Abstract
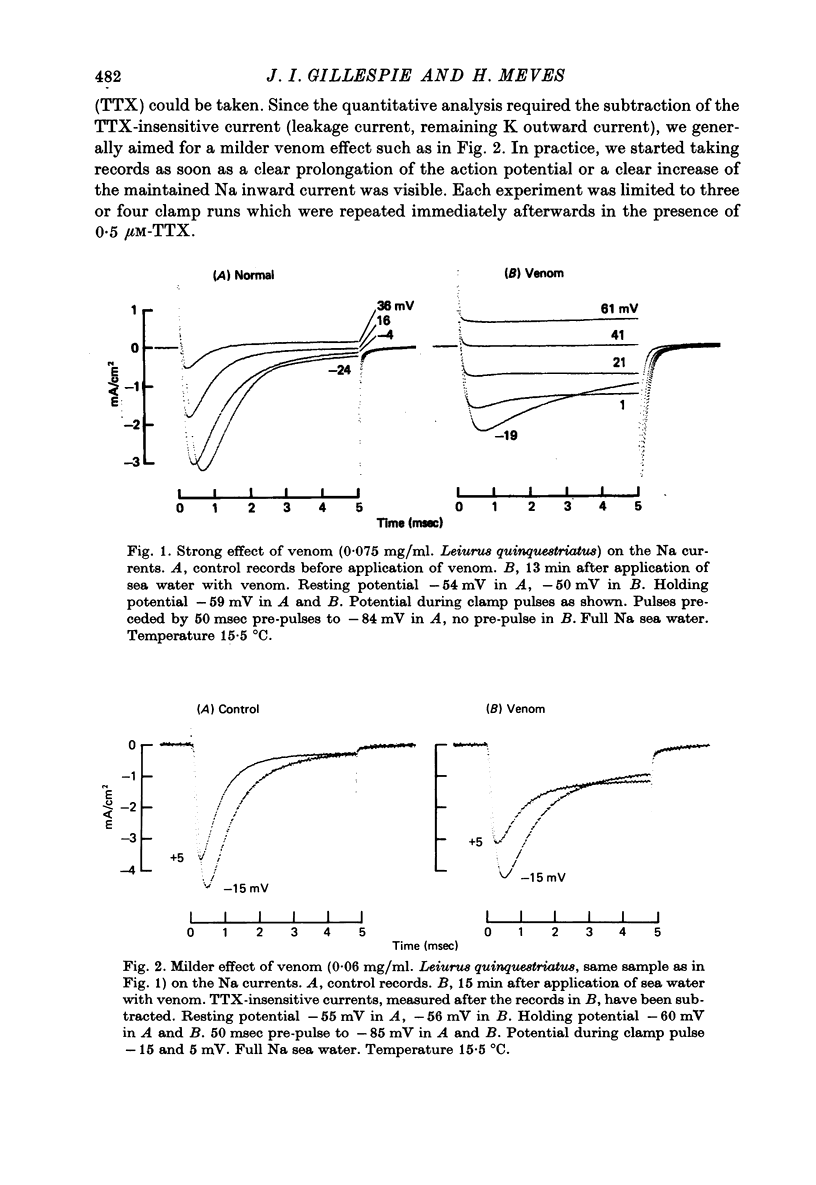
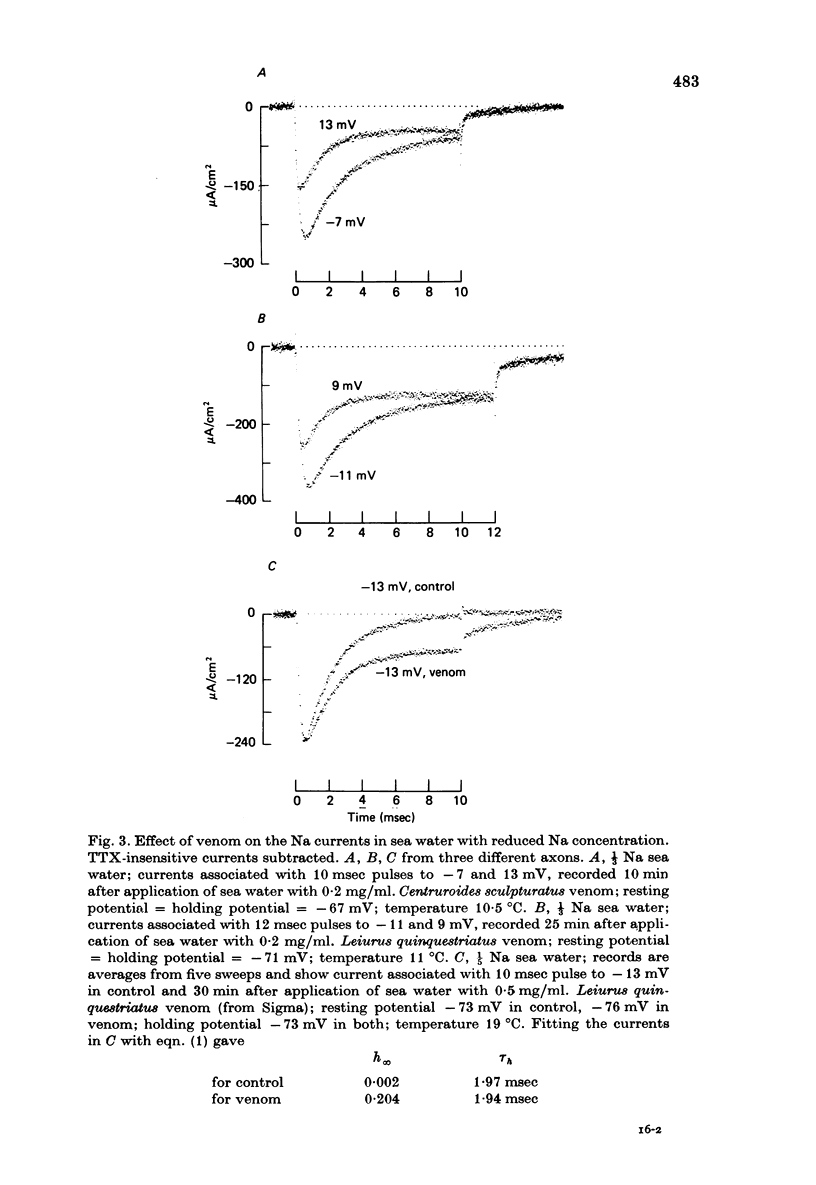
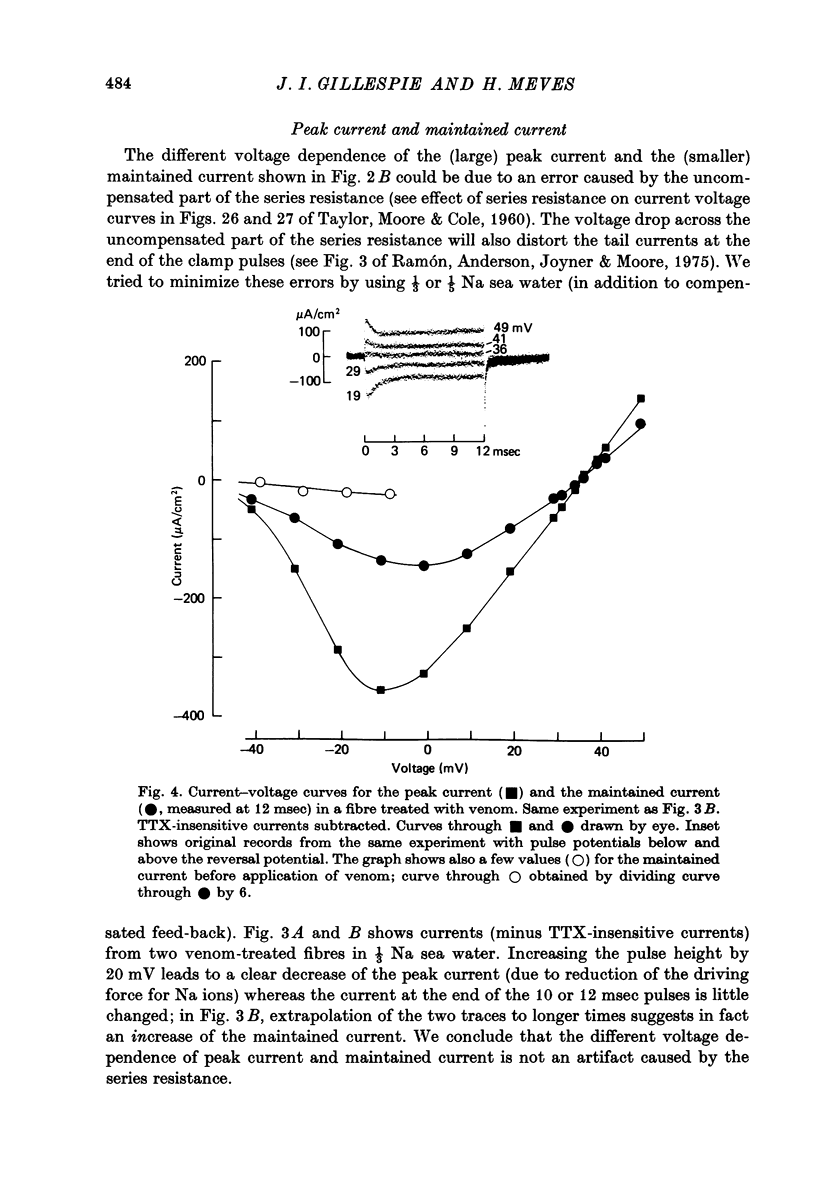
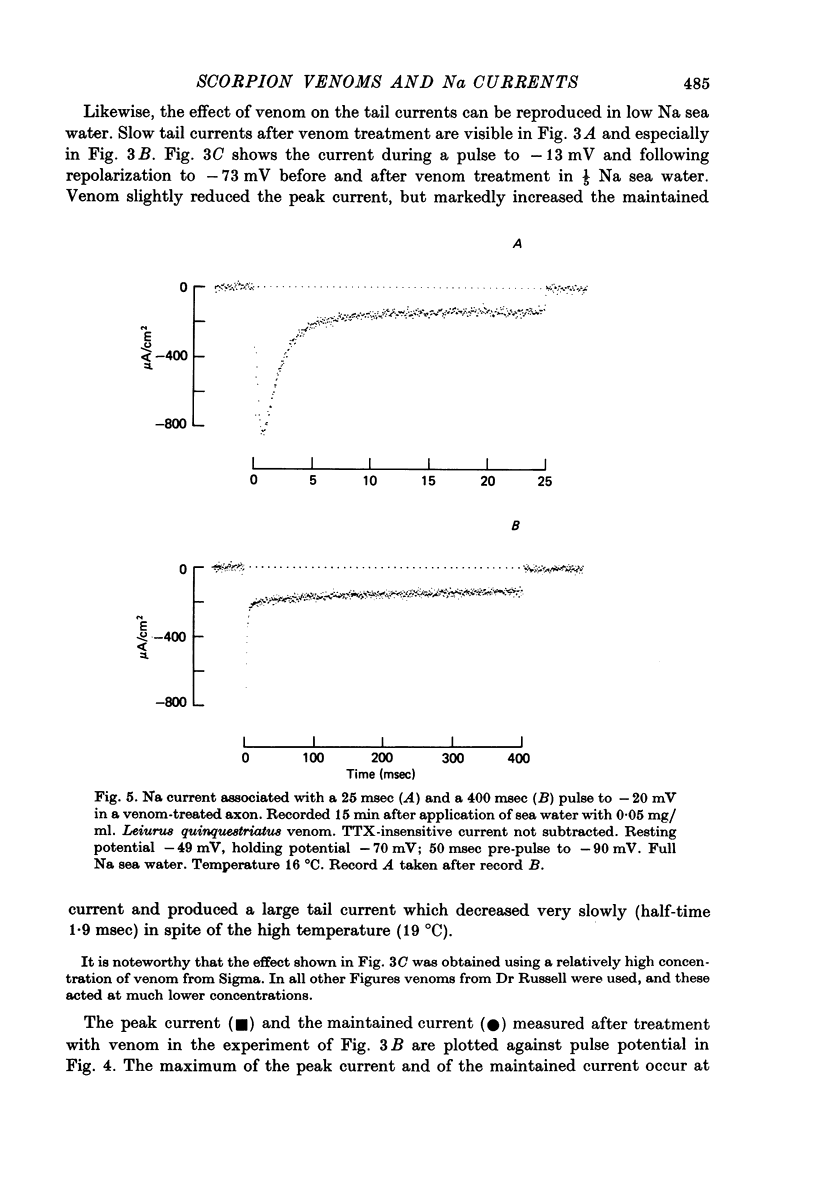
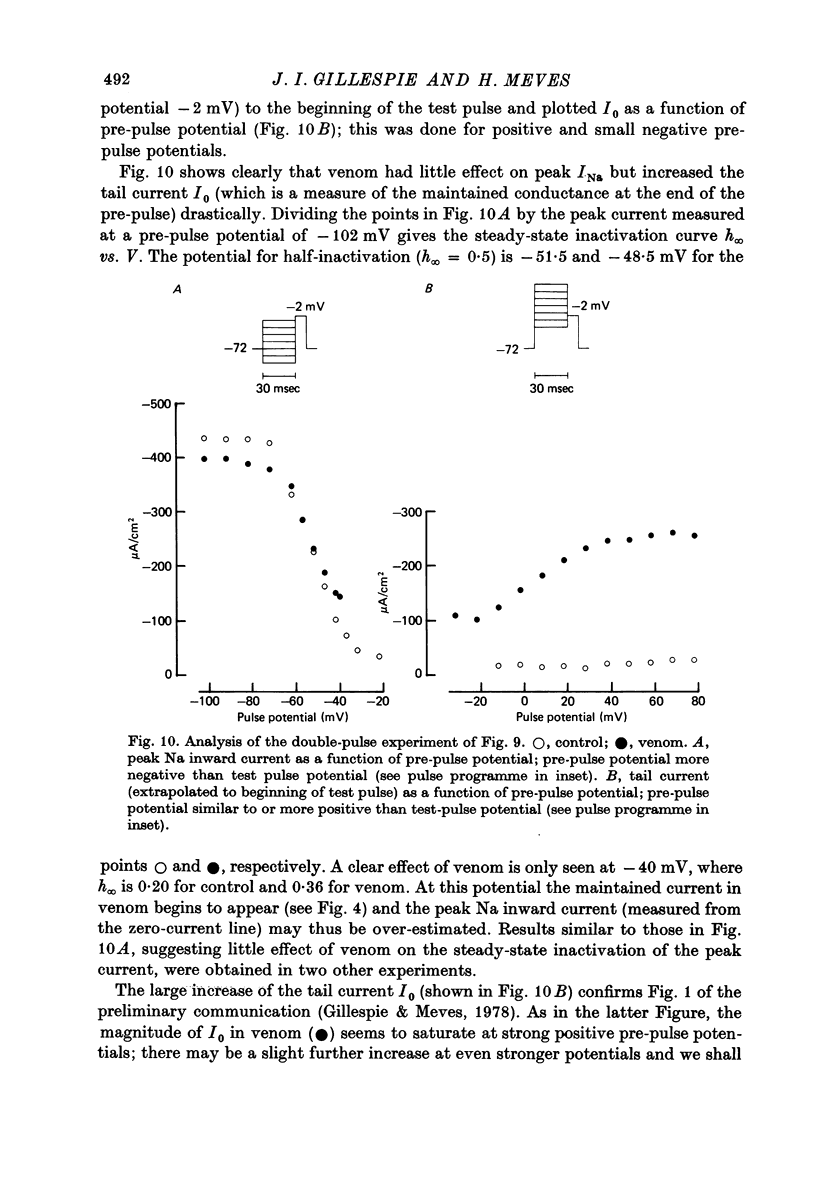
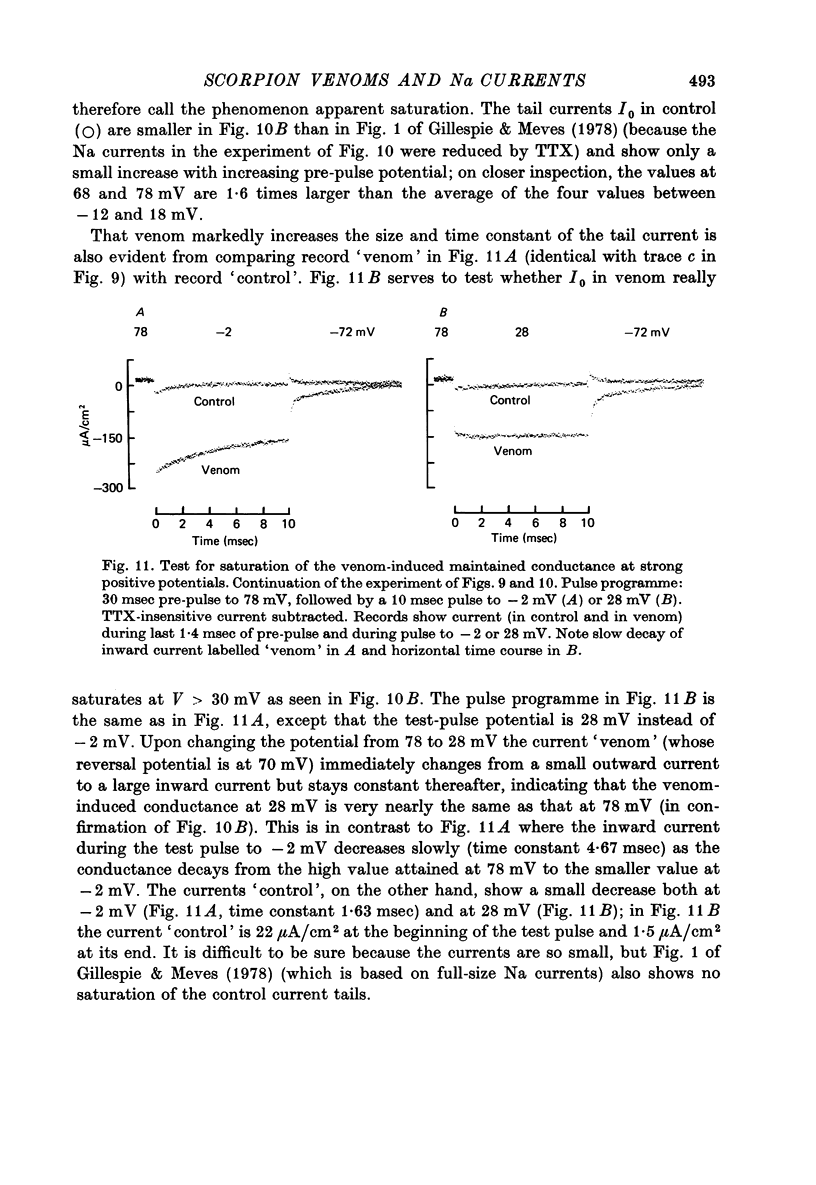
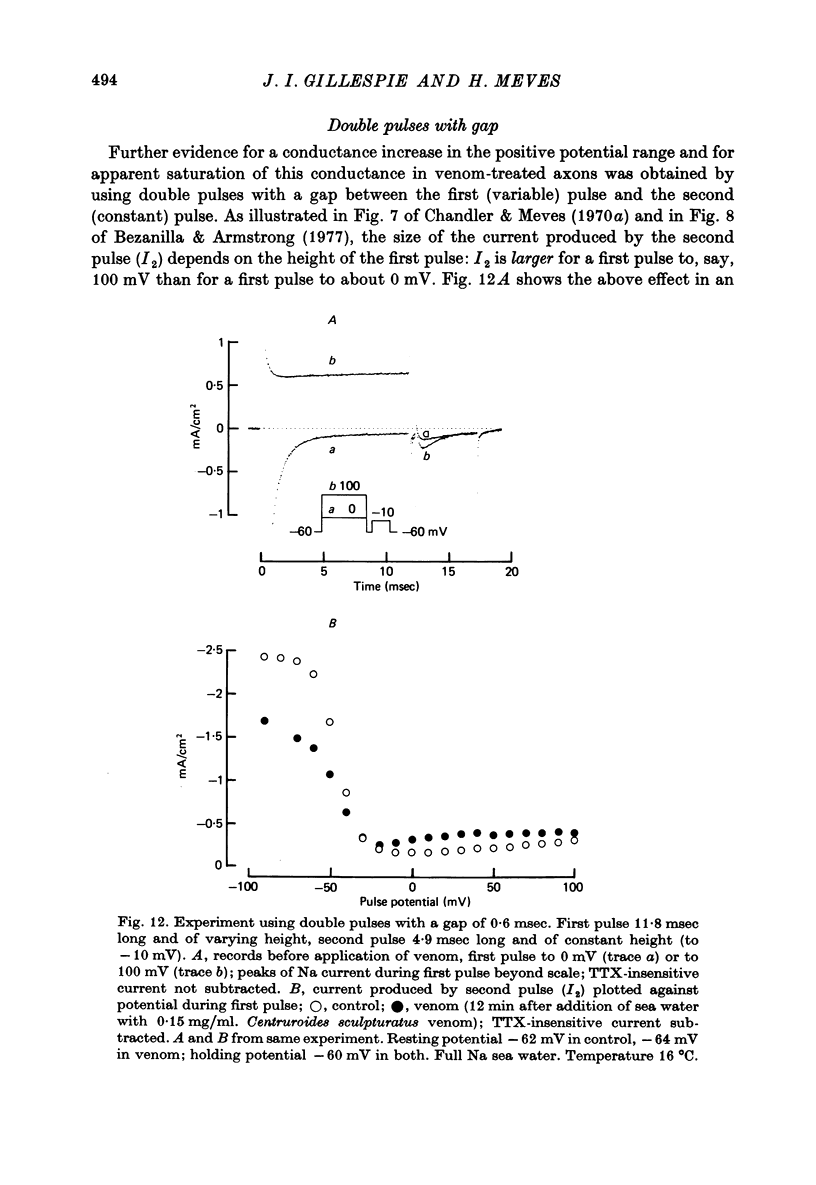
1. The effect of externally applied scorpion venoms (0.1--0.5 mg/ml., species Leiurus quinquestriatus and Centruroides sculpturatus) on the Na currents of intracellularly perfused squid giant axons has been studied with the voltage-clamp method. 2. The venoms from the two species had the same effect. They reduced the size of the peak conductance but had little effect on its kinetics (time to peak, time constant of inactivation) and on its steady-state inactivation. The venoms increased markedly, however, the maintained conductance and the time constants of its turning-on and turning-off. 3. The voltage dependence of the maintained conductance was determined (a) by fitting a modified Hodgkin--Huxley equation to the Na currents and (b) by measuring the tail currents at the end of depolarizing pulses. The maintained conductance rose with increasing depolarization from a minimum at -20 mV to a maximum at 40 mV. The peak conductance, by contrast, was constant in the positive potential range. 4. The ratio maintained conductance in venom to maintained conductance in control varied between 2 and 7 (depending on the venom concentration and the time of treatment) and was not significantly dependent on membrane potential. 5. Peak current and maintained current reversed sign at the same potential and were both blocked by tetrodotoxin. 6. During a pulse to -2 mV preceded by a pre-pulse to -42 mV the Na conductance showed a rapid initial increase followed by a slower decay and a subsequent slow increase, reflecting the activation and inactivation of the peak conductance and the slow development of the maintained conductance. 7. Many of the observations are compatible with the idea that scorpion venoms increase the number of channels which go from the peak conductance state into the maintained conductance state (open in equilibrium or formed from closed in equilibrium or formed from open transition of the inactivation gate, see Chandler & Meves (1970 a, b)). But the alternative hypothesis that peak conductance and maintained conductance reflect two separate populations of Na channels cannot be ruled out.
Full text
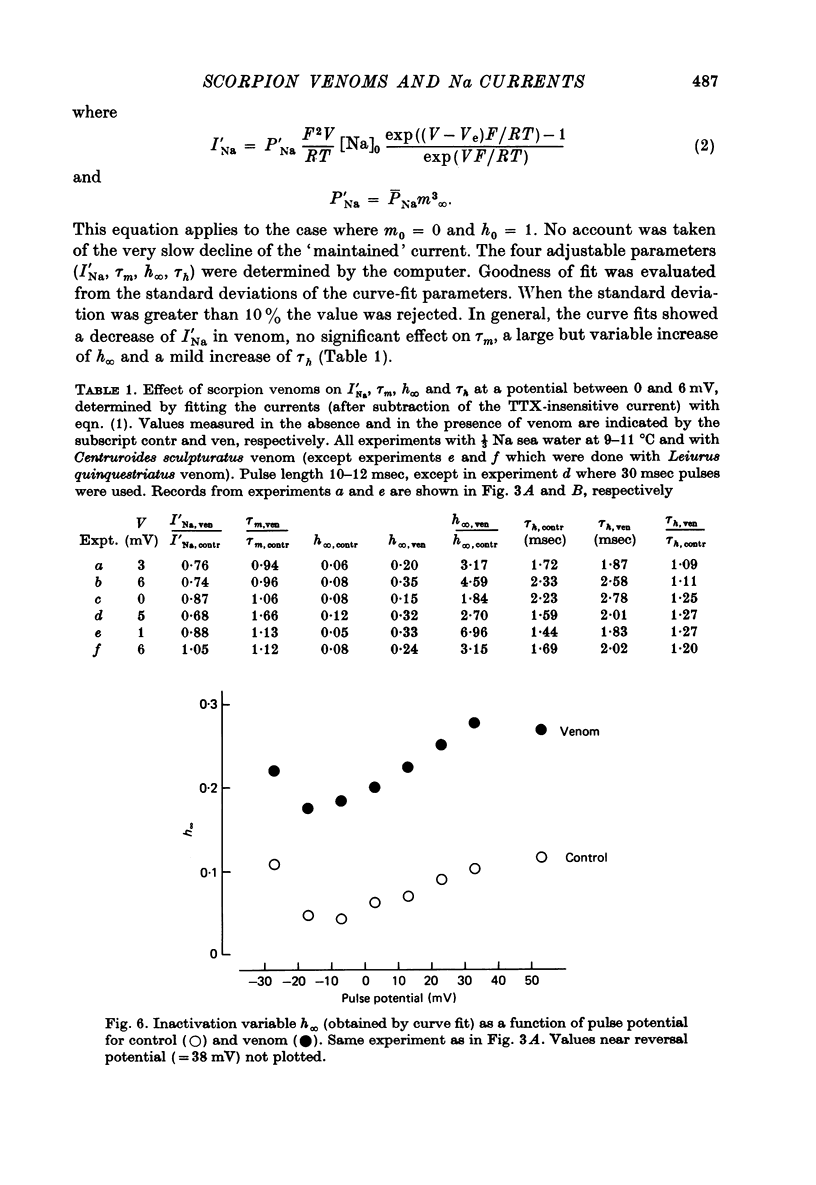
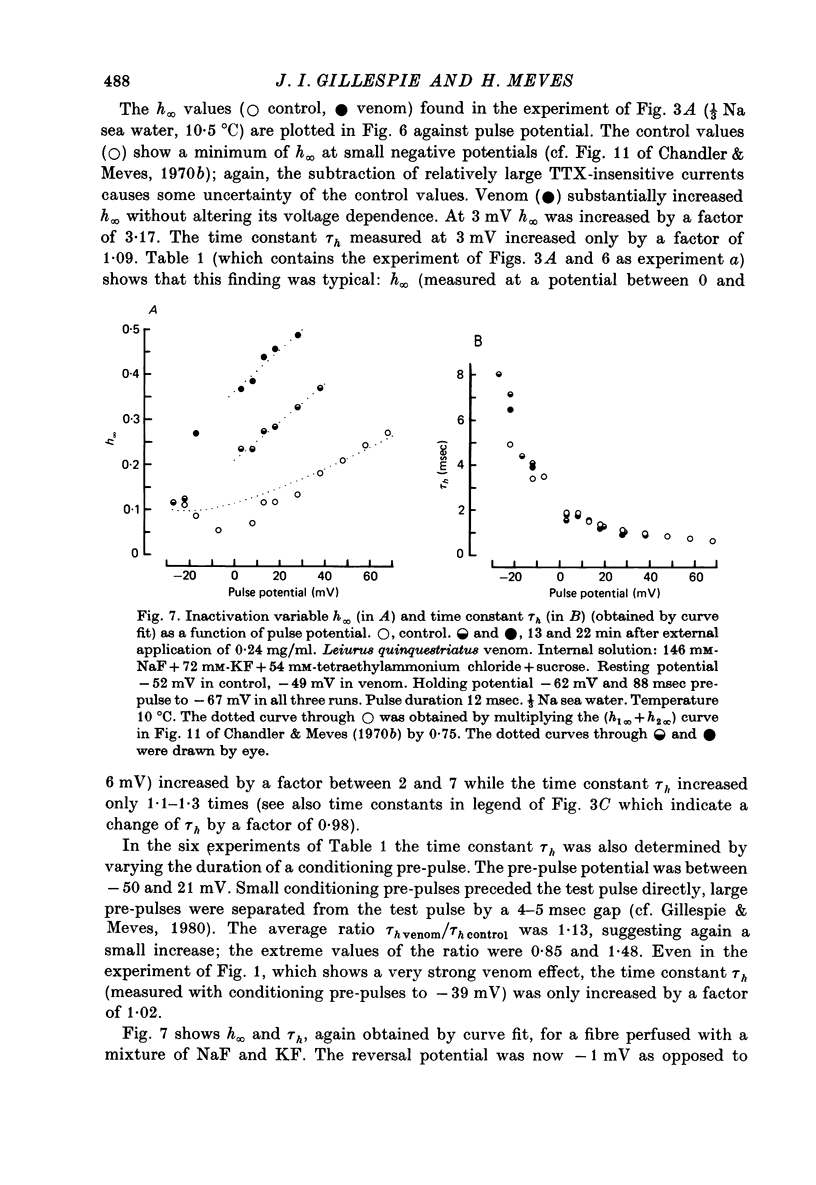
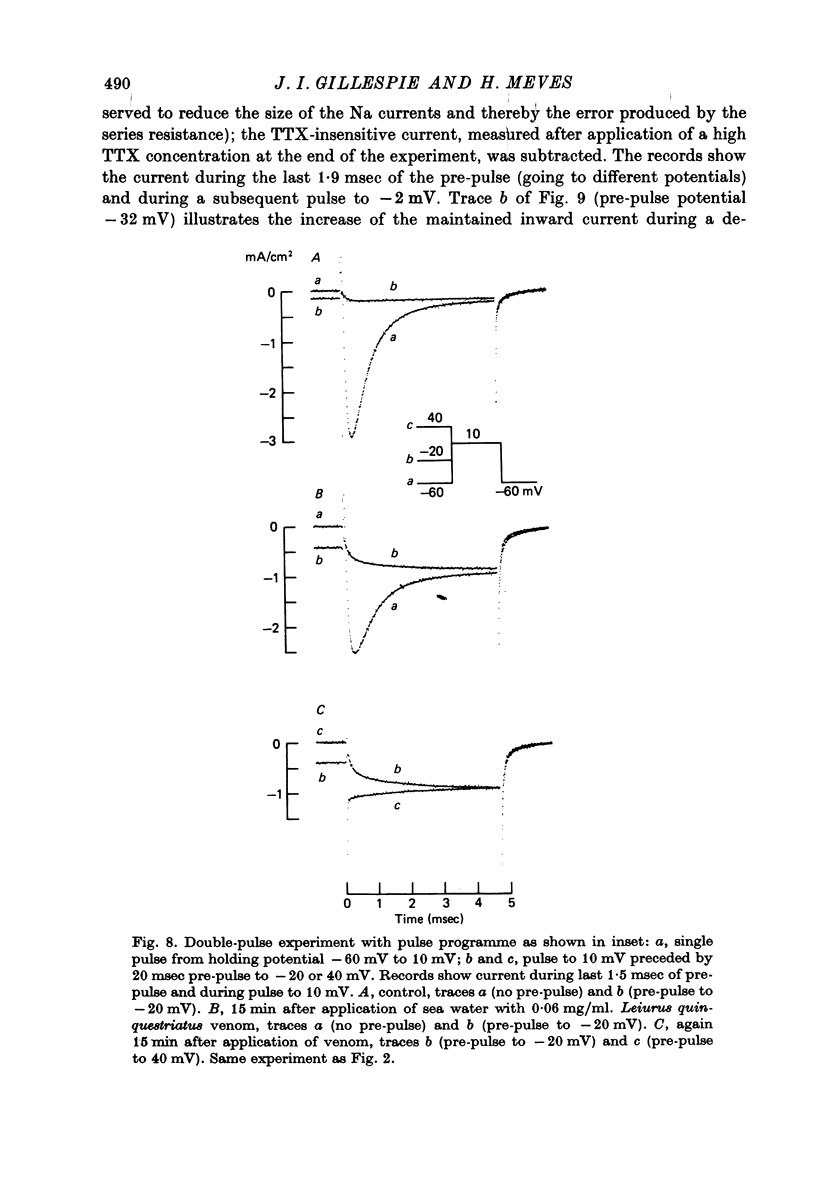
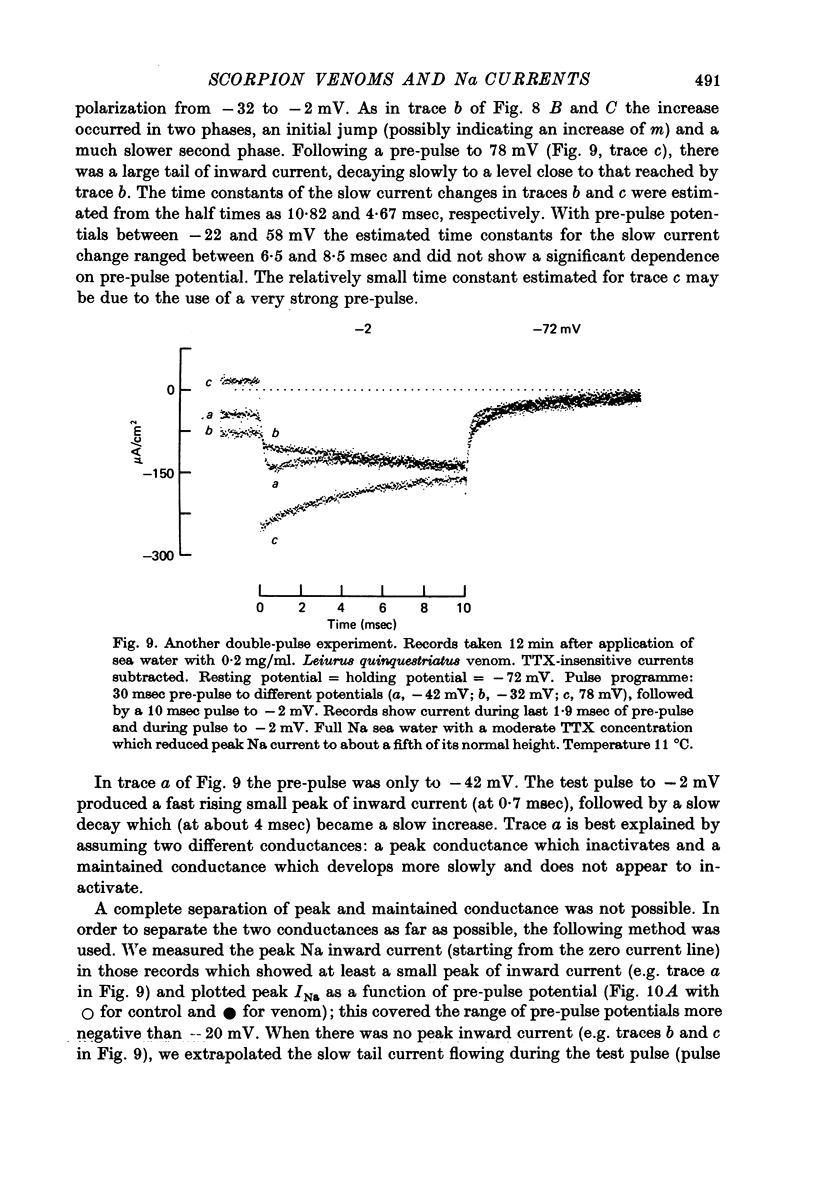
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAKER P. F., HODGKIN A. L., SHAW T. I. Replacement of the axoplasm of giant nerve fibres with artificial solutions. J Physiol. 1962 Nov;164:330–354. doi: 10.1113/jphysiol.1962.sp007025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babin D. R., Watt D. D., Goos S. M., Mlejnek R. V. Amino acid sequence of neurotoxin I from Centruroides sculpturatus Ewing. Arch Biochem Biophys. 1975 Jan;166(1):125–134. doi: 10.1016/0003-9861(75)90371-9. [DOI] [PubMed] [Google Scholar]
- Bezanilla F., Armstrong C. M. Inactivation of the sodium channel. I. Sodium current experiments. J Gen Physiol. 1977 Nov;70(5):549–566. doi: 10.1085/jgp.70.5.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanilla F., Rojas E., Taylor R. E. Sodium and potassium conductance changes during a membrane action potential. J Physiol. 1970 Dec;211(3):729–751. doi: 10.1113/jphysiol.1970.sp009301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binstock L., Adelman W. J., Jr, Senft P., Lecar H. Determination of the resistance in series with the membranes of giant axons. J Membr Biol. 1975 Apr 23;21(1-2):25–47. doi: 10.1007/BF01941060. [DOI] [PubMed] [Google Scholar]
- Chandler W. K., Meves H. Evidence for two types of sodium conductance in axons perfused with sodium fluoride solution. J Physiol. 1970 Dec;211(3):653–678. doi: 10.1113/jphysiol.1970.sp009298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W. K., Meves H. Rate constants associated with changes in sodium conductance in axons perfused with sodium fluoride. J Physiol. 1970 Dec;211(3):679–705. doi: 10.1113/jphysiol.1970.sp009299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler W. K., Meves H. Slow changes in membrane permeability and long-lasting action potentials in axons perfused with fluoride solutions. J Physiol. 1970 Dec;211(3):707–728. doi: 10.1113/jphysiol.1970.sp009300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti F., Hille B., Neumcke B., Nonner W., Stämpfli R. Conductance of the sodium channel in myelinated nerve fibres with modified sodium inactivation. J Physiol. 1976 Nov;262(3):729–742. doi: 10.1113/jphysiol.1976.sp011617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespi J. I., Meves H. Effect of scorpion venom on perfused squid axons [proceedings]. J Physiol. 1978 Sep;282:26P–27P. [PubMed] [Google Scholar]
- Gillespie J. I., Meves H. The time course of sodium inactivation in squid giant axons. J Physiol. 1980 Feb;299:289–307. doi: 10.1113/jphysiol.1980.sp013125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura J. E., Meves H. The effect of temperature on the asymmetrical charge movement in squid giant axons. J Physiol. 1979 Apr;289:479–500. doi: 10.1113/jphysiol.1979.sp012748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppenhöfer E., Schmidt H. Die Wirkung von Skorpiongift auf die Ionenströme des Ranvierschen Schnürrings. I. Die Permeabilitäten PNa und PK. Pflugers Arch. 1968;303(2):133–149. doi: 10.1007/BF00592631. [DOI] [PubMed] [Google Scholar]
- Koppenhöfer E., Schmidt H. Die Wirkung von Skorpiongift auf die Ionenströme des Ranvierschen Schnürrings. II. Unvollständiage Natrium-Inaktivierung. Pflugers Arch. 1968;303(2):150–161. doi: 10.1007/BF00592632. [DOI] [PubMed] [Google Scholar]
- Miranda F., Kupeyan C., Rochat H., Rochat C., Lissitzky S. Purification of animal neurotoxins. Isolation and characterization of eleven neurotoxins from the venoms of the scorpions Androctonus australis hector, Buthus occitanus tunetanus and Leiurus quinquestriatus quinquestriatus. Eur J Biochem. 1970 Nov;16(3):514–523. doi: 10.1111/j.1432-1033.1970.tb01111.x. [DOI] [PubMed] [Google Scholar]
- Mozhaeva G. N., Naumov A. P., Soldatov N. M., Grishin E. V. Deistvie toksinov skorpiona Buthus eupeus na natrievye kanaly membrany perekhvata Ranv'e. Biofizika. 1979 Mar-Apr;24(2):235–241. [PubMed] [Google Scholar]
- Mozhayeva G. N., Naumov A. P., Nosyreva E. D., Grishin E. V. Potential-dependent interaction of toxin from venom of the scorpion Buthus eupeus with sodium channels in myelinated fibre: voltage clamp experiments. Biochim Biophys Acta. 1980 Apr 24;597(3):587–602. doi: 10.1016/0005-2736(80)90230-8. [DOI] [PubMed] [Google Scholar]
- Narahashi T., Shapiro B. I., Deguchi T., Scuka M., Wang C. M. Effects of scorpion venom on squid axon membranes. Am J Physiol. 1972 Apr;222(4):850–857. doi: 10.1152/ajplegacy.1972.222.4.850. [DOI] [PubMed] [Google Scholar]
- Ramón F., Anderson N., Joyner R. W., Moore J. W. Axon voltage-clamp simulations. A multicellular preparation. Biophys J. 1975 Jan;15(1):55–69. doi: 10.1016/S0006-3495(75)85791-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romey G., Chicheportiche R., Lazdunski M., Rochat H., Miranda F., Lissitzky S. Scorpion neurotoxin - a presynaptic toxin which affects both Na+ and K+ channels in axons. Biochem Biophys Res Commun. 1975 May 5;64(1):115–121. doi: 10.1016/0006-291x(75)90226-0. [DOI] [PubMed] [Google Scholar]
- Schmitt O., Schmidt H. Influence of calcium ions on the ionic currents of nodes of Ranvier treated with scorpion venom. Pflugers Arch. 1972;333(1):51–61. doi: 10.1007/BF00586041. [DOI] [PubMed] [Google Scholar]
- TAYLOR R. E., MOORE J. W., COLE K. S. Analysis of certain errors in squid axon voltage clamp measurements. Biophys J. 1960 Nov;1:161–202. doi: 10.1016/s0006-3495(60)86882-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


