Abstract
The purpose of this study was to assess the effect of voriconazole on the blood tacrolimus concentration in a liver transplant recipient and to examine the interaction between voriconazole and tacrolimus by using human liver microsomes. Two subjects were enrolled in the clinical study: one received voriconazole, and the other received a placebo. Tacrolimus metabolism was evaluated in human liver microsomes at various concentrations in the absence and presence of various concentrations of voriconazole. Coadministration of voriconazole and tacrolimus resulted in elevated (nearly 10-fold-higher) trough tacrolimus blood concentrations in the liver transplant patient. In the in vitro study, voriconazole at a concentration of 10.4 ± 4.3 μg/ml inhibited the metabolism of tacrolimus by 50%. Clinically relevant concentrations of voriconazole inhibited the metabolism of tacrolimus in human liver microsomes. Close monitoring of the blood concentration and adjustment in the dose of tacrolimus are warranted in transplant recipients treated with voriconazole.
Organ transplant patients are susceptible to invasive fungal infections that necessitate treatment with antifungal agents including the azoles (9). Azole antifungal agents, e.g., ketoconazole, itraconazole, and fluconazole, are known to inhibit the metabolism of immunosuppressive drugs such as cyclosporine and tacrolimus (7, 8, 12). Voriconazole is a triazole antifungal agent that is currently undergoing phase III clinical trials for the treatment of a variety of fungal infections. Voriconazole is 4- to 16-fold more active than fluconazole and 2- to 8-fold more active than itraconazole against Candida species, including C. krusei and C. glabrata (1, 2, 10). Voriconazole is active against a wide range of filamentous fungi including Aspergillus species. In a randomized trial, voriconazole was more effective than amphotericin B as primary therapy for the treatment of invasive aspergillosis (R. Herbrecht et al., Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. J-680, p. 378, 2001). Voriconazole also appears to be a promising agent for the treatment of mycelial fungi that either are innately resistant or respond erratically to amphotericin B, such as hyaline molds and dematiaceous fungi. Availability in an intravenous and a highly bioavailable oral formulation renders voriconazole a potentially valuable drug for the treatment of invasive mycoses in transplant recipients.
Antifungal agents are known to inhibit cytochrome P450 3A4/5 (CYP3A4/5) enzymes. CYP3A4/5 is also involved in the metabolism of cyclosporine, tacrolimus, and sirolimus (3, 11, 13). Preliminary observations indicate that voriconazole at a dose of 200 mg twice a day increases the trough concentrations in blood of cyclosporine in transplant patients (P. Ghahramani, A. J. Romero, A. F. Lant, and M. J. Allen, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 845, p. 24, 2000). We hypothesized that voriconazole will alter the hepatic metabolism of tacrolimus as well. The objectives of the present study were to evaluate the interaction between voriconazole and tacrolimus clinically in transplant patients and in vitro by using human liver microsomes.
(These data were presented in part at the 41st Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, Ill., 16 to 19 December 2001.)
Clinical study.
The clinical study was designed as an open-labeled, randomized, two-period, two-treatment, placebo-controlled pharmacokinetic study in liver transplant recipients. The study protocol was approved by the Institutional Review Board of the VA Medical Center. Informed consent was obtained from the study participants. Prior to enrollment, patients were to be stabilized for at least 1 week on their present dose of tacrolimus. The first patient was randomized to voriconazole, and the second patient was randomized to placebo. Concurrent medications in patient 1 included fludrocortisone, insulin, and magnesium antacids, and those in patient 2 included felodipine, fludrocortisone, trimethoprim-sulfamethoxazole, and magnesium antacids. Tacrolimus concentrations were measured by a microparticulate enzyme immunoassay (MEIA) (6). Due to a significant increase in the trough tacrolimus blood concentrations in the first patient during the first few days after administration of voriconazole, the study was discontinued. Table 1 describes the dosing regimen of voriconazole and tacrolimus and the concentrations of tacrolimus in blood in the two subjects who participated in the study. The concentration of tacrolimus increased nearly 10-fold in the patient who received voriconazole. Voriconazole was discontinued and tacrolimus was withheld until the levels in blood returned to baseline values.
TABLE 1.
Clinical pharmacokinetic study summary
| Patient no. | Treatment drug regimen | Tacrolimus dose (mg) | Tacrolimus trough blood concn (ng/ml)
|
||
|---|---|---|---|---|---|
| Pretherapy | Day 3 | Day 5 | |||
| 1 | Voriconazole 200 mg orally twice a day (days 0 to 5) | 2 | 2.3 | 12.5 | 23.4 |
| 2 | Placebo (days 0 to 6) | 4 | 4.3 | 3.4 | 6.2 |
Human liver microsome study.
Human liver microsomes are commonly used to assess drug metabolism and drug-drug interactions. A human liver microsome was prepared by differential centrifugation from liver that was not used for organ transplantation. To evaluate the 50% inhibitory concentration (IC50) of voriconazole, tacrolimus (50 ng/ml) and voriconazole (0 to 100 μg/ml) were incubated with microsomal protein (0.02 to 0.08 mg/ml) in the presence of MgCl2 (10 mM) for 10 min at 37°C in 0.1 mM phosphate buffer (pH 7.4). The metabolism of tacrolimus was initiated with the addition of NADPH (1 mM). After 15 min, the reaction was terminated by placing the tubes into ice. Tacrolimus concentrations were measured immediately by a minor modification of the MEIA method used for blood samples.
For the calculation of Vmax (maximal velocity of tacrolimus metabolism) and Km values (the affinity of tacrolimus for the enzyme), tacrolimus was incubated at various concentrations (0 to 20 μM) in the absence and in the presence of 200 μg of voriconazole/ml. Tacrolimus with and without voriconazole was incubated with a human liver microsome at a protein concentration of 0.4 mg/ml, in the presence of MgCl2 (10 mM) for 10 min at 37°C in 0.1 mM phosphate buffer (pH 7.4). The metabolism of tacrolimus was initiated with the addition of NADPH (1 mM). After 30 min, the reaction was terminated by placing the tubes into ice. The tacrolimus concentration was measured by high-pressure liquid chromatography (HPLC). For HPLC analysis, 5 ml of cold ethyl ether was added to the cooled microsome. Cyclosporine (50 μl of a 0.1-mg/ml concentration in methanol) was added as the internal standard. Tacrolimus and cyclosporine were extracted into ethyl ether, and the ether layer was separated and evaporated under nitrogen. The residue obtained was reconstituted in acetonitrile-H2O (6:4) for HPLC analysis. The change in the concentration of tacrolimus was quantified by reverse-phase HPLC. A C18 column (3.9 by 150 mm; 10 μm; Bondapack; part no. 86684) was equipped with a C18 guard column and maintained at 70°C. A mobile phase consisting of acetonitrile-water (6:4) at a flow rate of 1.5 ml/min was used. Tacrolimus eluted at 4.5 min, and cyclosporine eluted at 7.0 min. The column eluent was monitored at 214 nm.
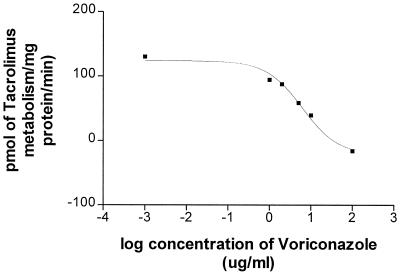
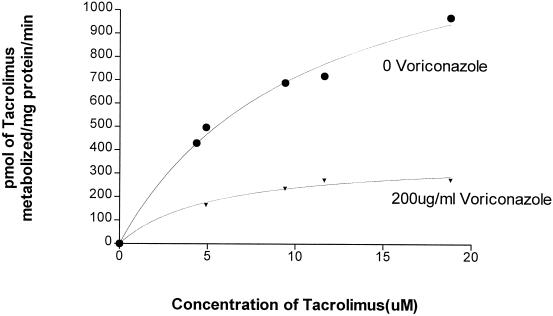
The concentration of voriconazole necessary to inhibit the metabolism of tacrolimus by 50% was calculated as the IC50 (Fig. 1). IC50, Vmax, and Km (Fig. 2) were calculated with Prism software (GraphPad Software Inc.). In three different sets of microsomes, the mean (± standard deviation) concentration of voriconazole needed to inhibit the metabolism of tacrolimus by 50% was 10.4 ± 4.3 μg/ml. The Vmax and Km for tacrolimus metabolism were 1.47 nmol/min/mg of protein and 10.64 μM in the absence and 0.37 nmol/min/mg of protein and 5.43 μM in the presence of voriconazole, respectively. This suggests that voriconazole inhibits the metabolism of tacrolimus by competitive and noncompetitive mechanisms.
FIG. 1.
Effect of voriconazole on tacrolimus metabolism. Tacrolimus (50 ng/ml) was incubated in the presence of various concentrations of voriconazole (0 to 100 μg/ml). The amount of tacrolimus metabolized per time in the absence and in the presence of various concentrations of voriconazole was measured by MEIA. At a concentration of 6.3 μg/ml, voriconazole inhibits the metabolism of tacrolimus by 50% in one microsome sample.
FIG. 2.
Metabolism of tacrolimus in the presence of voriconazole. Tacrolimus (0 to 20 μM) was incubated in the absence (•) or in the presence (▾) of 200 μg of voriconazole/ml, and the amount of tacrolimus metabolized was estimated by HPLC. The figure shows that voriconazole decreases the maximal velocity of tacrolimus metabolism (Vmax, 1.47 to 0.37 nmol/min/mg of protein) and changes the affinity of tacrolimus for the enzyme (Km, 10.64 to 5.43 μM).
The in vitro study qualitatively predicted the in vivo interaction between tacrolimus and voriconazole. However, the magnitude of the observed in vivo inhibition of tacrolimus metabolism appeared to be much greater than predicted based on the in vitro studies using concentrations of voriconazole comparable to those documented clinically in the serum. These data suggest that voriconazole may be present in higher concentrations in the gut and may inhibit gut metabolism of tacrolimus, thereby increasing the oral bioavailability of tacrolimus beyond its inhibitory effect on the hepatic metabolism of tacrolimus, as has been shown elsewhere for the interaction between ketoconazole and cyclosporine-tacrolimus (4, 5).
In conclusion, coadministration of voriconazole and tacrolimus resulted in a significant increase in the trough concentrations of tacrolimus in blood. The in vitro human liver microsomal study documented that, at clinically relevant concentrations of voriconazole, the metabolism of tacrolimus was inhibited. Monitoring of blood levels of tacrolimus and adjustment in its dosage are warranted in transplant patients receiving voriconazole.
REFERENCES
- 1.Barry, A. L., and S. D. Brown. 1996. In vitro studies of two triazole antifungal agents (UK-109,496 and fluconazole) against Candida species. Antimicrob. Agents Chemother. 40:1948-1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belanger, P., C. C. Nast, R. Fratti, H. Sanati, and M. Ghannoum. 1997. Voriconazole (UK-109,496) inhibits the growth and alters the morphology of fluconazole-susceptible and -resistant Candida species. Antimicrob. Agents Chemother. 41:1840-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Combalbert, J., I. Fabre, et al. 1989. Metabolism of cyclosporine A. IV. Purification and identification of rifampin inducible human liver cytochrome P450 as a product of P450IIIA gene subfamily. Drug Metab. Dispos. 17:197-207. [PubMed] [Google Scholar]
- 4.Floren, L. C., I. Bekersky, L. Z. Benet, Q. Mekki, D. Dressler, J. W. Lee, J. P. Roberts, and M. F. Hebert. 1997. Tacrolimus oral bioavailability doubles with coadministration of ketoconazole. Clin. Pharmacol. Ther. 62:41-49. [DOI] [PubMed] [Google Scholar]
- 5.Gomez, D. Y., V. J. Wacher, S. J. Tomlanovich, M. F. Hebert, and L. Z. Benet. 1995. The effects of ketoconazole on the intestinal metabolism and bioavailability of cyclosporine. Clin. Pharmacol. Ther. 58:15-19. [DOI] [PubMed] [Google Scholar]
- 6.Grenier, F. C., T. Luczkiw, M. Bergmann, S. Lunetta, M. Morrison, D. Blonski, K. Shoemaker, and M. Kobayashi. 1991. A whole blood FK506 assay for the IMx analyzer. Transplant. Proc. 23:2748-2749. [PubMed] [Google Scholar]
- 7.Iwasaki, K., H. Matsuda, K. Nagase, et al. 1993. Effects of twenty-three drugs on the metabolism of FK506 by human liver microsomes. Res. Commun. Chem. Pathol. Pharmacol. 32:209-217. [PubMed] [Google Scholar]
- 8.Lomaestro, B. M., and M. A. Piatek. 1998. Update on drug interactions with azole antifungal agents. Ann. Pharmacother. 32:915-928. [DOI] [PubMed] [Google Scholar]
- 9.Paterson, D. L., and N. Singh. 1999. Invasive aspergillosis in transplant recipients. Medicine 78:123-138. [DOI] [PubMed] [Google Scholar]
- 10.Pfaller, M. A., S. A. Messer, R. J. Hollis, R. N. Jones, G. V. Doern, M. E. Brandt, and R. A. Hajjeh. 1998. In vitro susceptibilities of Candida bloodstream isolates to the new triazole antifungal agents BMS-207147, Sch 56592, and voriconazole. Antimicrob. Agents Chemother. 42:3242-3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sattler, M., F. P. Guengrich, et al. 1992. Cytochrome P4503A enzymes are responsible for biotransformation of FK506 and rapamycin in man and rat. Drug Metab. Dispos. 20:753-761. [PubMed] [Google Scholar]
- 12.Venkataramanan, R., T. Prasad, A. Swaminathan, V. S. Warty, and G. J. Burckart. 1995. FK506. Clin. Pharmacokinet. 26:404-430. [DOI] [PubMed] [Google Scholar]
- 13.Vincent, S. H., B. V. Karanam, et al. 1992. In vitro metabolism of FK506 in rat, rabbit, and human microsomes: identification of a major metabolite and of cytochrome P450-3A as the major enzyme responsible for its metabolism. Arch. Biochem. Biophys. 294:454-460. [DOI] [PubMed] [Google Scholar]