Abstract
A recent study of hereditary prostate cancer has provided evidence for a prostate cancer–susceptibility locus, HPC20, which maps to 20q13. To assess further the potential contribution of this locus to prostate cancer susceptibility, we studied 172 unrelated families affected by prostate cancer, using 17 polymorphic markers across a 98.5-cM segment of chromosome 20 that contains the candidate region. Parametric analysis, allowing for heterogeneity, resulted in an overall HLOD score of 0.09 (P=.39) at D20S171, under the assumption of linkage in 6% of families. Mode-of-inheritance–free analysis of the entire data set resulted in a maximal Zlr score of 0.76 (LOD 0.13; P=.22) at the same location. The strongest evidence for linkage was seen in the subset of 16 black families (LOD 0.86; Zlr=1.99; P=.023) between markers D20S893 and D20S120, near the putative location of HPC20. Although some positive results were observed, our linkage study does not provide statistically significant support for the existence of a prostate cancer–susceptibility locus HPC20 at 20q13.
Prostate cancer (MIM 176807) is the most commonly diagnosed cancer among men in the United States (Greenlee et al. 2000) and the fourth-most-commonly diagnosed cancer in men worldwide (Parkin 1998). Despite this high prevalence, understanding of disease etiology is still emerging. Known risk factors for the disease are advanced age, black race, and an affected father or brother (Chan et al. 1998). Germline mutations account for ∼5%–10% of cases, and the rest are likely sporadic (Carter et al. 1992; Carter et al. 1993). A genetic basis for prostate cancer is indicated by studies demonstrating that the lifetime risk of developing prostate cancer increases by a factor of two- to threefold when at least one first-degree relative is affected (Steinberg et al. 1990; Narod et al. 1995). Having both a first- and a second-degree affected family member may provide as much as an eightfold increase in risk (Steinberg et al. 1990). Segregation analyses suggest that, in a small proportion of cases, a prostate cancer–susceptibility gene is passed in an autosomal dominant mode of inheritance (Carter et al. 1992; Gronberg et al. 1997; Schaid et al. 1998). However, epidemiologic studies show that affected brothers confer a higher risk of prostate cancer than do affected fathers, and that this result is consistent across multiple racial groups (Monroe et al. 1995; Narod et al. 1995). This pattern indicates possible X-linked or recessive modes of prostate cancer inheritance. Thus, genetic-epidemiology studies suggest that there likely will be more than one locus that contributes to the inherited predisposition to prostate cancer.
Five putative prostate cancer–predisposition genes have been identified by means of family-based linkage approaches: HPC1 (MIM 601518; Smith et al. 1996), PCAP (MIM 602759; Berthon et al. 1998), and CAPB (MIM 603688; Gibbs et al. 1999) on chromosome 1; HPC2 (MIM 605367; Tavtigian et al. 2000) on chromosome 17; and HPCX (MIM 300147; Xu et al. 1998) on the X chromosome. A recent linkage study by Berry et al. (2000) of 162 families affected by prostate cancer described evidence for a sixth prostate cancer predisposition gene, termed “HPC20,” on 20q13. The families studied in this report were identified at the Mayo Clinic in Rochester, MN; 161 of these families were white, and one family was of Hispanic descent.
The aim of this study is to confirm the potential contribution of the HPC20 locus to prostate cancer susceptibility in an independent set of families. Therefore, a replication study was undertaken using 172 families affected by prostate cancer who are participating in the University of Michigan Prostate Cancer Genetics Project (PCGP). The PCGP was initiated in 1995, with the goal of defining the molecular basis of hereditary prostate cancer.
Men with prostate cancer who have at least one living affected relative were asked to participate in the PCGP by providing a blood sample, extended family history information, and access to medical records. All participants gave written informed consent, and all research protocols and consent forms were approved by the institutional review board at the University of Michigan. Those families either with three or more confirmed cases of prostate cancer in first- or second-degree relatives or with two such cases occurring in men aged ⩽55 years were included in the current analysis. Blood samples collected from affected individuals and from unaffected individuals that were informative for linkage were used. DNA was isolated from nucleated blood cells by use of the Puregene kit (Gentra Systems).
The DNA samples from all 558 individuals (including 441 affected men) were genotyped using a panel of 17 polymorphic markers spanning 98.5 cM and containing the HPC20 candidate region at 20q13 (near D20S887). Markers were chosen and genotyping was performed as described elsewhere (Berry et al. 2000). Marker allele frequencies were estimated from the data using all genotyped individuals.
Data were analyzed using both parametric and mode-of-inheritance–free methods of linkage. Parametric linkage in the presence of heterogeneity was assessed using heterogeneity LOD (HLOD) scores. HLOD scores and their accompanying estimates of the percentage of linked families (α) were calculated using the statistical software package GENEHUNTER PLUS, version 1.2 (Kruglyak et al. 1996; Kong and Cox 1997) . These analyses require the specification of a disease-transmission model. For our study, we used a parametric model similar to that used by Berry et al. (2000) for the localization of HPC20. This parametric model (Model A) was introduced by Smith et al. (1996); it specifies an autosomal dominant mode of inheritance with a disease allele frequency of .003. The penetrance rate among affected men is .001 for noncarriers and 1.00 for carriers. In our model, all unaffected men and all women are considered uninformative (i.e., of unknown phenotype) for the analysis. HLODs follow a complex statistical distribution. To obtain significance estimates for the observed HLODs, the HLODs were first converted to a χ2, where χ2=4.6×HLOD. P values (P1) were then derived for χ2, using the χ2 distribution with one df. The P value for the HLOD score is .5×[1-(1-P1)(1-P1)] (Faraway 1993).
We performed multipoint mode-of-inheritance–free linkage analyses using the computer software package GENEHUNTER PLUS, version 1.2 (Kruglyak et al. 1996; Kong and Cox 1997). For these analyses, we utilized the Sall statistic (Whittemore and Halpern 1994) and the exponential likelihood-based allele-sharing model (ASM). Results are reported in terms of the Zlr statistic, the LOD score, and its associated one-sided P value. Under the null hypothesis of no linkage, the Zlr statistic is distributed asymptotically as a standard normal random variable.
For inclusion in the analyses, 558 individuals from 172 families (see table 1) were genotyped. There were 154 white, 16 black, and 2 Asian/Pacific Island families. The average age at diagnosis among all affected individuals genotyped for analysis was 62.7 years.
Table 1.
Characteristics of Participating Families
| Characteristic | No. ofFamilies | Average Age(years)at Diagnosisof GenotypedAffectedIndividuals (SD) | Average No.of AffectedMen perFamily (Range) | Average No.of AffectedMen Genotypedper Family(Range) |
| All families | 172 | 62.7 (9.43) | 3.8 (2–14) | 2.6 (2–7) |
| White | 154 | 63.1 (9.31) | 3.7 (2–13) | 2.6 (2–7) |
| Black | 16 | 59.7 (9.64) | 5.1 (2–14) | 2.5 (2–4) |
| Asian/Pacific Islander | 2 | 56.4 (12.82) | 5.0 (5) | 2.0 (2) |
| <5 affected members | 148 | 62.7 (9.60) | 3.3 (2–4) | 2.4 (2–4) |
| ⩾5 affected members | 24 | 62.4 (8.63) | 7.1 (5–14) | 3.3 (2–7) |
| Average age at diagnosis <66 years | 108 | 59.8 (8.98) | 3.7 (2–14) | 2.5 (2–5) |
| Average age at diagnosis ⩾66 years | 64 | 67.4 (8.14) | 4.1 (3–12) | 2.6 (2–7) |
| Male-to-male transmission | 122 | 62.7 (9.86) | 4.1 (2–14) | 2.6 (2–7) |
| No male-to-male transmission | 50 | 62.5 (8.26) | 3.1 (2–6) | 2.5 (2–5) |
Because prostate cancer is a heterogeneous disease, both parametric and mode-of-inheritance–free linkage analyses were performed on different stratifications of the data, as well as on the entire sample of families. In addition to race, families were stratified by criteria identical to those used by Berry et al. (2000). These criteria are defined as the number of affected family members (<5 vs. ⩾5), average age at diagnosis (<66 years vs. ⩾66 years), and by presence or absence of evidence for male-to-male transmission of prostate cancer within the family. There were 28 bilineal families included in our report; these families were included in the male-to-male transmission category.
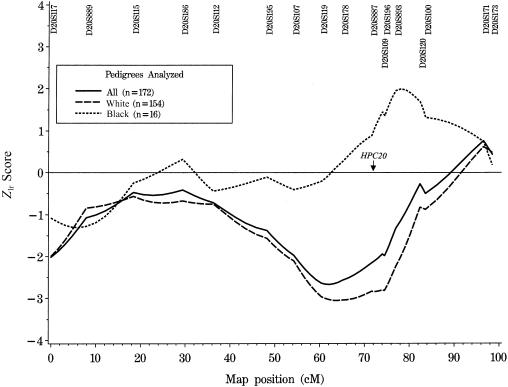
Results of both parametric and mode-of-inheritance–free linkage analyses are summarized in table 2. The overall maximum HLOD in the entire sample is 0.09 (P=.39) at 96.5 cM, under the assumption of linkage in 6% of families. Multipoint mode-of-inheritance–free linkage analysis of all 172 families results in a maximum Zlr score of 0.76 (LOD 0.13), with a corresponding one-sided P value of .22 at marker D20S171 (map position 96.5 cM; see table 2 and fig. 1). After stratification by race, marginally significant evidence for linkage is found using mode-of-inheritance–free methods to analyze the subset of 16 black families (Zlr=1.99; LOD 0.86; one-sided P value .023; between markers D20S893 and D20S120). The location of this peak Zlr score is within 4 cM of the marker that gave the most evidence for linkage in the entire data set in the initial HPC20 report (Berry et al. 2000; see fig. 1). It is of note that 6 black families from this data set provided the strongest evidence for linkage in our HPC1 confirmatory study (Cooney et al. 1997), whereas 11 of these 16 families did not support linkage to HPCX in our confirmatory report on this locus (Lange et al. 1999). Further analysis of these families, using data from multiple hereditary prostate cancer candidate regions, is in progress.
Table 2.
Summary of Parametric (Maximum HLOD) and Mode-of-Inheritance–Free (Maximum Multipoint LOD using ASM) Linkage Analysis Results
|
Parametric |
Mode-of-Inheritance–Free |
||||||||
| Subset | n | HLOD | α | Location(cM)(Nearest Marker) | P | Location(cM)(Nearest Marker) | MultipointLOD | Zlr | P |
| Full group (all races) | 172 | .09 | .06 | 96.5 (D20S171) | .39 | 96.5 (D20S171) | .13 | .76 | .22 |
| White | 154 | .05 | .05 | 96.5 (D20S171) | .43 | 96.5 (D20S171) | .084 | .62 | .27 |
| Black | 16 | .25 | .38 | 79.2 (D20S893–D20S120) | .24 | 78.1 (D20S893–D20S120) | .86 | 1.99 | .023 |
| <5 affected members | 148 | .20 | .09 | 96.5 (D20S171) | .28 | 96.5 (D20S171) | .19 | .93 | .18 |
| ⩾5 affected members | 24 | .08 | .08 | 82.4 (D20S120) | .39 | 32.2 (D20S186–D20S112) | .35 | 1.27 | .10 |
| Average age at diagnosis<66 years | 108 | .03 | .05 | 96.5 (D20S171) | .46 | 96.5 (D20S171) | .076 | .59 | .28 |
| Average age at diagnosis ⩾66 years | 64 | .13 | .10 | 98.5 (D20S173) | .34 | 98.5 (D20S173) | .13 | .77 | .22 |
| Male-to-male transmission | 122 | .19 | .09 | 96.5 (D20S171) | .29 | 96.5 (D20S171) | .32 | 1.21 | .12 |
| No male-to-male transmission | 50 | .02 | .06 | 29.4 (D20S186) | .47 | 18.4 (D20S115) | .020 | .30 | .38 |
| <5 affected members and average age at diagnosis ⩾66 years | 54 | .73 | .28 | 98.5 (D20S173) | .064 | 98.5 (D20S173) | .41 | 1.38 | .084 |
| ⩾5 affected members and average age at diagnosis <66 years | 13 | .54 | .43 | 80.3 (D20S893–D20S120) | .11 | 78.1 (D20S893–D20S120) | .29 | 1.16 | .11 |
| <5 affected and average age ⩾66 years and no male-to-male transmission | 18 | .79 | .50 | 7.9 (D20S889) | .055 | 7.9 (D20S889) | .49 | 1.51 | .066 |
| ⩾5 affected and average age <66 years and male-to-male transmission | 10 | .18 | .25 | 96.5 (D20S171) | .30 | 96.5 (D20S171) | .18 | .90 | .18 |
| <5 affected members and ⩾66 years and male-to-male transmission | 35 | 1.05 | .41 | 98.5 (D20S173) | .028 | 98.5 (D20S173) | .62 | 1.69 | .050 |
Figure 1.
Mode-of-inheritance–free linkage analysis on chromosome 20, showing Zlr scores calculated by the GENEHUNTER PLUS ASM, for all families, and stratified by race. (Note that the two Hispanic families were excluded from the race stratifications.) In the report by Berry et al. (2000), the maximum multipoint NPL Z score was observed at marker D20S887 when all families were analyzed together.
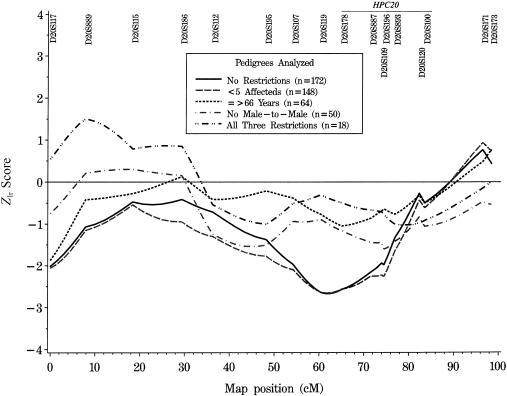
When the 172 families are stratified by number of affected members, average age at diagnosis, or male-to-male transmission, no statistically significant evidence for linkage to markers within the likely HPC20 candidate was observed in any of the subgroups, using either parametric or mode-of-inheritance–free methods (see table 2). In the original report by Berry et al. (2000), the strongest evidence for linkage occurred in the subset of families defined by no male-to-male transmission, fewer than five affected members, and age at onset ⩾66 years. In the current study, parametric analysis provides some evidence for linkage in the 18 families meeting these same criteria; however, the significance is marginal (HLOD 0.79 under the assumption that 50% of families are linked; P=.055 at marker D20S889). Results from the mode-of-inheritance–free analysis are consistent with the parametric findings (Zlr=1.51; LOD 0.49; P=.066 at marker D20S889). Interestingly, however, the maximum scores in our data set are at a marker 69.1 cM proximal to the marker with the maximum NPL score (D20S893) in the similarly defined subset reported by Berry et al. (2000). Our linkage results in this subgroup are negative in the putative region on 20q13 (near marker D20S887) that contains HPC20 (see fig. 2). Other strata produce some evidence for chromosome 20 linkage, but not in the HPC20 candidate interval. For example, the 35 families with fewer than five affected members, age at diagnosis ⩾66 years, and male-to-male transmission have a maximum HLOD of 1.05 at marker D20S173 (α=0.41; P=.028), which is ∼20 cM distal to the most likely HPC20 location.
Figure 2.
Mode-of-inheritance–free linkage analysis on chromosome 20, showing Zlr scores calculated by the GENEHUNTER PLUS ASM for all families and stratified by number of family members affected with prostate cancer; average age, within a family, at diagnosis of prostate cancer; and male-to-male transmission of prostate cancer. The maximum multipoint NPL Z scores in the stratified analyses performed by Berry et al. (2000) were located between markers D20S178 and D20S100.
In our study, neither parametric nor mode-of-inheritance–free linkage methods show significant support for the existence of a prostate cancer–susceptibility locus at 20q13. This may be explained in part by differences between the two studies in sample characteristics including family size, age of prostate cancer diagnosis, and ethnic composition. The University of Michigan PCGP families report significantly fewer affected members per family (3.8 vs. 4.4; P<.001). Furthermore, by comparison of the average of the mean age at diagnosis within families, the PCGP families were diagnosed at a significantly younger age (64.0 years vs. 66.5 years; P<.0001). Although both studies recruited families of prostate cancer patients identified at a tertiary-care medical center, the Michigan sample also includes families referred to the study from other sources within Michigan, as well as from elsewhere in the United States. Increased genetic heterogeneity in the Michigan sample is supported by the presence of black families, whereas all of the families in the Mayo study were white, with the exception of one Hispanic family.
Although it is possible that the findings by Berry et al. (2000) represent a false-positive result, it is also possible that their findings are real but will require a much larger sample size to replicate the linkage findings. It has been shown that, when a complex trait is caused by multiple susceptibility loci, the sample size needed for initial detection of linkage to any one of the loci is much smaller than the sample size required to confirm a particular chromosomal region (Suarez et al. 1994). Analyses using the computer program SIMLINK (Ploughman and Boehnke 1989) suggest that there is modest power to detect linkage to HPC20 in our entire collection of families. Using our parametric linkage model, we estimate that we have 20% power to measure an overall HLOD of ⩾1.0, conditional on the value of α, the proportion of families linked, reported by Berry et al. (2000). We have better power to detect an HLOD of ⩾1.0 when analyzing pedigrees with fewer than five affected members (0.67), average age at diagnosis <66 (0.70), and no male-to-male transmission (0.80), utilizing the corresponding values for α described in the original HPC20 report. However, these power estimates must be interpreted with caution, because the proportion of linked families (α) derived from the data of Berry et al. (2000) is not likely to be an accurate measure for other sets of pedigrees. Furthermore, although Model A (Smith et al. 1996) is a valid (unbiased) model for linkage analysis, it is not likely to be plausible for a complex disease. Decreasing the value of α or decreasing the relative risk between carriers and noncarriers likely will reduce the estimated power to detect linkage.
With the exception of the linkage result observed in the black families, our data fail to provide supportive evidence for the existence of a prostate cancer–susceptibility locus at chromosome 20q13. However, the data presented here follow some of the same trends with stratification as were observed in the original study (i.e., increased evidence of linkage in smaller families affected by prostate cancer with later disease onset), suggesting that HPC20 may represent a low-penetrance prostate cancer–predisposition gene. Additional studies of this region in other populations, using large sets of data, are necessary to validate HPC20 as a prostate cancer–susceptibility locus.
Acknowledgments
This work was supported by the following National Institutes of Health grants: Specialized Program of Research Excellence in Prostate Cancer P50 CA69568, T32 HG00040, CA79596, CA72818, and CA15083, as well as by the University of Michigan and the Mayo Clinic Comprehensive Cancer Centers.
Electronic-Database Information
Accession numbers and the URL for data in this article are as follows:
References
- Berry R, Schroeder JJ, French AJ, McDonnell SK, Peterson BJ, Cunningham JM, Thibodeau SN, Schaid DJ (2000) Evidence for a prostate cancer–susceptibility locus on chromosome 20. Am J Hum Genet 67:82–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthon P, Valeri A, Cohen-Akenine A, Drelon E, Paiss T, Wohr G, Latil A, et al (1998) Predisposing gene for early-onset prostate cancer, localized on chromosome 1q42.2-43. Am J Hum Genet 62:1416–1424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter BS, Beaty TH, Steinberg GD, Childs B, Walsh PC (1992) Mendelian inheritance of familial prostate cancer. Proc Natl Acad Sci USA 89:3367–3371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter BS, Bova SG, Beaty TH, Steinberg GD, Childs B, Isaacs WB, Walsh PC (1993) Hereditary prostate cancer: epidemiologic and clinical features. J Urol 150:797–802 [DOI] [PubMed] [Google Scholar]
- Chan JM, Stampfer MJ, Giovannucci EL (1998) What causes prostate cancer? A brief summary of the epidemiology. Semin Cancer Biol 8:263–273 [DOI] [PubMed] [Google Scholar]
- Cooney KA, McCarthy JD, Lange E, Huang L, Miesfeldt S, Montie JE, Oesterling JE, Sandler HM, Lange K (1997) Prostate cancer susceptibility locus on chromosome 1q: a confirmatory study. J Natl Cancer Inst 89:955–959 [DOI] [PubMed] [Google Scholar]
- Faraway JJ (1993) Distribution of the admixture test for the detection of linkage under heterogeneity. Genet Epidemiol 10:75–83 [DOI] [PubMed] [Google Scholar]
- Gibbs M, Stanford JL, McIndoe RA, Jarvik GP, Kolb S, Goode EL, Chakrabarti L, Schuster EF, Buckley VA, Miller EL, Brandzel S, Li S, Hood L, Ostrander EA (1999) Evidence for a rare prostate cancer-susceptibility locus at chromosome 1p36. Am J Hum Genet 64:776–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenlee RT, Murray T, Bolden S, Wingo PA (2000) Cancer statistics, 2000. CA Cancer J Clin 50:7–33 [DOI] [PubMed] [Google Scholar]
- Gronberg H, Damber L, Damber JE, Iselius L (1997) Segregation analysis of prostate cancer in Sweden: support for dominant inheritance. Am J Epidemiol 146:552–557 [DOI] [PubMed] [Google Scholar]
- Kong A, Cox NJ (1997) Allele-sharing models: LOD scores and accurate linkage tests. Am J Hum Genet 61:1179–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruglyak L, Daly MJ, Reeve-Daly MP, Lander ES (1996) Parametric and nonparametric linkage analysis: a unified multipoint approach. Am J Hum Genet 58:1347–1363 [PMC free article] [PubMed] [Google Scholar]
- Lange EM, Chen H, Brierley K, Perrone EE, Bock CH, Gillanders E, Ray ME, Cooney KA (1999) Linkage analysis of 153 prostate cancer families over a 30 cM region containing the putative susceptibility locus HPCX. Clin Cancer Res 5:4013–4020 [PubMed] [Google Scholar]
- Monroe KR, Yu MC, Kolonel LN, Coetzee GA, Wilkens LR, Ross RK, Henderson BE (1995) Evidence of an X-linked or recessive genetic component to prostate cancer risk. Nat Med 1:827–829 [DOI] [PubMed] [Google Scholar]
- Narod SA, Dupont A, Cusan L, Diamond P, Gomez JL, Suburu R, Labrie F (1995) The impact of family history on early detection of prostate cancer. Nat Med 1:99–101 [DOI] [PubMed] [Google Scholar]
- Parkin DM (1998) The global burden of cancer. Semin Cancer Biol 8:219–235 [DOI] [PubMed] [Google Scholar]
- Ploughman LM, Boehnke M (1989) Estimating the power of a proposed linkage study for a complex genetic trait. Am J Hum Genet 44:543–551 [PMC free article] [PubMed] [Google Scholar]
- Schaid DJ, McDonnell SK, Blute ML, Thibodeau SN (1998) Evidence for autosomal dominant inheritance of prostate cancer. Am J Hum Genet 62:1425–1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JR, Freije D, Carpten JD, Gronberg H, Xu J, Isaacs SD, Brownstein MJ, Bova GS, Guo H, Bujnovszky P, Nusskern DR, Damber JE, Bergh A, Emanuelsson M, Kallioniemi OP, Walker-Daniels J, Bailey-Wilson JE, Beaty TH, Meyers DA, Walsh PC, Collins FS, Trent JM, Isaacs WB (1996) Major susceptibility locus for prostate cancer on chromosome 1 suggested by a genome-wide search. Science 274:1371–1374 [DOI] [PubMed] [Google Scholar]
- Steinberg GD, Carter BS, Beaty TH, Childs B, Walsh PC (1990) Family history and the risk of prostate cancer. Prostate 17:337–347 [DOI] [PubMed] [Google Scholar]
- Suarez BK, Hampe BL, Van Eerdewegh P (1994) Problems of replicating linkage claims in psychiatry. In: Gershon ES, Cloninger ER (eds) Genetic approaches to mental disorders. American Psychiatric Press, Washington, DC [Google Scholar]
- Tavtigian SV, Simard J, Labrie F, Skolnick MH, Neuhausen SL, Rommens J, Cannon Albright LA (2000) A strong prostate cancer predisposition gene at chromosome 17p. Am J Hum Genet Suppl 67:11 [Google Scholar]
- Whittemore AS, Halpern J (1994) A class of tests of linkage using affected pedigree members. Biometrics 50:118–127 [PubMed] [Google Scholar]
- Xu J, Meyers D, Freije D, Isaacs S, Wiley K, Nusskern D, Ewing C, et al (1998) Evidence for a prostate cancer susceptibility locus on the X chromosome. Nat Genet 20:175–179 [DOI] [PubMed] [Google Scholar]