Abstract
Abilities of amoxicillin-clavulanate, cefpodoxime, cefprozil, azithromycin, and clarithromycin to select resistant mutants of Haemophilus influenzae were tested by multistep and single-step methodologies. For multistep studies, 10 random strains were tested: 5 of these were β-lactamase positive. After 50 daily subcultures in amoxicillin-clavulanate, MICs did not increase more than fourfold. However, cefprozil MICs increased eightfold for one strain. Clarithromycin and azithromycin gave a >4-fold increase in 8 and 10 strains after 14 to 46 and 20 to 50 days, respectively. Mutants selected by clarithromycin and azithromycin were associated with mutations in 23S rRNA and ribosomal proteins L4 and L22. Three mutants selected by clarithromycin or azithromycin had alterations in ribosomal protein L4, while five had alterations in ribosomal protein L22. Two mutants selected by azithromycin had mutations in the gene encoding 23S rRNA: one at position 2058 and the other at position 2059 (Escherichia coli numbering), with replacement of A by G. One clone selected by clarithromycin became hypersusceptible to macrolides. In single-step studies azithromycin and clarithromycin had the highest mutation rates, while amoxicillin-clavulanate had the lowest. All resistant clones were identical to parents as observed by pulsed-field gel electrophoresis. The MICs of azithromycin for azithromycin-resistant clones were 16 to >128 μg/ml, and those of clarithromycin for clarithromycin-resistant clones were 32 to >128 μg/ml in multistep studies. For strains selected by azithromycin, the MICs of clarithromycin were high and vice versa. After 50 daily subcultures in the presence of drugs, MICs of amoxicillin-clavulanate and cefpodoxime against H. influenzae did not rise more than fourfold, in contrast to cefprozil, azithromycin, and clarithromycin, whose MICs rose to variable degrees.
Haemophilus influenzae is a major cause, together with Streptococcus pneumoniae and Moraxella catarrhalis, of community-acquired respiratory infections in children and adults, including pneumonia, acute exacerbations of chronic bronchitis, sinusitis, and otitis media. In countries such as the United States where the H. influenzae type b vaccine is widely used, untypeable H. influenzae strains cause the majority of infections (14).
The major resistance mechanism in H. influenzae is β-lactamase production (TEM-1, ROB-1). A recent study in the United States has documented the incidence of β-lactamase production in 1,676 H. influenzae strains isolated throughout the United States to be 41.6% (11). The incidence of β-lactamase-negative ampicillin-resistant (BLNAR) strains in the United States is <1%. Of available β-lactams for treatment of infections caused by this organism, cefixime and cefpodoxime are the most active from a potency as well as a pharmacokinetic and pharmacodynamic point of view, followed by amoxicillin-clavulanate, cefuroxime, and cefdinir. Other oral cephalosporins such as cefprozil, cefaclor, and loracarbef are less active against these organisms (21). Among macrolides and azalides, azithromycin yields the lowest MIC for H. influenzae, followed by erythromycin and clarithromycin. However, the pharmacokinetic and pharmacodynamic properties of these compounds cast doubt on their clinical efficacy against H. influenzae (5, 10).
It is well known that antimicrobial abuse leads to development of resistance. Baquero has postulated that abuse of azithromycin and clarithromycin has played an important part in the development of macrolide resistance in S. pneumoniae in Spain, Italy, and France (1, 2). The present study attempts to shed further light on this phenomenon in H. influenzae by using single and multistep resistance selection studies to examine the effect of amoxicillin-clavulanate, cefpodoxime, cefprozil, azithromycin, and clarithromycin.
MATERIALS AND METHODS
Bacteria.
Ten recent clinical isolates of untypeable H. influenzae were tested. Of these, five were β-lactamase positive (by Cefinase disk methodology; BBL Microbiology Systems, Cockeysville, Md.) and five were β-lactamase negative. Strains were stored in double-strength skim milk (Difco Laboratories, Detroit, Mich.) at −70°C before testing and examined for purity throughout the study by culture and Gram stain methods.
MIC methodology and antimicrobials.
Microdilution MICs of all strains were determined as recommended by the NCCLS (13) using freshly prepared Haemophilus test medium (HTM), with inoculum checks performed in each case. Standard quality control strains were included in each run. Trays were incubated for 16 to 20 h in ambient air. Drugs were obtained from their respective manufacturers.
Multistep mutation studies.
The method previously used by our group for pneumococci (6, 7, 12, 15) was modified as follows. Serial passages in freshly prepared HTM were performed daily for each strain in subinhibitory concentrations of all antimicrobials. For each subsequent daily passage, an inoculum was taken from the tube containing antimicrobial at 1 to 2 dilutions below the MIC. The latter inoculum was used to determine the next MIC. Daily passages were performed until a significant increase (more than fourfold) was obtained. A minimum of 14 passages were performed in each case. For cefprozil, three β-lactamase-positive strains for which the MICs were 128 were not tested. The maximal number of passages was 50. Control MICs were also determined daily in the original strain without serial passages. Stability of the acquired resistance was determined after 10 daily passages of the clone on chocolate agar (BBL) without antibiotics.
Single-step mutations.
The frequency of spontaneous single-step mutations was determined by spreading approximately 1010 CFU/ml in 100-μl aliquots on HTM plates containing one, two, four, and eight times the MIC of each compound. After 24 to 72 h of incubation, resistant colonies were confirmed by replica plating on media with antibiotics. The resistance frequency was calculated as the number of resistant colonies per inoculum. Strains 4, 5, and 7, for which the MIC of cefprozil was 128, were not tested for single-step mutation for this antibiotic.
Determination of macrolide and penicillin resistance mechanisms.
Presence of known resistance genes for macrolides mef(A/E), erm(B), erm(A) [subclass erm(TR)], and ere(A) was tested by PCR as described previously (16). Alterations in the genes coding for 23S rRNA and ribosomal proteins L4 and L22 were verified by sequencing of these genes after amplification by PCR. The sequences for 23S rRNA and ribosomal proteins L4 and L22 in H. influenzae were obtained from The Institute for Genomic Research website (http://www.tigr.org). Specific primers used to amplify the region involved in the peptidyl-transferase center of 23S rRNA were HF23-1 (5′CGGCGGCCGTAACTATAACG3′) and HF23-2 (5′GATGTGATGAGCCGACATCG3′), which amplify the region from positions 1902 to 2520 in 23S rRNA (H. influenzae numbering). Specific primers were designed to amplify entire genes that code for ribosomal proteins L4 and L22. The L22 gene was amplified with primers HL22-1 (5′CGGCAGATAAGAAAGCTAAG3′) and HL22-2 (5′TGGATGTACTTTTTGACCC3′). The L4 gene was amplified with primers HL4-1 (5′TTAAGCCGGCAGTTAAAGC3′) and HF4-2 (5′CACTTAGCAAACGTTCTTG3). All PCRs were done as follows: 1 cycle of 94°C (5 min); 35 cycles of 94°C (1 min), 50°C (1 min), and 72°C (1 min); and 1 cycle of 72°C (7 min). Mutations in the penicillin binding protein PBP 3 were investigated in β-lactam-resistant mutants by amplification of the ftsI gene as described by Ubukata et al. (18). The PCR products after amplification of the 23S rRNA, L22, L4, and fstI genes were purified using a QIAquick PCR purification kit (QIAGEN, Valencia, Calif.) and sequenced using an Applied Biosystems model 373 DNA sequencer.
PFGE.
All strains were tested by pulsed-field gel electrophoresis (PFGE) before and after resistance selection. PFGE was performed using a CHEF DR III apparatus (Bio-Rad, Hercules, Calif.) as described previously (12).
Transformation.
Total DNA was extracted from mutant H. influenzae strains and used for transformation of the H. influenzae Rd (ATCC 51907) strain as described previously by Barcak et al. (3). Transformants were selected on brain heart infusion agar (Becton Dickinson, Cockeysville, Md.), supplemented with hemin (2 μg/ml) and NAD (2 μg/ml) (sBHI), that contained erythromycin (15 μg/ml).
RESULTS
Multistep resistance selection.
The results of multistep resistance selection experiments are shown in Table 1. After 50 daily subcultures in amoxicillin-clavulanate, MICs did not rise more than fourfold, and the highest MIC was 4 μg/ml. After 50 days, the MICs of cefpodoxime for two strains rose to the level seen in low-BLNAR strains (18), with a fourfold increase to 0.25 μg/ml. These strains were not analyzed further, because they did not meet the mutant selection criteria (>4-fold increase) and remained susceptible according to the NCCLS breakpoint of 2.0 μg/ml (13). Cefprozil could not be tested for three β-lactamase-positive strains for which the MICs were 128 μg/ml, and one of seven strains tested yielded higher MICs. Only one β-lactamase-negative strain yielded a >4-fold increase after 32 days and became resistant (MIC ≥32 μg/ml). The MIC for this strain was 4 μg/ml and became 64 μg/ml after selection.
TABLE 1.
Multistep resistance selection studies
| Strain | β-Lactamase | Selecting drug(s) | No. of passages | MIC (μg/ml) of drug for parent and selected strains after 10 antibiotic-free subcultures
|
||||
|---|---|---|---|---|---|---|---|---|
| Amoxicillin-clavulanic acid | Cefpodoxime | Cefprozil | Azithromycin | Clarithromycin | ||||
| 1 | − | 0.5 | 0.06 | 4 | 2 | 8 | ||
| Amoxicillin-clavulanic acid | 50 | —a | — | — | — | — | ||
| Cefpodoxime | 50 | — | — | — | — | — | ||
| Cefprozil | 32 | 0.5 | 0.06 | 64 | 4 | 16 | ||
| Azithromycin | 29 | 0.5 | 0.06 | 8 | 32 | 64 | ||
| Clarithromycin | 14 | 0.5 | 0.03 | 4 | 64 | 128 | ||
| 2 | − | 0.5 | 0.06 | 2 | 1 | 8 | ||
| Amoxicillin-clavulanic acid | 50 | — | — | — | — | — | ||
| Cefpodoxime | 50 | — | — | — | — | — | ||
| Cefprozil | 50 | — | — | — | — | — | ||
| Azithromycin | 29 | 0.5 | 0.06 | 2 | >128 | >128 | ||
| Clarithromycin | 24 | 0.5 | 0.06 | 2 | 16 | 128 | ||
| 3 | − | 0.5 | 0.125 | 16 | 1 | 8 | ||
| Amoxicillin-clavulanic acid | 50 | — | — | — | — | — | ||
| Cefpodoxime | 50 | — | — | — | — | — | ||
| Cefprozil | 50 | — | — | — | — | — | ||
| Azithromycin | 22 | 0.5 | 0.125 | 32 | 64 | 64 | ||
| Clarithromycin | 32 | 1 | 0.125 | 8 | 8 | 64 | ||
| 4 | + | 2 | 0.06 | 128 | 1 | 8 | ||
| Amoxicillin-clavulanic acid | 50 | — | — | — | — | — | ||
| Cefpodoxime | 50 | — | — | — | — | — | ||
| Cefprozil | NDb | |||||||
| Azithromycin | 50 | 1 | 0.06 | 64 | 32 | 64 | ||
| Clarithromycin | 50 | — | — | — | — | — | ||
| 5 | + | 1 | 0.03 | 128 | 1 | 8 | ||
| Amoxicillin-clavulanic acid | 50 | — | — | — | — | — | ||
| Cefpodoxime | 50 | — | — | — | — | — | ||
| Cefprozil | ND | |||||||
| Azithromycin | 23 | 1 | 0.03 | 32 | 16 | 64 | ||
| Clarithromycin | 24 | 1 | 0.03 | 16 | >128 | >128 | ||
| 6 | − | 0.5 | 0.06 | 4 | 2 | 4 | ||
| Amoxicillin-clavulanic acid | 50 | — | — | — | — | — | ||
| Cefpodoxime | 50 | — | — | — | — | — | ||
| Cefprozil | 50 | — | — | — | — | — | ||
| Azithromycin | 49 | 0.5 | 0.06 | 2 | 16 | 64 | ||
| Clarithromycin | 46 | 0.5 | 0.06 | 2 | 8 | 32 | ||
| 7 | + | 2 | 0.125 | 128 | 2 | 8 | ||
| Amoxicillin-clavulanic acid | 50 | — | — | — | — | — | ||
| Cefpodoxime | 50 | — | — | — | — | — | ||
| Cefprozil | ND | |||||||
| Azithromycin | 22 | 2 | 0.125 | 32 | >128 | >128 | ||
| Clarithromycin | 50 | |||||||
| 8 | + | 1 | 0.06 | 64 | 1 | 8 | ||
| Amoxicillin-clavulanic acid | 50 | — | — | — | — | — | ||
| Cefpodoxime | 50 | — | — | — | — | — | ||
| Cefprozil | 50 | — | — | — | — | — | ||
| Azithromycin | 24 | 1 | 0.125 | >128 | 16 | 32 | ||
| Clarithromycin | 14 | 2 | 0.125 | >128 | 32 | 128 | ||
| 9 | + | 1 | 0.06 | 8 | 2 | 32 | ||
| Amoxicillin-clavulanic acid | 50 | — | — | — | — | — | ||
| Cefpodoxime | 50 | — | — | — | — | — | ||
| Cefprozil | 50 | — | — | — | — | — | ||
| Azithromycin | 20 | 1 | 0.25 | 4 | 16 | >128 | ||
| Clarithromycin | 32 | 1 | 0.03 | 16 | 32 | >128 | ||
| 10 | − | 0.5 | 0.06 | 2 | 2 | 16 | ||
| Amoxicillin-clavulanic acid | 50 | — | — | — | — | — | ||
| Cefpodoxime | 50 | — | — | — | — | — | ||
| Cefprozil | 50 | — | — | — | — | — | ||
| Azithromycin | 32 | 0.5 | 0.125 | 2 | >128 | >128 | ||
| Clarithromycin | 14 | 0.5 | 0.06 | 2 | 16 | 128 | ||
—, no mutant was selected after 50 days of subculture.
ND, not done. For three strains for which the MICs of cefprozil were >128 μg/ml, no selection was done.
Azithromycin gave a >4-fold increase in MICs for all 10 strains after 20 to 50 days, and clarithromycin gave a >4-fold increase in MICs for 8 of 10 strains after 14 to 46 days. Parent 1 strain yielded two clarithromycin-resistant clones: one became hypersusceptible to macrolides during the 10 days of daily drug-free subcultures. The MICs of erythromycin, azithromycin, clarithromycin, clindamycin, and quinupristin-dalfopristin for this hypersusceptible mutant were 0.5, 0.06, 0.12, 0.06, and 0.06 μg/ml, instead of 8, 2, 8, 32, and 2 μg/ml, respectively, in the parent strain. All resistant clones were identical to parents by PFGE. MICs of azithromycin for azithromycin-resistant clones were 16 to >128 μg/ml, and those of clarithromycin for clarithromycin-resistant clones were 32 to >128 μg/ml. For strains selected by azithromycin, the MICs of clarithromycin were high and vice versa, but for the one strain selected by a β-lactam (cefprozil) the azithromycin and clarithromycin MICs were the same as those for the parent strain.
Single-step mutations.
Single-step mutation results are shown in Table 2. The mutation frequencies for cultures grown with MICs of the antimicrobials were 2.0 × 10−3 to 2.0 × 10−9 (clarithromycin), 6.0 × 10−4 to 1.3 × 10−9 (azithromycin), 1.7 × 10−7 to <2.5 × 10−10 (cefprozil for seven strains tested), 1.5 × 10−7 to <1.0 × 10−10 (cefpodoxime), and <6.7 × 10−10 to <1.0 × 10−10 (amoxicillin-clavulanate). Clarithromycin produced the highest mutation rates, followed by azithromycin, cefprozil, cefpodoxime, and amoxicillin-clavulanate.
TABLE 2.
Frequencies of single-step mutation for 10 H. influenzae strains used for multistep study
| Strain | Selecting drug(s) | MICa (μg/ml) of drug
|
|||
|---|---|---|---|---|---|
| Initial | 2× | 4× | 8× | ||
| 1 | Amoxicillin-clavulanic acid | 0.5 | <5.0 × 10−10 | <5.0 × 10−10 | <5.0 × 10−10 |
| Cefpodoxime | 0.06 | 1.3 × 10−8 | <1.3 × 10−10 | <1.3 × 10−10 | |
| Cefprozil | 4 | 4.0 × 10−10 | <1.3 × 10−10 | <1.3 × 10−10 | |
| Azithromycin | 2 | <1.0 × 10−10 | <1.0 × 10−10 | <1.0 × 10−10 | |
| Clarithromycin | 8 | <1.0 × 10−10 | <1.0 × 10−10 | <1.0 × 10−10 | |
| 2 | Amoxicillin-clavulanic acid | 0.5 | <6.7 × 10−10 | <6.7 × 10−10 | <6.7 × 10−10 |
| Cefpodoxime | 0.06 | <1.3 × 10−10 | <1.3 × 10−10 | <1.3 × 10−10 | |
| Cefprozil | 2 | <2.0 × 10−10 | <2.0 × 10−10 | <2.0 × 10−10 | |
| Azithromycin | 1 | 4.0 × 10−9 | 2.0 × 10−9 | <2.0 × 10−10 | |
| Clarithromycin | 8 | 1.7 × 10−8 | 6.7 × 10−9 | 2.7 × 10−9 | |
| 3 | Amoxicillin-clavulanic acid | 0.5 | <5.0 × 10−10 | <5.0 × 10−10 | <5.0 × 10−10 |
| Cefpodoxime | 0.125 | <1.8 × 10−10 | <1.8 × 10−10 | <1.8 × 10−10 | |
| Cefprozil | 16 | 1.5 × 10−8 | <1.8 × 10−10 | <1.8 × 10−10 | |
| Azithromycin | 1 | 6.7 × 10−9 | <3.3 × 10−10 | <3.3 × 10−10 | |
| Clarithromycin | 8 | 5.0 × 10−9 | <5.0 × 10−10 | <5.0 × 10−10 | |
| 4 | Amoxicillin-clavulanic acid | 2 | <5.0 × 10−10 | <5.0 × 10−10 | <5.0 × 10−10 |
| Cefpodoxime | 0.06 | <5.0 × 10−10 | <5.0 × 10−10 | <5.0 × 10−10 | |
| Cefprozil | 128 | NDb | ND | ND | |
| Azithromycin | 1 | <5.0 × 10−10 | <5.0 × 10−10 | <5.0 × 10−10 | |
| Clarithromycin | 8 | 8.0 × 10−9 | <5.0 × 10−10 | <5.0 × 10−10 | |
| 5 | Amoxicillin-clavulanic acid | 1 | <3.3 × 10−10 | <3.3 × 10−10 | <3.3 × 10−10 |
| Cefpodoxime | 0.03 | <3.3 × 10−10 | <3.3 × 10−10 | <3.3 × 10−10 | |
| Cefprozil | 128 | ND | ND | ND | |
| Azithromycin | 1 | <3.3 × 10−10 | <3.3 × 10−10 | <3.3 × 10−10 | |
| Clarithromycin | 8 | <3.3 × 10−10 | <3.3 × 10−10 | <3.3 × 10−10 | |
| 6 | Amoxicillin-clavulanic acid | 0.5 | <5.0 × 10−10 | <5.0 × 10−10 | <5.0 × 10−10 |
| Cefpodoxime | 0.06 | <1.0 × 10−10 | <1.0 × 10−10 | <1.0 × 10−10 | |
| Cefprozil | 4 | <5.0 × 10−10 | <5.0 × 10−10 | <5.0 × 10−10 | |
| Azithromycin | 2 | 3.0 × 10−9 | <5.0 × 10−10 | <5.0 × 10−10 | |
| Clarithromycin | 4 | 5.0 × 10−9 | 1.0 × 10−9 | <5.0 × 10−10 | |
| 7 | Amoxicillin-clavulanic acid | 2 | <1.0 × 10−10 | <1.0 × 10−10 | <1.0 × 10−10 |
| Cefpodoxime | 0.125 | <1.0 × 10−10 | <1.0 × 10−10 | <1.0 × 10−10 | |
| Cefprozil | 128 | ND | ND | ND | |
| Azithromycin | 2 | 3.0 × 10−9 | 2.0 × 10−9 | 3.0 × 10−10 | |
| Clarithromycin | 8 | 2.0 × 10−9 | 2.0 × 10−10 | <1.0 × 10−10 | |
| 8 | Amoxicillin-clavulanic acid | 1 | <5.0 × 10−10 | <5.0 × 10−10 | <5.0 × 10−10 |
| Cefpodoxime | 0.06 | <5.0 × 10−10 | <5.0 × 10−10 | <5.0 × 10−10 | |
| Cefprozil | 64 | ND | ND | ND | |
| Azithromycin | 1 | 3.5 × 10−9 | <5.0 × 10−10 | <5.0 × 10−10 | |
| Clarithromycin | 8 | 1.5 × 10−9 | 1.5 × 10−9 | <5.0 × 10−10 | |
| 9 | Amoxicillin-clavulanic acid | 1 | <3.3 × 10−10 | <3.3 × 10−10 | <3.3 × 10−10 |
| Cefpodoxime | 0.06 | <3.3 × 10−10 | <3.3 × 10−10 | <3.3 × 10−10 | |
| Cefprozil | 8 | 6.7 × 10−8 | <3.3 × 10−10 | <3.3 × 10−10 | |
| Azithromycin | 2 | 1.0 × 10−9 | <3.3 × 10−10 | <3.3 × 10−10 | |
| Clarithromycin | 32 | 6.7 × 10−9 | 6.7 × 10−10 | <3.3 × 10−10 | |
| 10 | Amoxicillin-clavulanic acid | 0.5 | <1.3 × 10−10 | <1.3 × 10−10 | <1.3 × 10−10 |
| Cefpodoxime | 0.06 | <1.3 × 10−10 | <1.3 × 10−10 | <1.3 × 10−10 | |
| Cefprozil | 2 | <1.3 × 10−10 | <1.3 × 10−10 | <1.3 × 10−10 | |
| Azithromycin | 2 | <4.0 × 10−10 | <4.0 × 10−10 | <4.0 × 10−10 | |
| Clarithromycin | 16 | 1.3 × 10−9 | 6.3 × 10−10 | 2.5 × 10−10 | |
2×, 4×, and 8×, two, four, and eight times the MIC, respectively.
ND, not done. For strains 4, 5, 7, and 8, for which the MICs of cefprozil were high, no selection was done with cefprozil.
Mechanism of resistance to macrolides.
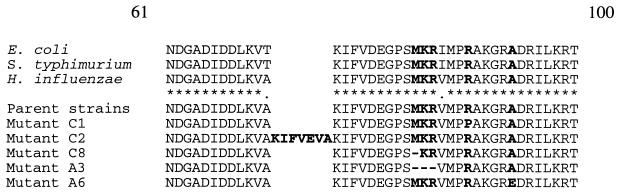
No parent or macrolide- or azalide-resistant strain had erm(B), mef(A/E), erm(A), or ere(A) genes. Of 18 mutants selected by clarithromycin and azithromycin, 2 had alterations in 23S rRNA, 5 had alterations in ribosomal protein L22, and three had alterations in ribosomal protein L4 (Tables 3 to 5). The alignments of amino acid sequence of deduced ribosomal protein sequences of L4 and L22 proteins of mutant resistant strains are shown in Fig. 1 and 2 (H. influenzae numbering). Two mutants, A2 and A7, selected by azithromycin had an A-to-C substitution at position 2059 and an A-to-G substitution at position 2058 in 23S rRNA, respectively (E. coli numbering). The MICs of erythromycin, azithromycin, clarithromycin, and clindamycin for these mutants were >128 μg/ml (Table 3). Three strains selected by clarithromycin had deletions or one-amino-acid substitutions in the highly conserved region of ribosomal protein L4 (Fig. 1). Mutant C3 had a replacement of Gly by Asp at position 65. Mutant 5 had a deletion of two amino acids, Gly and Arg, after position 65, and mutant C10 had a change of Gly to Ala at position 53 and a deletion of Arg and Ala after position 66 in ribosomal protein L4. These mutations in L4 were associated with a >4-fold increase in the MICs of clarithromycin and azithromycin (Table 4). The alterations in ribosomal protein L22 were in the highly conserved region of this protein by substitution of one amino acid, or by amino acid insertions or deletions (Fig. 2). Mutants C1 and A6 had one amino acid change at positions 88 and 93: Arg to Pro and Ala to Glu, respectively. The mutant C2 had seven amino acid insertions after position 72 in L22. Mutants C8 and A3 had one and three amino acid deletions, respectively, after position 82. The increase in MICs of clarithromycin and azithromycin for mutants with L22 alterations was at least eightfold (Table 5).
TABLE 3.
Susceptibilities of parent H. influenzae strains and their selected mutants with alterations in rRNA 23Sa
| Strain | MIC (μg/ml)
|
Detected mutation | ||||
|---|---|---|---|---|---|---|
| ERY | AZM | CLR | CLI | Q-D | ||
| Parent 2 | 8 | 2 | 8 | 16 | 2 | |
| A2 | >128 | >128 | >256 | 256 | 4 | A2059C |
| Parent 7 | 8 | 2 | 8 | 32 | 4 | |
| A7 | >128 | >128 | >256 | >256 | 4 | A2058G |
See footnotes a and b to Table 4.
TABLE 5.
Susceptibilities of parent H. influenzae strains and their selected mutants with alterations in ribosomal protein L22a
| Strain | MIC (μg/ml)
|
Detected mutation | ||||
|---|---|---|---|---|---|---|
| ERY | AZM | CLR | CLI | Q-D | ||
| Parent 1 | 8 | 2 | 8 | 32 | 2 | |
| C1 | 128 | 64 | 128 | 64 | 16 | R88P |
| Parent 2 | 8 | 2 | 8 | 16 | 2 | |
| C2 | 64 | 16 | 64 | 4 | 8 | Insb 72KIFVEVA |
| Parent 8 | 8 | 2 | 8 | 2 | 2 | |
| C8 | 64 | 32 | 128 | 16 | 8 | Del 82M |
| Parent 3 | 8 | 2 | 4 | 32 | 2 | |
| A3 | 64 | 64 | 64 | 32 | 4 | Del 82MKR |
| Parent 6 | 8 | 2 | 8 | 8 | 4 | |
| A6 | 32 | 16 | 64 | 16 | 32 | A94E |
See footnotes to Table 4.
Ins, insertion.
FIG. 1.
Alignment of deduced amino acid sequences of the highly conserved region of ribosomal protein L4 from E. coli, Salmonella enterica serovar Typhimurium, and H. influenzae and parent strains and macrolide-resistant mutant H. influenzae strains obtained by in vitro multistep resistance selection. Alterations are indicated in boldface type.
FIG. 2.
Alignment of deduced amino acid sequences of the highly conserved region of ribosomal protein L22 from E. coli, S. enterica Typhimurium, and H. influenzae and the parent strains and macrolide-resistant mutant H. influenzae strains obtained by in vitro multistep resistance selection. Alterations are indicated in boldface type.
TABLE 4.
Susceptibilities of parent H. influenzae strains and their selected mutants with alterations in ribosomal protein L4a
| Strain | MIC (μg/ml)
|
Detected mutation(s) | ||||
|---|---|---|---|---|---|---|
| ERY | AZM | CLR | CLI | Q-D | ||
| Parent 3 | 8 | 2 | 4 | 32 | 2 | |
| C3b | 64 | 8 | 64 | 256 | 8 | G65D |
| Parent 5 | 4 | 2 | 8 | 16 | 2 | |
| C5 | 32 | >128 | 32 | 32 | 32 | Delc 65GR |
| Parent 10 | 16 | 4 | 16 | 16 | 4 | |
| C10 | 128 | 16 | 128 | 4 | 8 | G53A, Del 66RA |
Abbreviations: ERY, erythromycin; AZM, azithromycin; CLR, clarithromycin; CLI, clindamycin; Q-D, quinupristin-dalfopristin.
Mutant strain designations are formed by the first letter of the antibiotic (A for AZM and C for CLR) and the number of parent strain used for selection.
Del, deletion.
Total DNAs from C1 with the L22 mutation and C10 with the L4 mutation were used for transformation of H. influenzae Rd. After transformation resistant strains were selected on sBHI agar with erythromycin (15 μg/ml). Two transformants were studied further. Transformants C1/Rd (L22) and C10/Rd (L4) had the same PFGE pattern as the Rd strain after digestion by SmaI. The MICs of erythromycin for Rd strains rose from 2 μg/ml to 64 and 32 μg/ml after transformation with total DNA from mutants C1 and C10, respectively. The sequence analysis of the genes coding for 23S rRNA and ribosomal proteins L4 and L22 were amplified from transformants and the Rd strain and was sequenced. The transformants C1/Rd and C10/Rd had the same mutations as the mutants C1 and C10, respectively (Table 6).
TABLE 6.
Susceptibility of H. influenzae Rd strains transformed with total DNA from C1 with mutation in ribosomal protein L22 and C10 with mutation in ribosomal protein L4a
| Strain | MIC (μg/ml)
|
Detected mutation(s) | |||
|---|---|---|---|---|---|
| ERY | AZM | CLR | CLI | ||
| Rd | 2 | 2 | 4 | 1 | |
| C1 | 128 | 64 | 128 | 64 | R88P in L22 |
| C1/Rdb | 64 | 16 | 64 | 4 | R88P in L22 |
| C10 | 128 | 16 | 128 | 4 | G53A, Del 66RA in L4 |
| C10/Rdb | 32 | 8 | 64 | 1 | G53A, Del 66RA in L4 |
See footnotes to Table 4.
C1/Rd and C10/Rd, Rd strain transformed with total DNA from mutant C1 or mutant C10, respectively.
Among 18 resistant mutant strains selected by azithromycin and clarithromycin, no modification was found in ribosomal proteins L4 or L22 or the studied portion of 23S rRNA for eight strains. These strains are currently being studied for their resistance mechanisms.
Three randomly selected single-step mutants, selected at two times MIC by azithromycin and clarithromycin, were analyzed for alterations in 23S rRNA, and ribosomal proteins L4 and L22. Strains selected by azithromycin from parents 6 and 8 had deletions in ribosomal protein L22: two amino acids, Arg and Ile, at positions 95 and 96 from strain 6 and Asp and Glu at positions 77 and 78 from strain 8, were deleted. No modification was found in the other strains.
Mechanism of resistance to cefprozil.
The gene ftsI that codes for PBP 3 from parent 1 and the mutant strain selected by cefprozil were amplified and sequenced. The analysis of these sequences and alignment of deduced amino acids showed two mutations, which led to Ala-to-Val and Ser-to-Asn substitutions at positions 271 and 357, respectively. These mutations were associated with a 16-fold increase in the cefprozil MIC; however, the activity of amoxicillin-clavulanate and cefpodoxime was not affected (Table 1).
DISCUSSION
The main mechanism of resistance detected in wild-type H. influenzae strains is the production of β-lactamase, with an overall prevalence of 13.4% in Europe (9) and up to 41.6% in the United States (11). In Japan, the prevalence of β-lactamase production is low (13.9%). However, the prevalence of BLNAR strains is 28.8% (18), while in the United States and Europe this phenotype is rare (<1%) (9, 11). This study tested the ability of five antibiotics, three β-lactams and two macrolides, to select resistant mutants in vitro in H. influenzae. No resistant mutants were selected by amoxicillin-clavulanate or cefpodoxime. Cefprozil MICs rose in one β-lactamase-negative strain. This MIC increase for cefprozil was associated with two amino acid changes, Ala-271 to Val and Ser-357 to Asn in PBP 3. The contribution of the Ser-357-to-Asn mutation to resistance to β-lactam antibiotics with decrease of β-lactam affinity has already been shown with transformation by Ubukata et al. (18). The low frequency of selection of resistant mutants by the β-lactam antibiotics tested in this study correlates with the low prevalence of BLNAR strains in the United States. However, in vitro selection of highly resistant mutants by azithromycin and clarithromycin does not correspond with the low prevalence of such resistance levels seen among clinical H. influenzae strains (0.5 and 1.9%, respectively) (8). Clarithromycin and azithromycin selected a total of 18 resistant mutants from 10 strains tested. Multistep azithromycin and clarithromycin exposure resulted in selection of resistant mutants in all 10 strains tested and 8 of these strains, respectively. Macrolide and azalide MICs for these mutants were 16.0 to >128.0 μg/ml. Such high MICs are not commonly seen in clinical H. influenzae isolates, and their clinical significance is unclear at the present time.
To our knowledge, no resistance mechanisms have been described for highly macrolide-resistant H. influenzae strains. In this study we characterized mutations in 23S rRNA and in ribosomal proteins L4 and L22 in H. influenzae mutants for which the macrolide and azalide MICs are high. Macrolide resistance as a result of alterations in 23S rRNA have been found in different gram-negative and -positive species (19). For two resistant mutants selected by exposure to azithromycin, with A2059C and A2058G mutations in 23S rRNA, the MICs of azithromycin, clarithromycin, erythromycin, and clindamycin were >128 μg/ml. The mutations found in ribosomal proteins L4 and L22 were in the highly conserved region of these proteins (Fig. 1 and 2). These mutations probably cause a conformational change in the ribosome that decreases the affinity of macrolides. Resistance as a result of mutations in L4 and L22 have been reported in in vitro mutants of E. coli (20). L4 mutation has been detected as the cause of resistance in clinical isolates of S. pneumoniae (17). In laboratory mutants of S. pneumoniae, alterations in ribosomal protein L4 and L22 conferring resistance to macrolides were also shown (4). This study showed that mutations in ribosomal proteins in L4 and L22 in H. influenzae were associated with increases in the MICs of erythromycin, clarithromycin, and azithromycin and contributed to increases of macrolide MICs in H. influenzae Rd strain, as observed in transformation studies. However MICs of clindamycin and quinupristin-dalfopristin were not raised in all of these mutants. Susceptibility to quinupristin-dalfopristin seems to be affected by alterations in ribosomal proteins L4 and L22 far more than to clindamycin. In some mutants a fourfold decrease in clindamycin MICs was observed. The significance and explanation for these lower clindamycin MICs are unknown at this time. In clinical strains of S. pneumoniae, mutations in L4 do not affect the susceptibility of the strains to clindamycin (17). The isolation of an H. influenzae clone that was hypersusceptible to macrolides is of great interest. The mechanism of macrolide susceptibility in this hypersusceptible strain is currently under investigation. For eight resistant mutants no mechanism of resistance was detected. The absence of modifications in the studied portion of 23S rRNA and in ribosomal proteins L4 and L22 shows that other ribosomal regions or proteins are likely to be involved in macrolide resistance. Investigation of the mechanisms of resistance are being conducted in our laboratory.
In summary, repeated daily subcultures in subinhibitory concentrations of amoxicillin-clavulanate and cefpodoxime did not lead to development of resistant clones, although cefpodoxime MICs of some strains rose to the levels seen in low-BLNAR strains. Only one resistant mutant was selected by cefprozil from a β-lactamase-negative strain. Sequential subculture in azithromycin and clarithromycin led to extremely high MICs, and in some strains these increases in MICs were associated with alterations in 23S rRNA, or in ribosomal proteins L4 or L22. One initially resistant mutant strain became hypersusceptible to macrolides during subculture without antibiotic. The clinical significance of these findings is not known, and this aspect is currently being investigated by our group.
Acknowledgments
This study was supported by a grant from GlaxoSmithKline Laboratories, Collegeville, Pa.
We thank Kimiko Ubukata for helpful discussions.
REFERENCES
- 1.Baquero, F. 1999. Evolving resistance patterns of Streptococcus pneumoniae: a link with long-acting macrolide consumption? J. Chemother. 11:35-43. [DOI] [PubMed] [Google Scholar]
- 2.Baquero, F. 1996. Trends in antibiotic resistance of respiratory pathogens: an analysis and commentary on a collaborative surveillance study. J. Antimicrob. Chemother. 38(Suppl. A):117-132. [DOI] [PubMed] [Google Scholar]
- 3.Barcak, G. J., M. S. Chandler, R. J. Redfield, and J. F. Tomb. 1991. Genetic systems in Haemophilus influenzae. Methods Enzymol. 204:321-342. [DOI] [PubMed] [Google Scholar]
- 4.Canu, A., B. Malbruny, M. Coquemont, T. A. Davies, P. C. Appelbaum, and R. Leclercq. 2002. Diversity of ribosomal mutations conferring resistance to macrolides, clindamycin, streptogramin, and telithromycin in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 46:125-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Craig, W. A. 2001. The hidden impact of antibacterial resistance in respiratory tract infection. Re-evaluating current antibiotic therapy. Respir. Med. 95(Suppl. A):S12-9, S26-S27. [DOI] [PubMed] [Google Scholar]
- 6.Davies, T. A., B. E. Dewasse, M. R. Jacobs, and P. C. Appelbaum. 2000. In vitro development of resistance to telithromycin (HMR 3647), four macrolides, clindamycin, and pristinamycin in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 44:414-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davies, T. A., G. A. Pankuch, B. E. Dewasse, M. R. Jacobs, and P. C. Appelbaum. 1999. In vitro development of resistance to five quinolones and amoxicillin-clavulanate in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 43:1177-1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doern, G. V., A. B. Brueggemann, G. Pierce, H. P. Holley, Jr., and A. Rauch. 1997. Antibiotic resistance among clinical isolates of Haemophilus influenzae in the United States in 1994 and 1995 and detection of beta-lactamase-positive strains resistant to amoxicillin-clavulanate: results of a national multicenter surveillance study. Antimicrob. Agents Chemother. 41:292-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Felmingham, D., and R. N. Gruneberg. 2000. The Alexander Project 1996-1997: latest susceptibility data from this international study of bacterial pathogens from community-acquired lower respiratory tract infections. J. Antimicrob. Chemother. 45:191-203. [DOI] [PubMed] [Google Scholar]
- 10.Jacobs, M. R. 2001. Optimisation of antimicrobial therapy using pharmacokinetic and pharmacodynamic parameters. Clin. Microbiol. Infect. 7:589-596. [DOI] [PubMed] [Google Scholar]
- 11.Jacobs, M. R., S. Bajaksouzian, A. Zilles, G. Lin, G. A. Pankuch, and P. C. Appelbaum. 1999. Susceptibilities of Streptococcus pneumoniae and Haemophilus influenzae to 10 oral antimicrobial agents based on pharmacodynamic parameters: 1997 USA surveillance study. Antimicrob. Agents Chemother. 43:1901-1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagai, K., T. A. Davies, G. A. Pankuch, B. E. Dewasse, M. R. Jacobs, and P. C. Appelbaum. 2000. In vitro selection of resistance to clinafloxacin, ciprofloxacin, and trovafloxacin in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 44:2740-2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Committee for Clinical Laboratory Standards. 1997. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. NCCLS publication no. M7-A4. National Committee for Clinical Laboratory Standards. Wayne, Pa.
- 14.Needham, C. A. 1988. Haemophilus influenzae antibiotic susceptibility. Clin. Microbiol. Rev. 1:218-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pankuch, G. A., S. A. Jueneman, T. A. Davies, M. R. Jacobs, and P. C. Appelbaum. 1998. In vitro selection of resistance to four β-lactams and azithromycin in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 42:2914-2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sutcliffe, J., T. Grebe, A. Tait-Kamradt, and L. Wondrack. 1996. Detection of erythromycin-resistant determinants by PCR. Antimicrob. Agents Chemother. 40:2562-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tait-Kamradt, A., T. A. Davies, P. C. Appelbaum, F. Depardieu, P. Courvalin, J. Petitpas, L. Wondrack, A. Walker, M. R. Jacobs, and J. Sutcliffe. 2000. Two new mechanisms of macrolide resistance in clinical strains of Streptococcus pneumoniae from Eastern Europe and North America. Antimicrob. Agents Chemother. 44:3395-3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ubukata, K., Y. Shibasaki, K. Yamamoto, N. Chiba, K. Hasegawa, Y. Takeuchi, K. Sunakawa, M. Inoue, and M. Konno. 2001. Association of amino acid substitutions in penicillin-binding protein 3 with β-lactam resistance in beta-lactamase-negative ampicillin-resistant Haemophilus influenzae. Antimicrob Agents Chemother. 45:1693-1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vester, B., and S. Douthwaite. 2001. Macrolide resistance conferred by base substitutions in 23S rRNA. Antimicrob. Agents Chemother. 45:1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weisblum, B. 1995. Erythromycin resistance by ribosome modification. Antimicrob. Agents Chemother. 39:577-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeckel, M. L., K. D. Jacobson, F. J. Guerra, D. G. Therasse, and D. Farlow. 1992. Loracarbef (LY 163892) versus amoxicillin/clavulanate in the treatment of acute bacterial exacerbations of chronic bronchitis. Clin. Ther. 14:214-229. [PubMed] [Google Scholar]