Abstract
We report a new locus for familial exudative vitreoretinopathy (FEVR), on chromosome 11p12-13 in a large autosomal dominant pedigree. Statistically significant linkage was achieved across a 14-cM interval flanked by markers GATA34E08 and D11S4102, with a maximum multipoint LOD score of 6.6 at D11S2010. FEVR is a disease characterized by the failure of development of peripheral retinal blood vessels, and it is difficult to diagnose clinically because of the wide spectrum of fundus abnormalities associated with it. The identification of a new locus is important for genetic counseling and potentiates further studies aimed toward the identification of a gene with an important role in angiogenesis within neuroepithelial tissues. Such a gene may also have a role in the genetic predisposition to retinopathy of prematurity, a sporadic disorder with many clinical similarities to FEVR.
Familial exudative vitreoretinopathy (FEVR [MIM 133780]) is a bilateral disorder of the retinal vasculature, characterized by an abrupt cessation of the growth of peripheral retinal capillaries (Criswick and Schepens 1969; Miyakubo et al. 1984). Autosomal dominant inheritance was the first mode of segregation to be described and is the most common form of this disease, although recessive and X-linked pedigrees are also seen (Criswick and Schepens 1969; Fullwood et al. 1993; De Crecchio et al. 1998). The phenotype is variable; in severe cases, the formation of fibrovascular vitreous membranes that cause retinal traction may result in the displacement of the macula, the presence of retinal folds, and/or the detachment of the retina, any of which may cause a visual-acuity decrease sufficient to result in legal blindness. Retinal detachment may also occur as a result of vascular exudation (Criswick and Schepens 1969; Benson 1995). Patients with mild forms of the disease are asymptomatic, and their only disease-related abnormality is an arc of avascular retina in the extreme temporal periphery (Criswick and Schepens 1969; Ober et al. 1980). These individuals are difficult to diagnose with certainty. Fluorescein angiography of the peripheral fundus is the definitive investigation; however, because it is an invasive procedure that is not routinely performed, many individuals in published pedigrees who are designated as normal may be affected. Figures for penetrance are therefore speculative but are thought to approach 100% when pedigrees are tested by angiography (Ober et al. 1980).
Mutations have been identified in the gene for Norrie disease (ND) (Xp11.4-p11.3) in several X-linked FEVR pedigrees, and, as a consequence, the Norrie gene locus is designated “EVR2” (Chen et al. 1993a; Shastry et al. 1995). This gene encodes a protein involved in neuronal cell interactions during embryonic vascular development (Meindl et al. 1992; Chen et al. 1993b). A single locus (EVR1) has been identified for autosomal dominant disease, on 11q13-23 (Li et al. 1992; Muller et al. 1994). We recently published exclusion of this locus in a large autosomal dominant pedigree (Bamashmus et al. 2000), proving locus heterogeneity within autosomal dominant FEVR. In the present report, we describe the discovery—in the same family—of a new FEVR locus, designated “EVR3,” on chromosome 11p12-13, ∼30 cM from the known locus, EVR1.
The individuals studied here are members of a large autosomal dominant pedigree originating in western Scotland. A detailed description of their clinical phenotype has been published elsewhere (Bamashmus et al. 2000). Essentially, they show the usual variations in expression of the disease, with defective peripheral retinal vascularization being the feature common to all affected individuals. Retinal detachment was a frequent finding, as was macular ectopia, with >50% of patients having a Snellen acuity ⩽6/60 in at least one eye. In addition, small atrophic peripheral retinal holes were an unusually prevalent feature of the phenotype in this pedigree. The more severely affected family members usually presented in childhood with poor vision, owing to macular traction. Slit-lamp biomicroscopy and indirect ophthalmoscopy were performed on all individuals who were genotyped. Fluorescein angiography of the fundus was not routinely performed but was used—whenever possible and after informed consent had been obtained—to clarify the diagnosis in mildly affected individuals.
Genomic DNA was extracted from peripheral blood leukocytes obtained from 5–10 ml of venous blood, by standard techniques. A cohort of 24 family members was screened for linkage across the whole genome, using a panel of fluorescence-tagged microsatellite markers spaced at 10-cM intervals. PCR reactions with these markers were performed on an MWG Roboseq 4200 robot. Genotyping was then undertaken using an ABI 377 automated DNA sequencer (Applied Biosystems). The products were identified and sized using GENESCAN (version 2.0.2) and GENOTYPER (version 1.1.1) software.
Alleles were initially screened by eye, to detect markers suggesting linkage. For any such marker, data files prepared on the LINKSYS (version 3.1) data-management package were transferred to the LINKAGE (version 5.1) suite of programs. In all analyses, we assumed autosomal dominant inheritance with 90% penetrance. Allele frequencies were estimated from the chromosomes of normal partners of affected family members, and, when possible, these estimates were compared with those available from The Genome Database. This screen identified several distinct chromosomal regions that appeared to cosegregate with FEVR. One of the regions contained marker D11S1392, which gave a LOD score of 1.99 in this panel of patients. When the linkage panel was extended to include seven additional family members, the LOD score with this marker increased to 3.27, with no recombination. By genotyping this extended panel with further nearby markers, we obtained a maximum two-point LOD score of 6.43 at D11S2010, again with no recombination, using the program MLINK. Three-point linkage analysis, using the LINKMAP program with both these markers, gives a maximum LOD score of 6.6 at D11S2010, proving beyond reasonable doubt the existence, at chromosome 11p12-13, of a second locus for autosomal dominant FEVR.
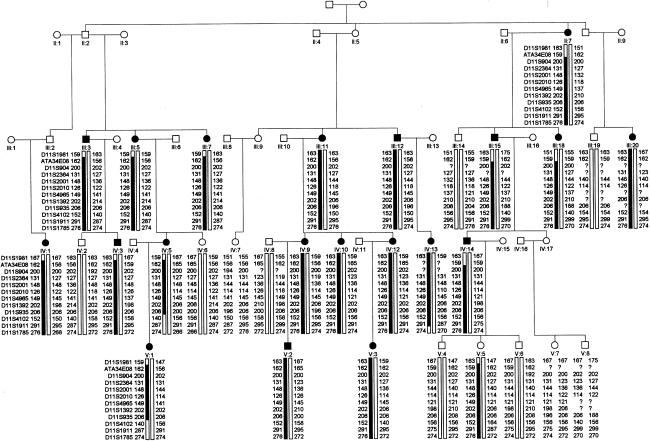
Haplotype analysis with markers in this region (fig. 1) places the involved gene within a 14-cM interval that is defined by markers GATA34E08 (distal) and D11S4102 (proximal). This locus is distinct from those of an assortment of other inherited retinal diseases that map on chromosome 11, including the initial autosomal FEVR locus EVR1 (see fig. 2). One clinically normal individual (V:5) has inherited the affected haplotype. This individual has not undergone fluorescein angiography of the peripheral fundus, because of the practical difficulties of performing such a procedure on a child. Therefore, this result may have occurred because of the variability in expression of the disease—exemplified by the mild phenotype in this patient—rather than because of a discrepancy in genetic analysis. Two-point LOD scores for markers typed across the region are shown in table 1.
Figure 1.
Haplotypes for 31 members of the large pedigree with autosomal dominant FEVR. Blackened symbols indicate affected individuals; blackened bars indicate the disease haplotype. The proximal crossover is seen in individual IV:5 and was detected by D11S4102 and other markers more proximal to it. The distal crossover is in individual II:15 and was detected by marker GATA34E08 and another, more-distal marker.
Figure 2.
Chromosome 11 ideogram showing the approximate positions of genes implicated in various retinal phenotypes, mapped according to their distance from the 11p telomere. Distances are composites derived from the databases of Retnet, OMIM, and the Centre for Medical Genetics, Marshfield Medical Research Foundation. AA = locus for atrophia areata; BBS1 = locus for Bardet-Biedl syndrome locus; EVR1 = locus for autosomal dominant familial exudative vitreoretinopathy locus; EVR3 = locus for the new locus described in the present article; MYO7A = locus for Usher syndrome 1B; ROM1 = gene involved in digenic and, possibly, in dominant retinitis pigmentosa; USH1C = locus for Usher syndrome 1C; and VNR1 = locus for autosomal dominant inflammatory vitreoretinopathy.
Table 1.
Two-Point LOD Scores for 11p13 Markers for FEVR, for 90% Penetrance
| LOD score at θa = |
||||||||
| Marker | Distance from 11pter(cM) | .0 | .01 | .05 | .1 | .2 | .3 | .4 |
| D11S1981 | 21.5 | −4.70 | −.83 | .44 | .84 | .93 | .70 | .35 |
| ATA34E08 | 33.0 | −1.59 | 1.54 | 1.91 | 1.81 | 1.33 | .78 | .31 |
| D11S2364 | 33.6 | 4.18 | 4.10 | 3.77 | 3.34 | 2.42 | 1.46 | .58 |
| D11S2001 | 37.6 | 5.18 | 5.10 | 4.77 | 4.29 | 3.21 | 2.03 | .85 |
| D11S2010 | 40.9 | 6.43 | 6.32 | 5.89 | 5.32 | 4.05 | 2.62 | 1.13 |
| D11S4965 | 41.0 | −1.56 | .35 | 1.46 | 1.68 | 1.47 | 1.00 | .48 |
| D11S1392 | 43.2 | 3.27 | 3.25 | 3.11 | 2.85 | 2.17 | 1.38 | .61 |
| D11S935 | 45.9 | 1.99 | 1.95 | 1.80 | 1.60 | 1.21 | .81 | .40 |
| D11S4102 | 47.6 | .87 | 4.37 | 4.71 | 4.49 | 3.61 | 2.43 | 1.13 |
| D11S4966 | 48.7 | 3.16 | 3.10 | 2.86 | 2.53 | 1.84 | 1.15 | .53 |
| D11S1911 | 50.9 | 3.42 | 3.38 | 3.21 | 2.92 | 2.19 | 1.36 | .57 |
| D11S905 | 52.0 | .74 | .77 | .84 | .85 | .73 | .51 | .25 |
| D11S1785 | 53.9 | −5.94 | .58 | 1.75 | 2.01 | 1.81 | 1.27 | .62 |
| D11S903 | 54.7 | −3.81 | −.96 | −.13 | .21 | .37 | .25 | .05 |
Values were calculated using MLINK. Maximum LOD scores for each marker are underlined.
The region between markers GATA34E08 and D11S4102 contains ∼100 expressed-sequence tags, several of which show retinal expression, together with ⩾14 characterized genes (GeneMap’98). Candidate genes for FEVR are expected to have a role in peripheral retinal vascular development. In particular, proteins involved in retinal angiogenesis, rather than vasculogenesis, are implicated. Proteins involved in angiogenesis are implicated because central retinal vessels are thought to arise as a result of vasculogenesis, whereas the extreme retinal periphery, which is defective in FEVR, is formed by angiogenesis and vascular remodeling (Hughes et al. 2000). Genes that have been characterized in this region and that may be relevant to FEVR include the gene for brain-derived neurotrophic factor, PAX6 (a homeobox gene and an important coordinator of ocular development), and the genes for basic transcription factor 3, rhombotin 2, and solute-carrier family 1, member 2 (SLC1A2).
The molecular defect that underlies FEVR is unclear. Failure of peripheral retinal vascularization is the common feature in an otherwise variable phenotype, leading to speculation that vessel development is defective. Of significance is the existence of the sporadic disorder retinopathy of prematurity (ROP), which is an excellent phenocopy for FEVR (Benson 1995). Affected premature infants invariably show poor peripheral retinal vascularization, which, in a small percentage, may lead to fibrovascular proliferation and tractional retinal detachment, producing a clinical appearance identical to that seen in FEVR. Several environmental factors, of which exposure to high oxygen tensions is historically the most significant, have a role in determining which neonates are at risk. However, extreme prematurity and low birth weight are also significant risk factors for the disease (Gibson 1989). As a consequence, the incidence of ROP is increasing, despite careful monitoring of oxygen therapy, as more premature infants survive. Evidence of a genetic basis of ROP comes from an observed racial bias in disease incidence (Saunders et al. 1997), together with the recent tentative demonstration of mutations in the ND gene in a small cohort of infants with severe ROP (Shastry et al. 1997).
The identification of the other genes that are defective in FEVR is therefore important, because these may contain polymorphisms that produce a genetic predisposition to severe ROP. Characterization of such genes will also provide valuable information regarding the molecular pathways involved in angiogenesis within neuroepithelial tissues.
Acknowledgments
We gratefully acknowledge the contributions of L. Patterson and C. G. Woods, for research support, and of J. Tolmie, for coordination of DNA extraction. We would like to express our gratitude to the Wellcome Trust for funding this research (grants 055145/Z/98 and 035535/Z/96).
Electronic-Database Information
Accession numbers and URLs for data in this article are as follows:
- Centre for Medical Genetics, Marshfield Medical Research Foundation, http://research.marshfieldclinic.org/genetics/
- GeneMap ’98, http://www.ncbi.nlm.nih.gov/genemap98/
- Genome Database, The, http://www.gdb.org/
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim (for FEVR [MIM 133780]) [PubMed]
- Retnet, http://www.sph.uth.tmc.edu/RetNet/
References
- Bamashmus MA, Downey LM, Inglehearn CF, Gupta SR, Mansfield DC (2000) Genetic heterogeneity in familial exudative vitreoretinopathy: exclusion of the EVR1 locus on chromosome 11q in a large autosomal dominant pedigree. Br J Ophthalmol 84:358–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson WE (1995) Familial exudative vitreoretinopathy. Trans Am Ophthalmol Soc 93:473–521 [PMC free article] [PubMed] [Google Scholar]
- Chen ZY, Battinelli EM, Fielder A, Bundey S, Sims K, Breakefield XO, Craig IW (1993a) A mutation in the Norrie disease gene (NDP) associated with X-linked familial exudative vitreoretinopathy. Nat Genet 5:180–183 [DOI] [PubMed] [Google Scholar]
- Chen ZY, Battinelli EM, Hendriks RW, Powell JF, Middleton-Price H, Sims KB, Breakefield XO, Craig IW (1993b) Norrie disease gene: characterisation of deletions and possible function. Genomics 16:533–535 [DOI] [PubMed] [Google Scholar]
- Criswick VG, Schepens CL (1969) Familial exudative vitreoretinopathy. Am J Ophthalmol 68:578–594 [DOI] [PubMed] [Google Scholar]
- De Crecchio G, Simonelli F, Nunziata G, Mazzeo S, Greco GM, Rinaldi E, Ventruto V, Ciccodicola A, Miano MG, Testa F, Curci A, D'Urso M, Rinaldi MM, Cavaliere ML, Castelluccio P (1998) Autosomal recessive familial exudative vitreoretinopathy: evidence for genetic heterogeneity. Clin Genet 54:315–320 [DOI] [PubMed] [Google Scholar]
- Fullwood P, Jones J, Bundey S, Dudgeon J, Fielder AR, Kilpatrick M (1993) X-linked exudative vitreoretinopathy: clinical features and genetic linkage analysis. Br J Ophthalmol 77:168–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson DL, Sheps SB, Schechter MT, Wiggins S, McCormick AQ (1989) Retinopathy of prematurity: a new epidemic? Pediatrics 83:486–492 [PubMed] [Google Scholar]
- Hughes S, Yang H, Chan-Ling T (2000) Vascularisation of the human fetal retina: roles of vasculogenesis and angiogenesis. Invest Ophthalmol Vis Sci 41:1217–1228 [PubMed] [Google Scholar]
- Li Y, Muller B, Fuhrmann C, van Nouhuys CE, Laqua H, Humphries P, Schwinger E, Gal A (1992) The autosomal dominant familial exudative vitreoretinopathy locus maps on 11q and is closely linked to D11S533. Am J Hum Genet 51:749–754 [PMC free article] [PubMed] [Google Scholar]
- Meindl A, Berger W, Meitinger T, van de Pol D, Achatz H, Dorner C, Haasemann M, Hellebrand H, Gal A, Cremers F (1992) Norrie disease is caused by mutations in an extracellular protein resembling C-terminal globular domain of mucins. Nat Genet 2:139–143 [DOI] [PubMed] [Google Scholar]
- Miyakubo H, Hashimoto K, Miyakubo S (1984) Retinal vascular pattern in familial exudative vitreoretinopathy. Ophthalmology 91:1524–1530 [DOI] [PubMed] [Google Scholar]
- Muller B, Orth U, van Nouhuys CE, Duvigneau C, Fuhrmann C, Schwinger E, Laqua H, Gal A (1994) Mapping of autosomal dominant exudative vitreoretinopathy locus (EVR1) by multipoint linkage analysis in four families. Genomics 20:317–319 [DOI] [PubMed] [Google Scholar]
- Ober RR, Bird AC, Hamilton AM, Sehmi K (1980) Autosomal dominant exudative vitreoretinopathy. Br J Ophthalmol 64:112–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders RA, Donahue ML, Christmann LM, Pakalnis AV, Tung B, Hardy RJ, Phelps DL (1997) Racial variation in retinopathy of prematurity. The Cryotherapy for Retinopathy of Prematurity Cooperative Group. Arch Ophthalmol 115:604–608 [DOI] [PubMed] [Google Scholar]
- Shastry BS, Hejtmancik JF, Plager DA, Hartzer MK, Trese MT (1995) Linkage and candidate gene analysis of X-linked familial exudative vitreoretinopathy. Genomics 27:341–344 [DOI] [PubMed] [Google Scholar]
- Shastry BS, Pendergast SD, Hartzer MK, Liu X, Trese MT (1997) Identification of missense mutations in the Norrie disease gene associated with advanced retinopathy of prematurity. Arch Ophthalmol 115:651–655 [DOI] [PubMed] [Google Scholar]