Abstract
1. Electrical coupling between rod photoreceptors was studied in the eyecup preparation of the snapping turtle, Chelydra serpentina, using intracellular micro-electrodes.
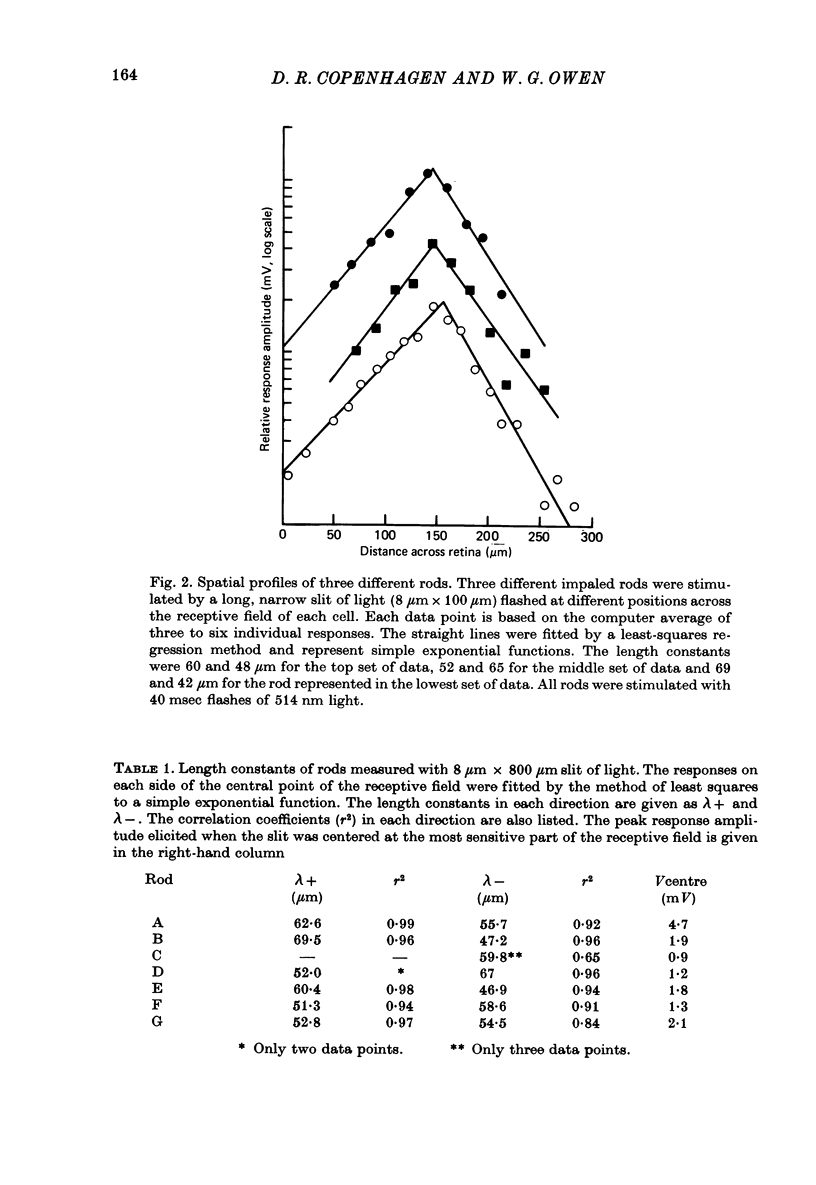
2. The spatial profiles of rod responses to a long narrow slit of light were determined. The peak response amplitudes were found to decline exponentially as the slit was moved from the most sensitive position in the receptive field of each rod. The mean length constant was 55·7 μm.
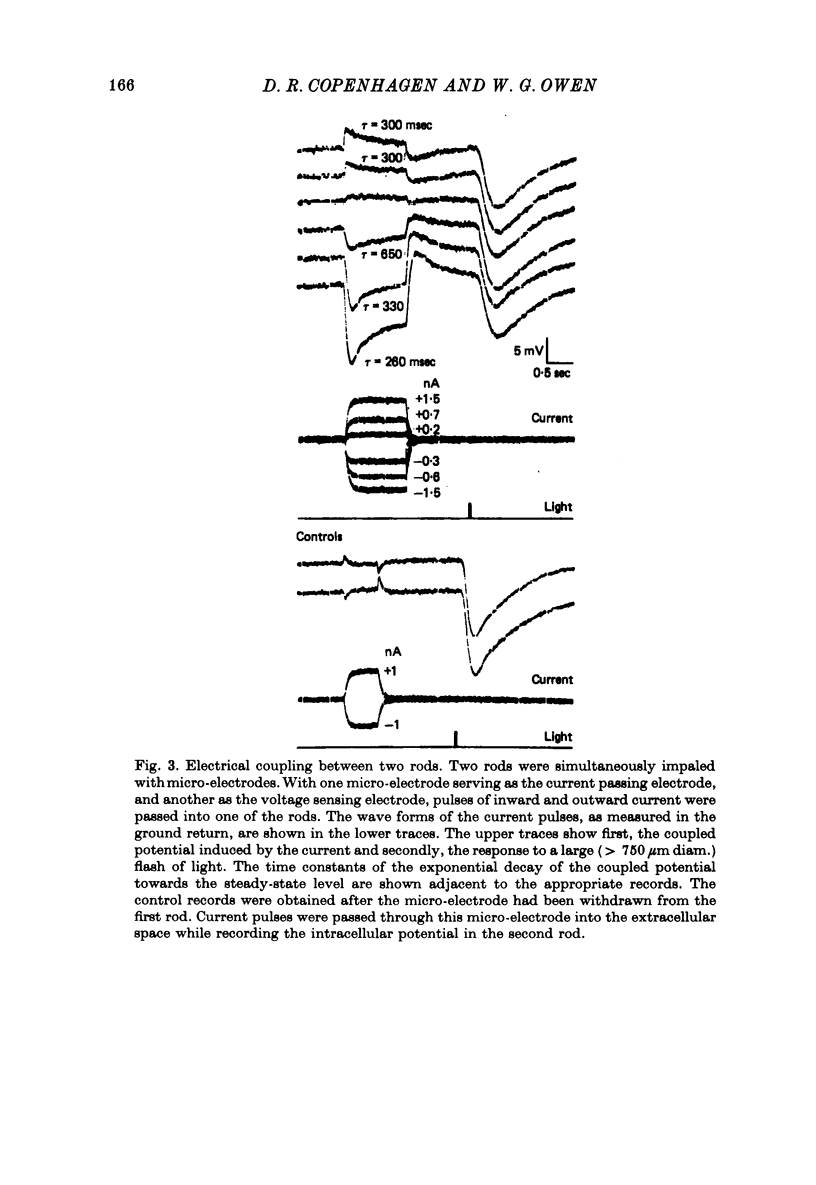
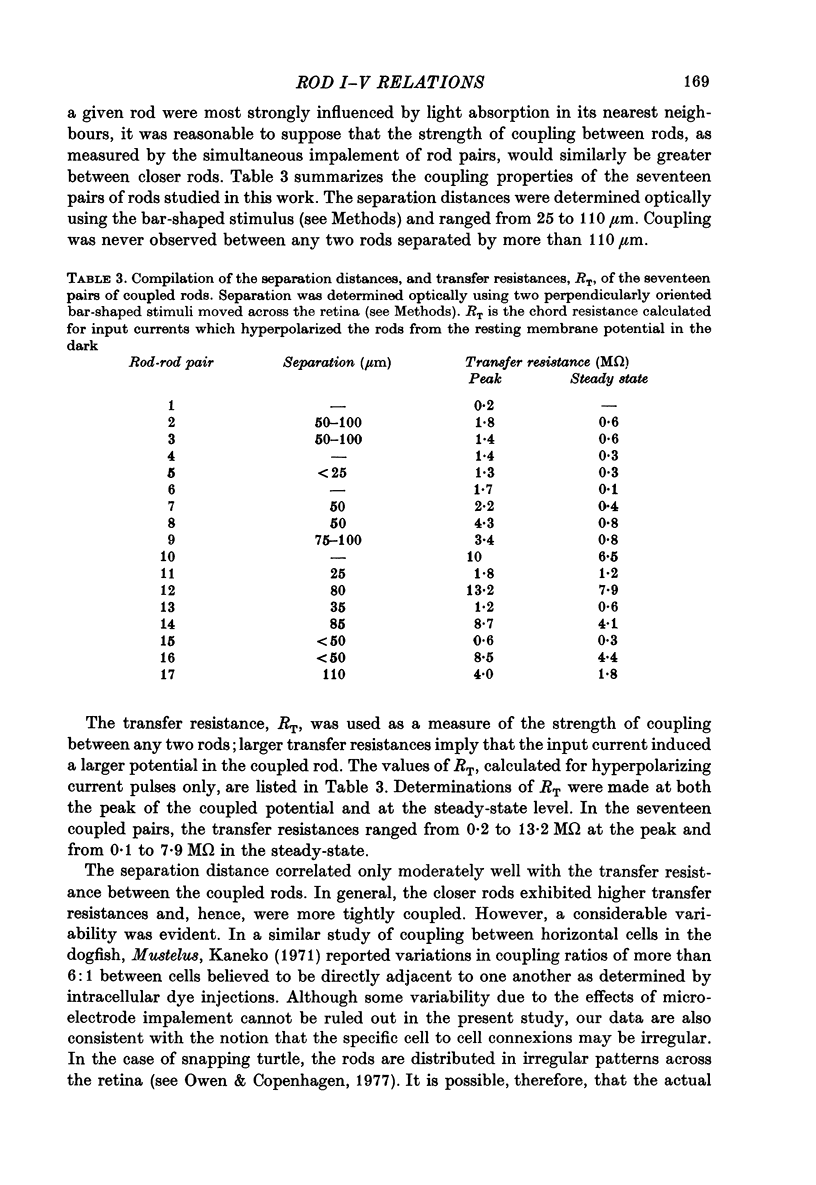
3. Rods were simultaneously impaled in pairs and electrical coupling was demonstrated between the rods in seventeen of these pairs. No coupling was observed between rods separated by more than 110 μm. The transfer resistance, defined as the ratio of potential in the second rod (coupled potential) to the current injected into the first rod, varied from 0·2 to 13·2 MΩ.
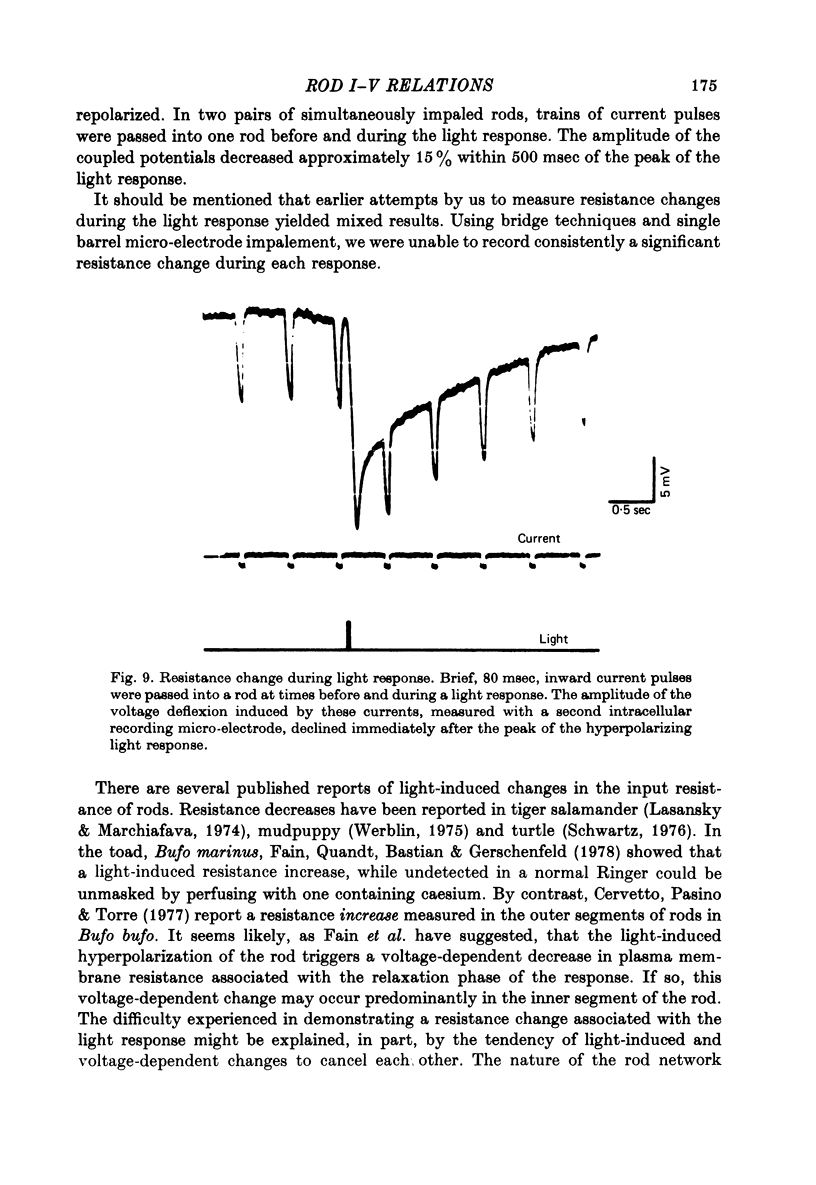
4. The waveform of the coupled potential was time varying, exhibiting a peak and subsequent relaxation phase. The time course of the relaxation phase was voltage-dependent. At the cessation of current, the coupled potential rebounded beyond the resting potential and then decayed to the dark potential.
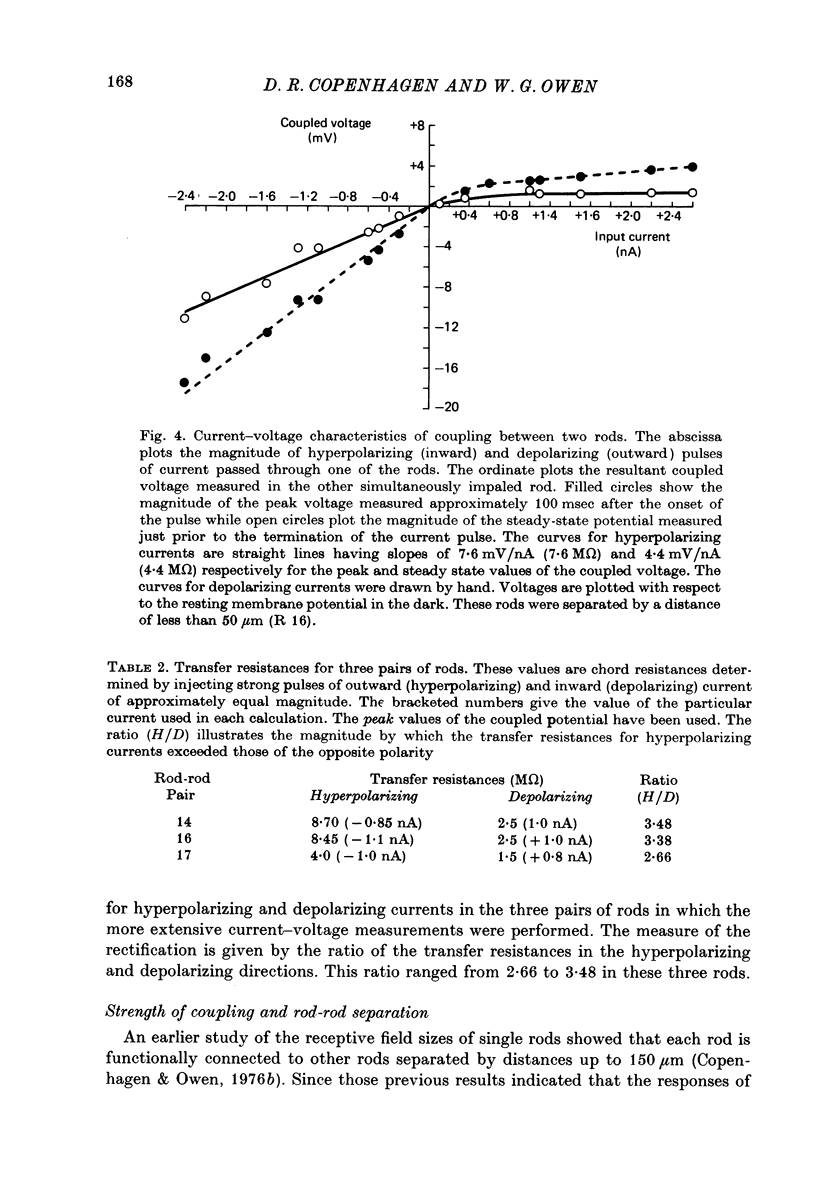
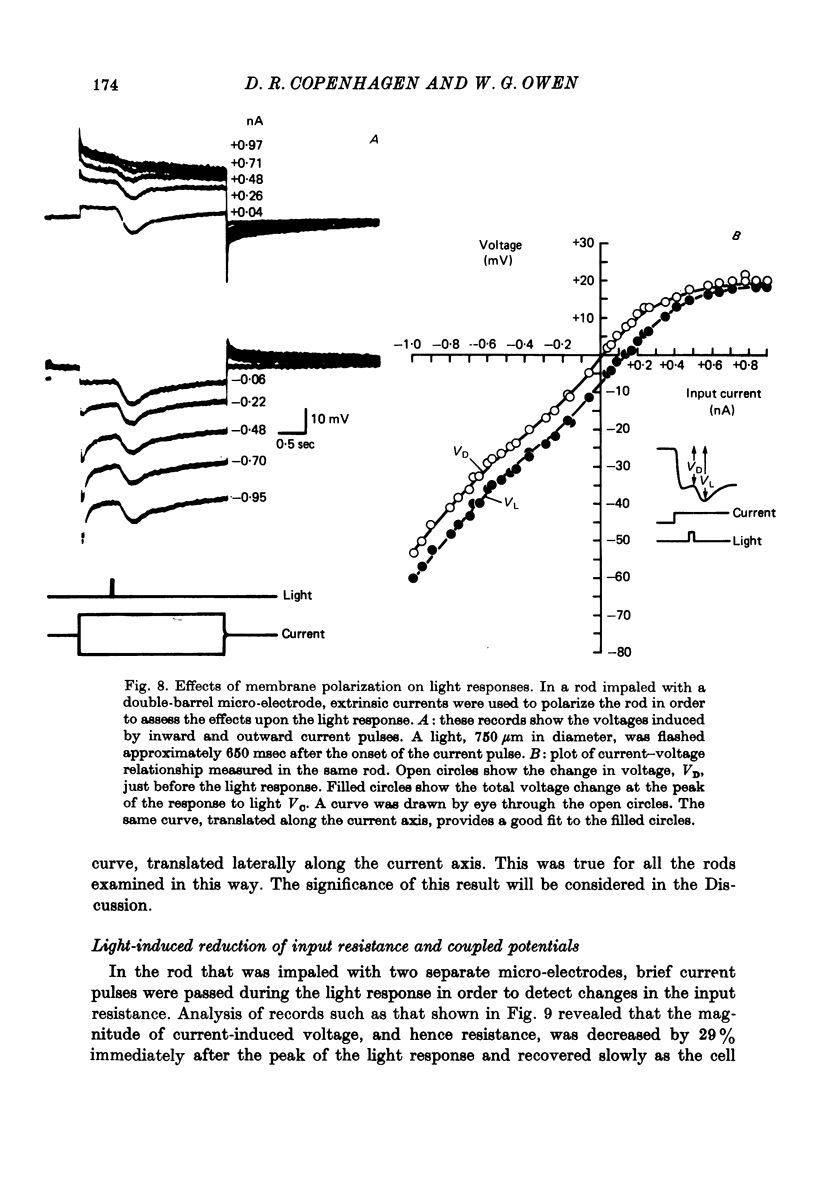
5. Plots of input current versus coupled potential showed strong outward-going rectification, chord transfer resistances being as much as 3·5 times lower for depolarizing currents.
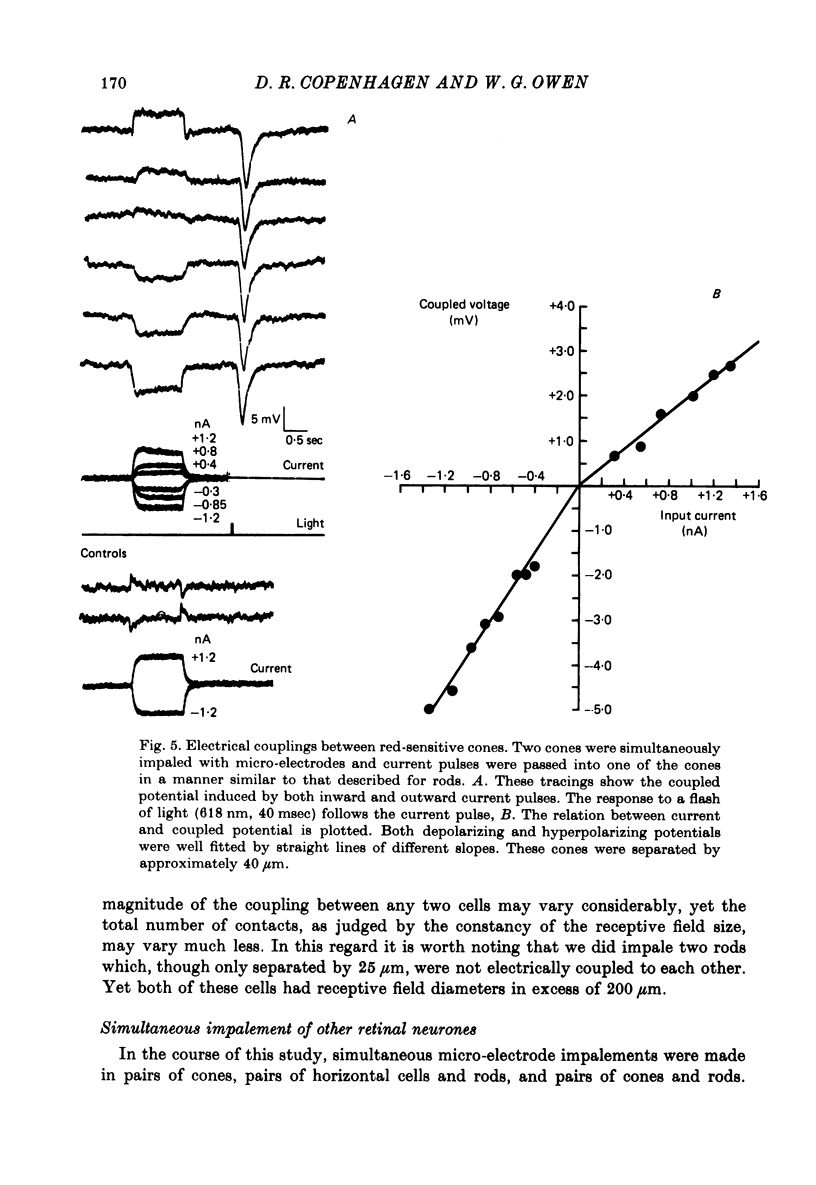
6. Simultaneous impalements were made of pairs of neighbouring red-sensitive cones, of horizontal cells and rods, and of red-sensitive cones and rods. No evidence of coupling between cones and rods were found, nor could feed-back from horizontal cells onto rods be demonstrated; however, coupling between red-sensitive cones was found. This coupling exhibited neither the marked time-varying nor voltage-dependent properties that characterize the rod-rod coupling.
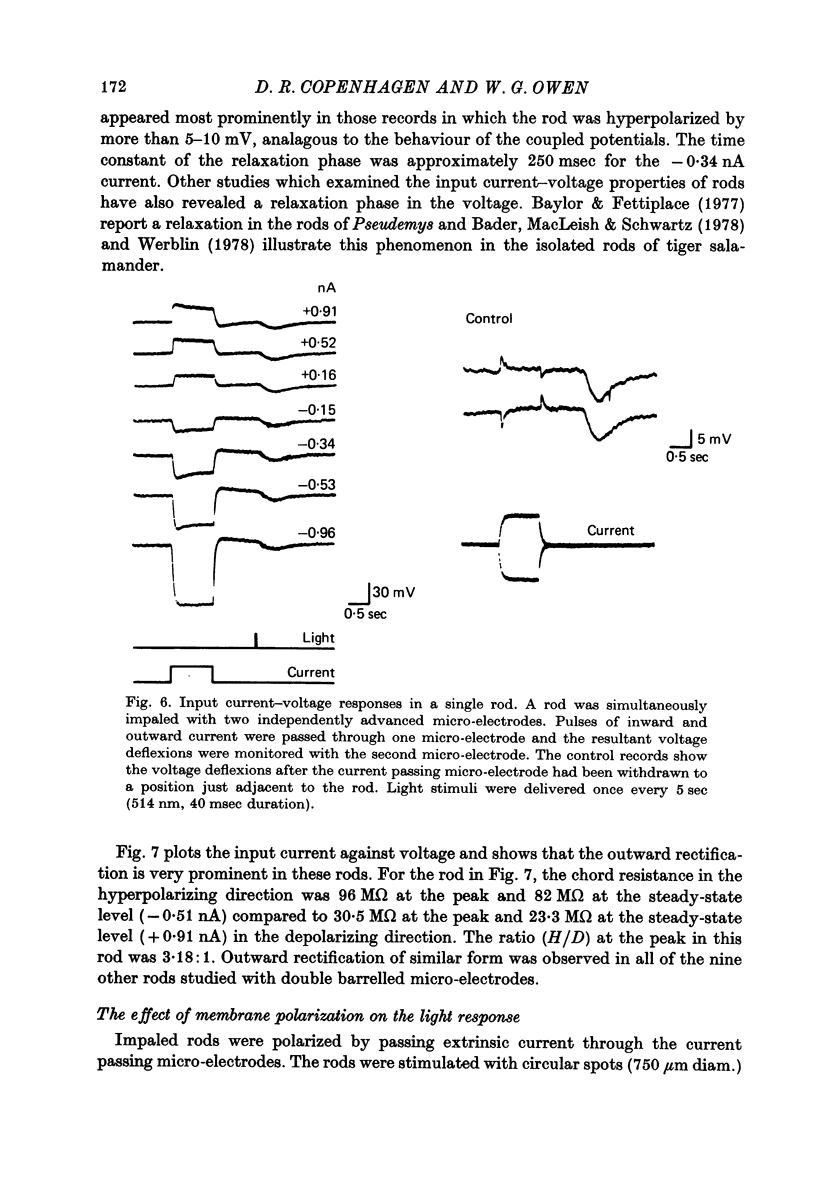
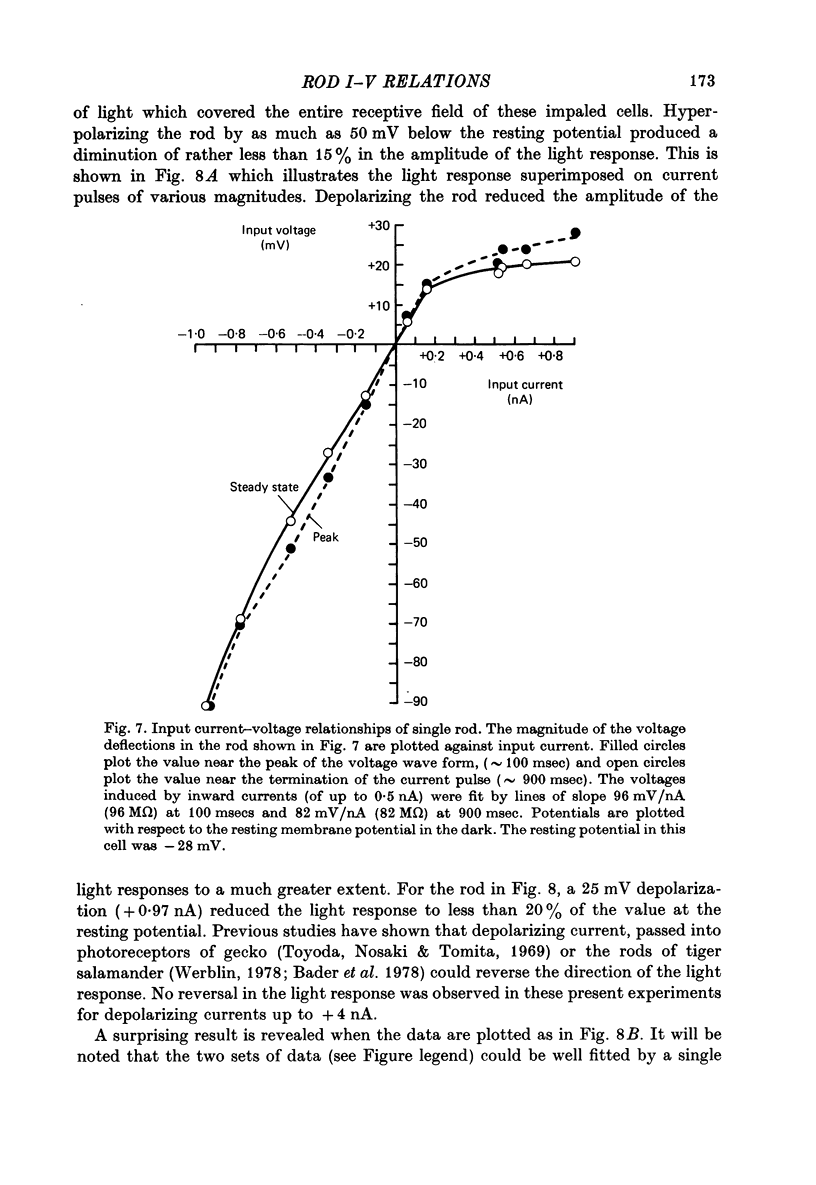
7. Individual rods were impaled with independent current passing and voltage sensing micro-electrodes. Pulses of current produced time-varying potentials having relaxation and rebound phases. Current—voltage measurements showed a strong outward-going rectification. Input resistances at the resting potential ranged up to 96 MΩ.
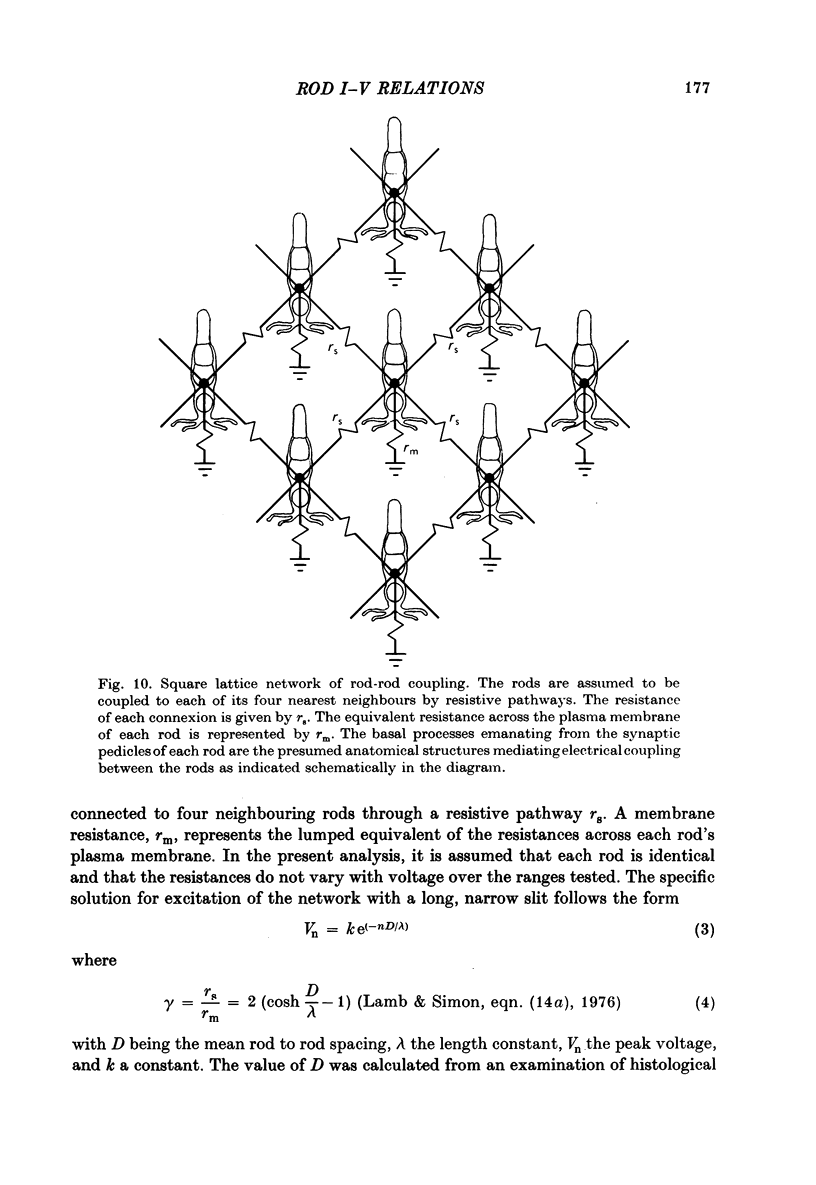
8. Square grid and hexagonal lattice models of ohmic electrical coupling were applied to the results. Using the measured values for the length constants and input resistances of single rods, we calculate that the plasma membrane resistance of each rod is approximately 1000 MΩ at the resting potential and that the coupling resistances are 272 and 444 MΩ for the square grid and hexagonal models, respectively.
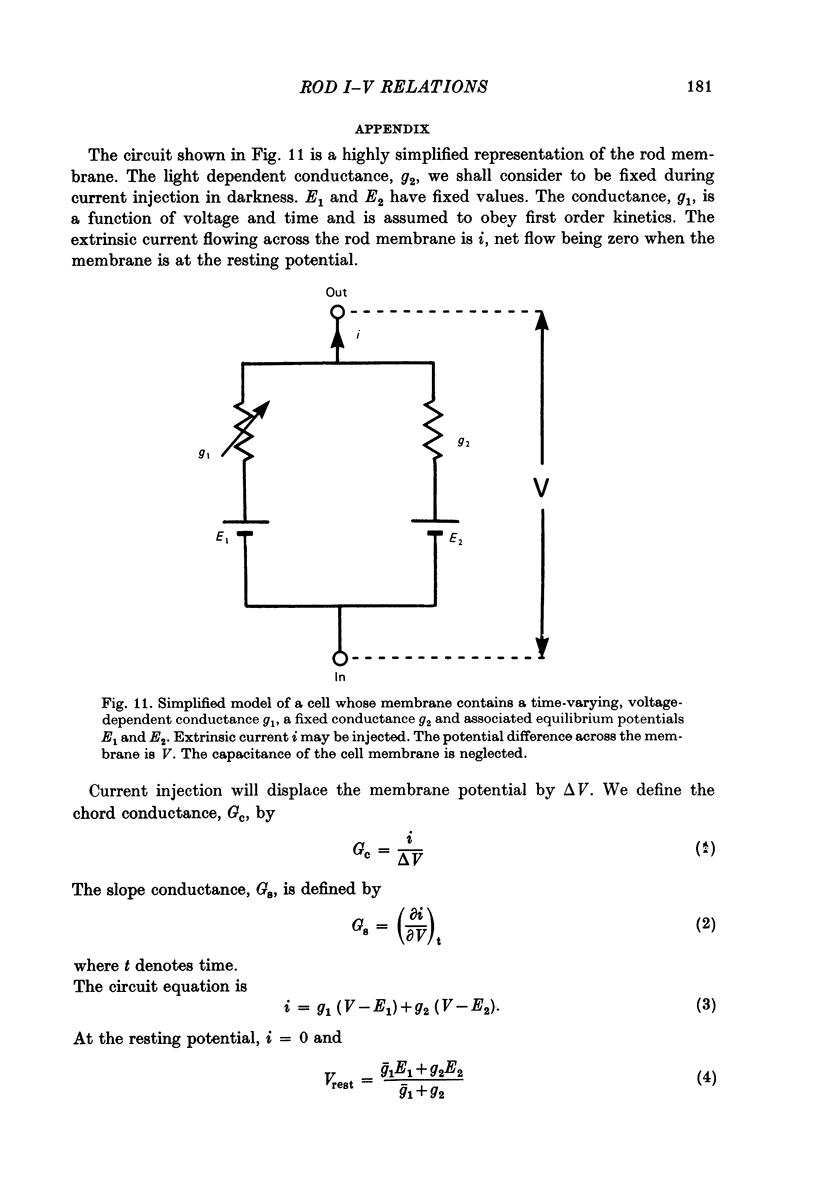
9. The time-varying and voltage-dependent properties observed at the input and in the coupling between rods appear to reflect characteristics of the rod's plasma membrane and not of the coupling pathways between the rods. Both the outward-going rectification and relaxation phase of the response appear to involve voltage-dependent conductance increases in the rod's plasma membrane.
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bader C. R., MacLeish P. R., Schwartz E. A. Responses to light of solitary rod photoreceptors isolated from tiger salamander retina. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3507–3511. doi: 10.1073/pnas.75.7.3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader C. R., Macleish P. R., Schwartz E. A. A voltage-clamp study of the light response in solitary rods of the tiger salamander. J Physiol. 1979 Nov;296:1–26. doi: 10.1113/jphysiol.1979.sp012988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Fettiplace R. Kinetics of synaptic transfer from receptors to ganglion cells in turtle retina. J Physiol. 1977 Oct;271(2):425–448. doi: 10.1113/jphysiol.1977.sp012007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Fuortes M. G., O'Bryan P. M. Receptive fields of cones in the retina of the turtle. J Physiol. 1971 Apr;214(2):265–294. doi: 10.1113/jphysiol.1971.sp009432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. V., Nakajima Y., Pappas G. D. Physiology and ultrastructure of electrotonic junctions. I. Supramedullary neurons. J Neurophysiol. 1967 Mar;30(2):161–179. doi: 10.1152/jn.1967.30.2.161. [DOI] [PubMed] [Google Scholar]
- Brink P., Barr L. The resistance of the septum of the median giant axon of the earthworm. J Gen Physiol. 1977 May;69(5):517–536. doi: 10.1085/jgp.69.5.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervetto L., Pasino E., Torre V. Electrical responses of rods in the retina of Bufo marinus. J Physiol. 1977 May;267(1):17–51. doi: 10.1113/jphysiol.1977.sp011799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copenhagen D. R., Owen W. G. Coupling between rod photoreceptors in a vertebrate retina. Nature. 1976 Mar 4;260(5546):57–59. doi: 10.1038/260057a0. [DOI] [PubMed] [Google Scholar]
- Copenhagen D. R., Owen W. G. Functional characteristics of lateral interactions between rods in the retina of the snapping turtle. J Physiol. 1976 Jul;259(2):251–282. doi: 10.1113/jphysiol.1976.sp011465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detwiler P. B., Hodgkin A. L., McNaughton P. A. A surprising property of electrical spread in the network of rods in the turtle's retina. Nature. 1978 Aug 10;274(5671):562–565. doi: 10.1038/274562a0. [DOI] [PubMed] [Google Scholar]
- FURSHPAN E. J., POTTER D. D. Transmission at the giant motor synapses of the crayfish. J Physiol. 1959 Mar 3;145(2):289–325. doi: 10.1113/jphysiol.1959.sp006143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain G. L., Gold G. H., Dowling J. E. Receptor coupling in the toad retina. Cold Spring Harb Symp Quant Biol. 1976;40:547–561. doi: 10.1101/sqb.1976.040.01.051. [DOI] [PubMed] [Google Scholar]
- Fain G. L., Quandt F. N., Bastian B. L., Gerschenfeld H. M. Contribution of a caesium-sensitive conductance increase to the rod photoresponse. Nature. 1978 Mar 30;272(5652):466–469. doi: 10.1038/272467a0. [DOI] [PubMed] [Google Scholar]
- Fain G. L., Quandt F. N., Gerschenfeld H. M. Calcium-dependent regenerative responses in rods. Nature. 1977 Oct 20;269(5630):707–710. doi: 10.1038/269707a0. [DOI] [PubMed] [Google Scholar]
- Fuortes M. G., Simon E. J. Interactions leading to horizontal cell responses in the turtle retina. J Physiol. 1974 Jul;240(1):177–198. doi: 10.1113/jphysiol.1974.sp010606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold G. H. Photoreceptor coupling in retina of the toad, Bufo marinus. II. Physiology. J Neurophysiol. 1979 Jan;42(1 Pt 1):311–328. doi: 10.1152/jn.1979.42.1.311. [DOI] [PubMed] [Google Scholar]
- Grundfest H. Heterogeneity of excitable membrane: electrophysiological and pharmacological evidence and some consequences. Ann N Y Acad Sci. 1966 Jul 14;137(2):901–949. doi: 10.1111/j.1749-6632.1966.tb50208.x. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F., KATZ B. Measurement of current-voltage relations in the membrane of the giant axon of Loligo. J Physiol. 1952 Apr;116(4):424–448. doi: 10.1113/jphysiol.1952.sp004716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M., Oshima T. Electrical behaviour of the motoneurone membrane during intracellularly applied current steps. J Physiol. 1965 Oct;180(3):607–635. doi: 10.1113/jphysiol.1965.sp007720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko A. Electrical connexions between horizontal cells in the dogfish retina. J Physiol. 1971 Feb;213(1):95–105. doi: 10.1113/jphysiol.1971.sp009370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb T. D., Simon E. J. Analysis of electrical noise in turtle cones. J Physiol. 1977 Nov;272(2):435–468. doi: 10.1113/jphysiol.1977.sp012053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb T. D., Simon E. J. The relation between intercellular coupling and electrical noise in turtle photoreceptors. J Physiol. 1976 Dec;263(2):257–286. doi: 10.1113/jphysiol.1976.sp011631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb T. D. Spatial properties of horizontal cell responses in the turtle retina. J Physiol. 1976 Dec;263(2):239–255. doi: 10.1113/jphysiol.1976.sp011630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasansky A., Marchiafava P. L. Light-induced resistance changes in retinal rods and cones of the tiger salamander. J Physiol. 1974 Jan;236(1):171–191. doi: 10.1113/jphysiol.1974.sp010429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeper H. F., Normann R. A., Copenhagen D. R. Evidence for passive electrotonic interactions in red rods of toad retina. Nature. 1978 Sep 21;275(5677):234–236. doi: 10.1038/275234b0. [DOI] [PubMed] [Google Scholar]
- Nelson R. Cat cones have rod input: a comparison of the response properties of cones and horizontal cell bodies in the retina of the cat. J Comp Neurol. 1977 Mar 1;172(1):109–135. doi: 10.1002/cne.901720106. [DOI] [PubMed] [Google Scholar]
- Schwartz E. A. Electrical properties of the rod syncytium in the retina of the turtle. J Physiol. 1976 May;257(2):379–406. doi: 10.1113/jphysiol.1976.sp011374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz E. A. Responses of single rods in the retina of the turtle. J Physiol. 1973 Aug;232(3):503–514. doi: 10.1113/jphysiol.1973.sp010283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz E. A. Rod-rod interaction in the retina of the turtle. J Physiol. 1975 Apr;246(3):617–638. doi: 10.1113/jphysiol.1975.sp010907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spray D. C., Harris A. L., Bennett M. V. Voltage dependence of junctional conductance in early amphibian embryos. Science. 1979 Apr 27;204(4391):432–434. doi: 10.1126/science.312530. [DOI] [PubMed] [Google Scholar]
- Toyoda J., Nosaki H., Tomita T. Light-induced resistance changes in single photoreceptors of Necturus and Gekko. Vision Res. 1969 Apr;9(4):453–463. doi: 10.1016/0042-6989(69)90134-5. [DOI] [PubMed] [Google Scholar]
- Werblin F. S. Regenerative hyperpolarization in rods. J Physiol. 1975 Jan;244(1):53–81. doi: 10.1113/jphysiol.1975.sp010784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werblin F. S. Transmission along and between rods in the tiger salamander retina. J Physiol. 1978 Jul;280:449–470. doi: 10.1113/jphysiol.1978.sp012394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiederhold M. L. Rectification in Aplysia statocyst receptor cells. J Physiol. 1977 Mar;266(1):139–156. doi: 10.1113/jphysiol.1977.sp011760. [DOI] [PMC free article] [PubMed] [Google Scholar]


