Tsukahara, W. Satoh, and T. Tsuruo. 1990. Cross-resistance to anti-HIV nucleoside analogs in multidrug-resistant human cells. Biochem. Biophys. Res. Commun. 169:986-990. [DOI] [PubMed] [Google Scholar]
- 54.Zaretsky, M. D. 1995. AZT toxicity and AIDS prophylaxis: is AZT beneficial for HIV+ asymptomatic persons with 500 or more T4 cells per cubic millimeter? Genetica 95:91-101. [DOI] [PubMed] [Google Scholar]