Abstract
1. A material that powerfully inhibits the bovine retractor penis and rat anococcygeus muscles has been extracted from these muscles. The inhibitory activity is unaffected by atropine 10(-6) M, phentolamine 5 x 10(-6) M or propranolol 5 x 10(-6) M. 2. This inhibitory material exists in two forms, a stable but inactive form and an unstable inhibitory form. As isolated the material is in the stable, inactive form and is converted into the active form by a brief exposure to acid. The optimum for conversion is pH 2.0 and the active form, after neutralization, reverts with time to the inactive but can be reactivated by a further exposure to acid. The reversion to the inactive form is temperature sensitive, and is rapid at 37 degrees C. 3. The inhibitory material, both active and inactive, is irreversibly destroyed by 2 min in a boiling water bath or by exposure to U.V. irradiation. 4. The inhibitory material is not confined to tissues known to possess a non-adrenergic non-cholinergic innervation. Similar activity has been detected in extracts os skeletal and cardiac muscle and of the liver. The poorly innervated rat uterus and the non-innervated human umbilical artery, however, gave only small and variable amounts. The possible relationship of this material to non-adrenergic non-cholinergic nerves is discussed.
Full text
PDF

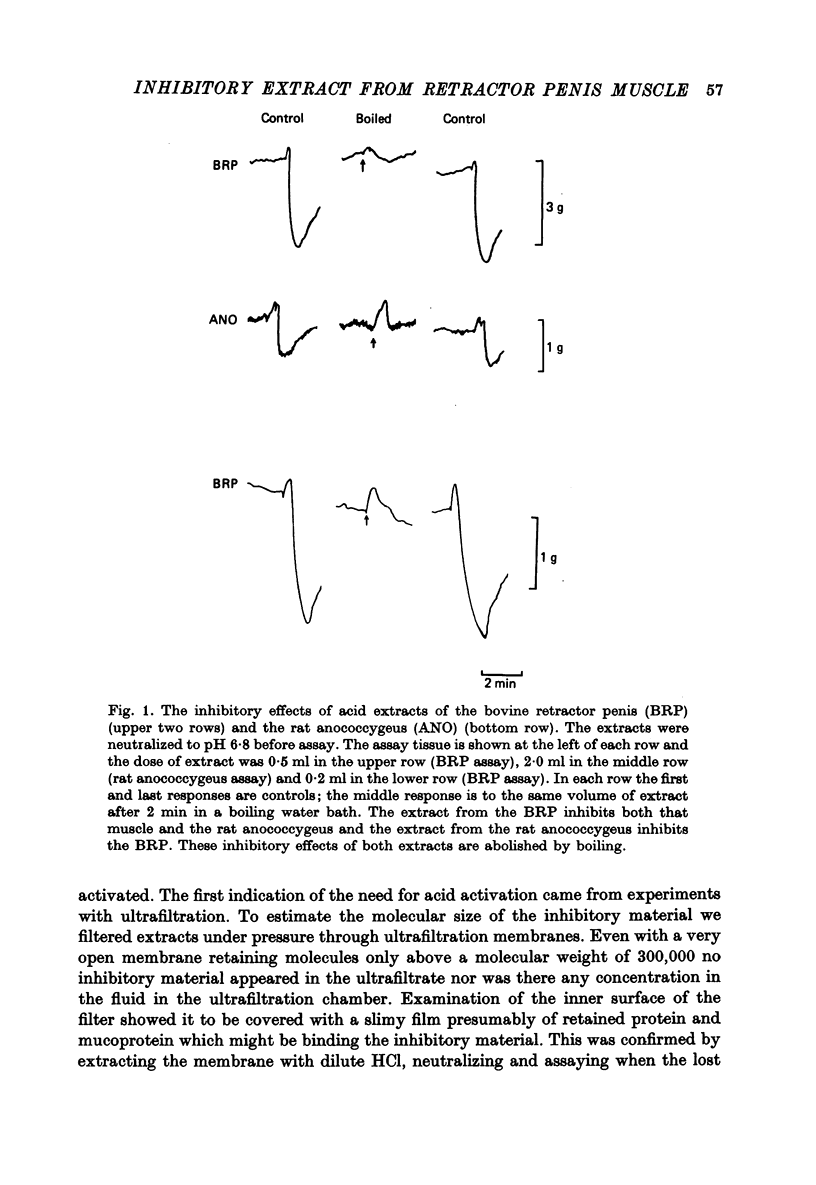
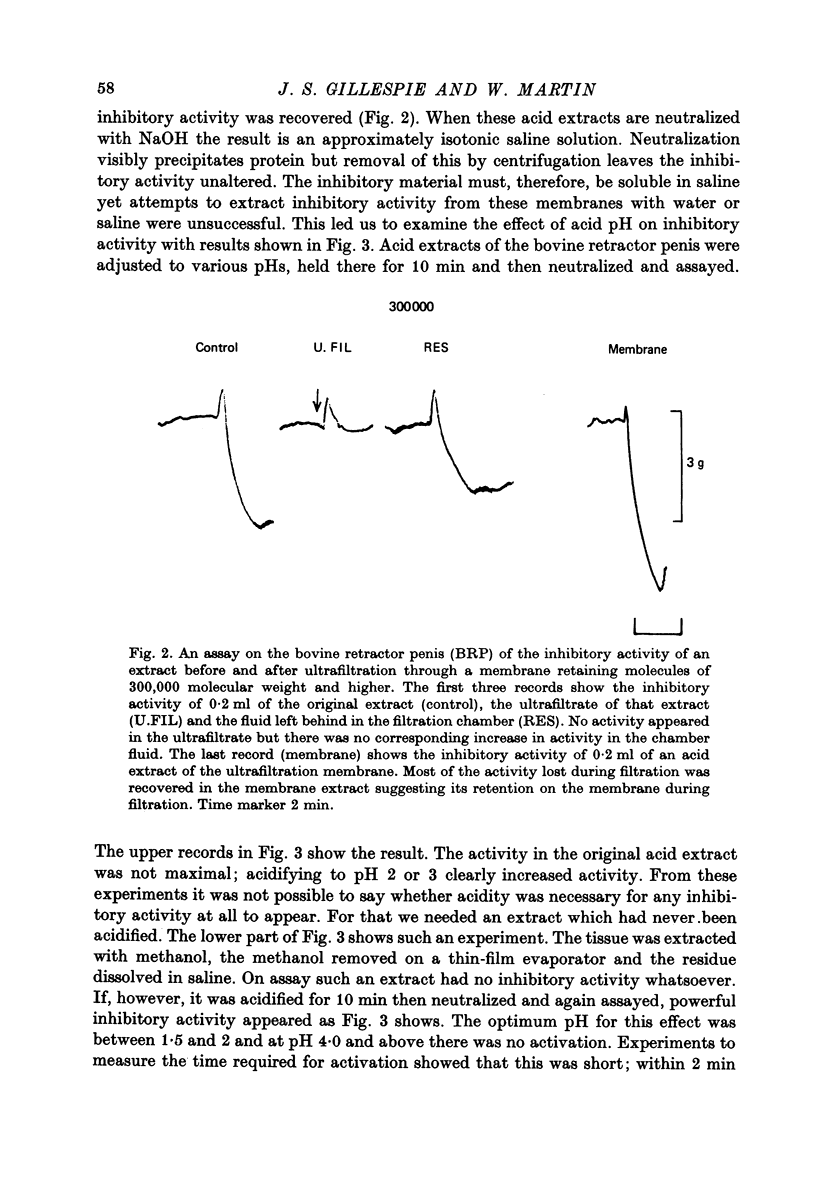
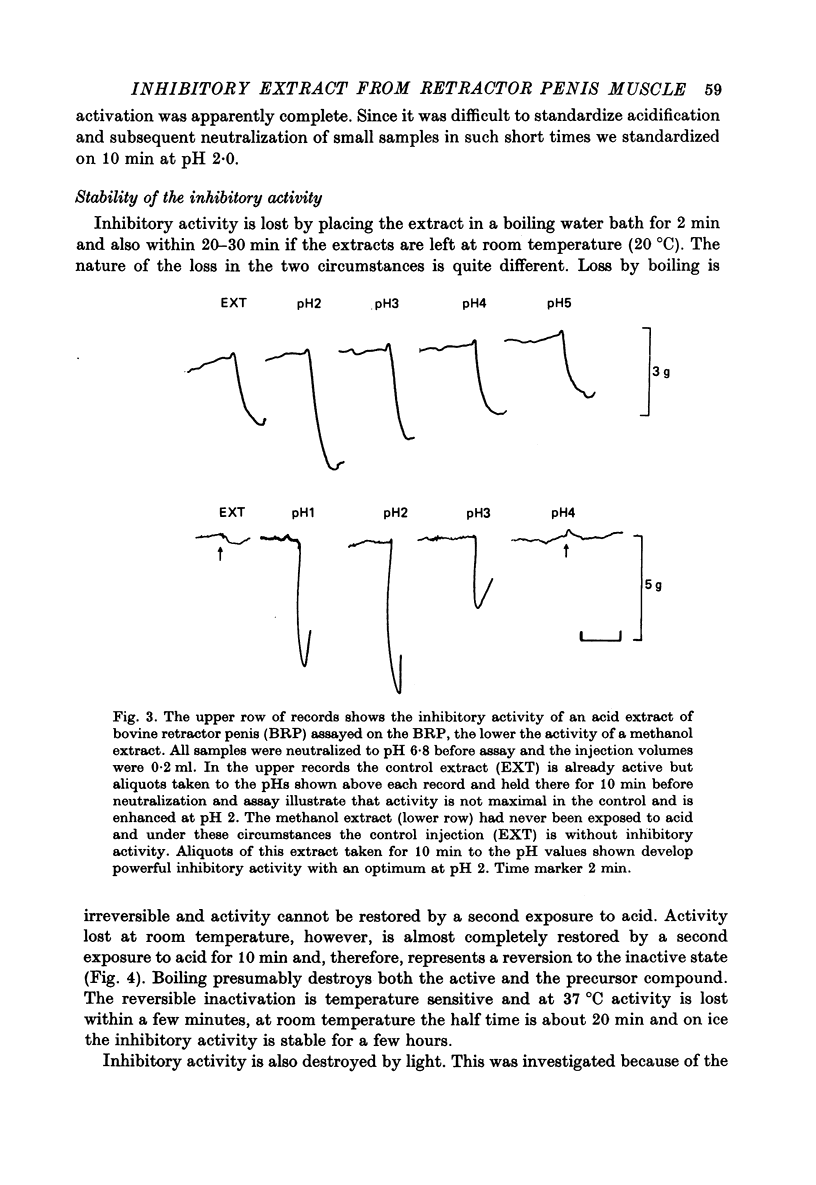
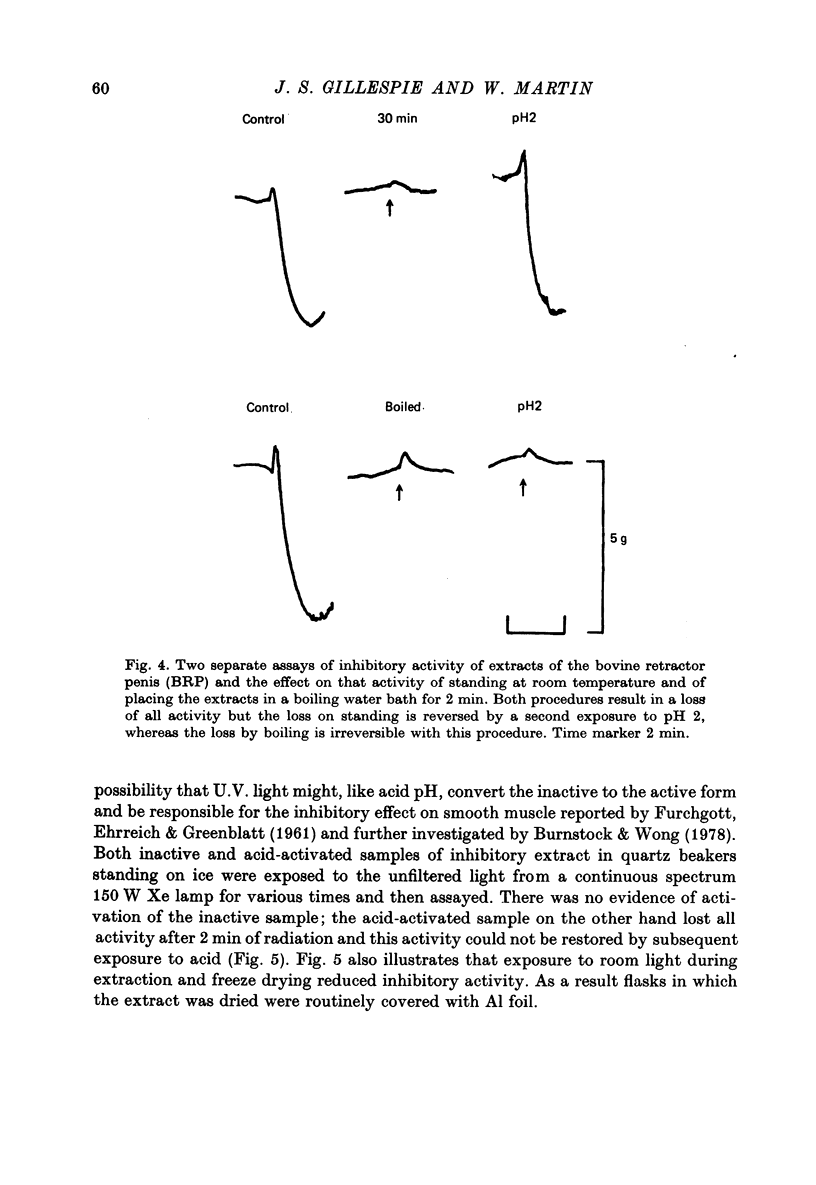


Selected References
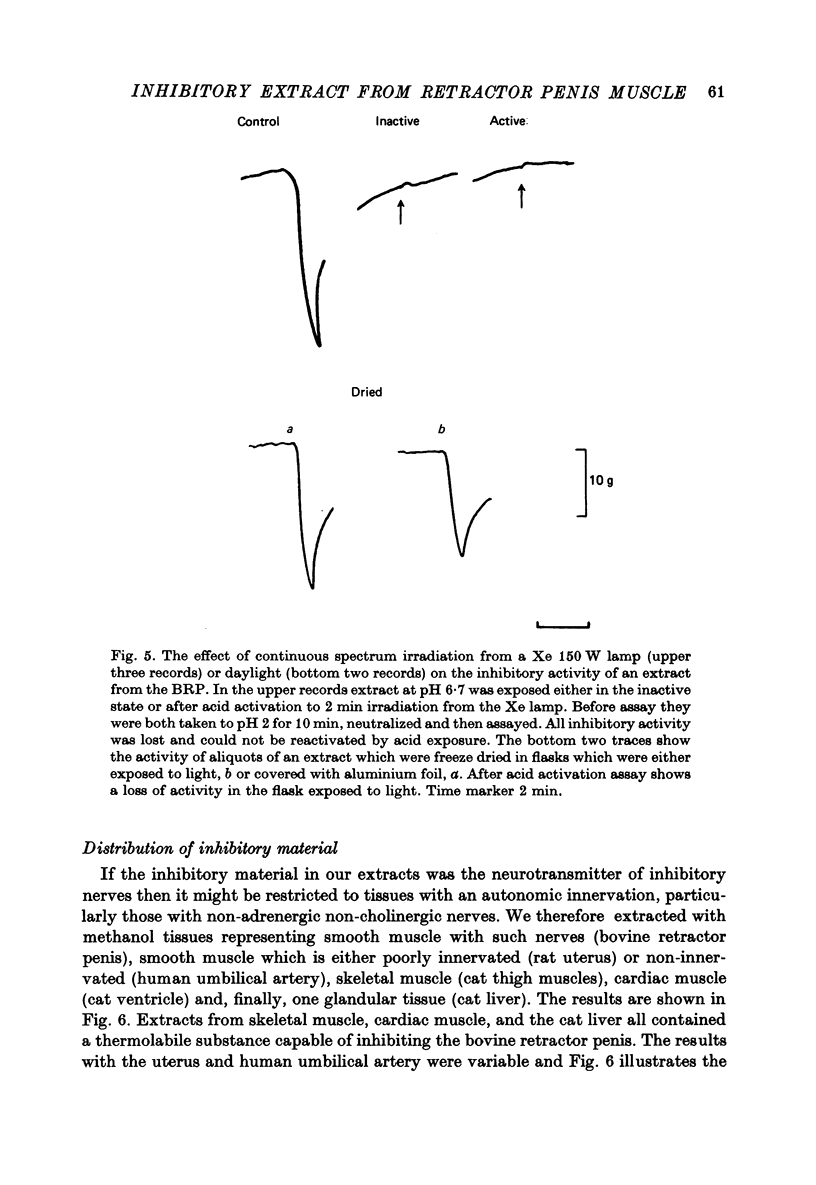
These references are in PubMed. This may not be the complete list of references from this article.
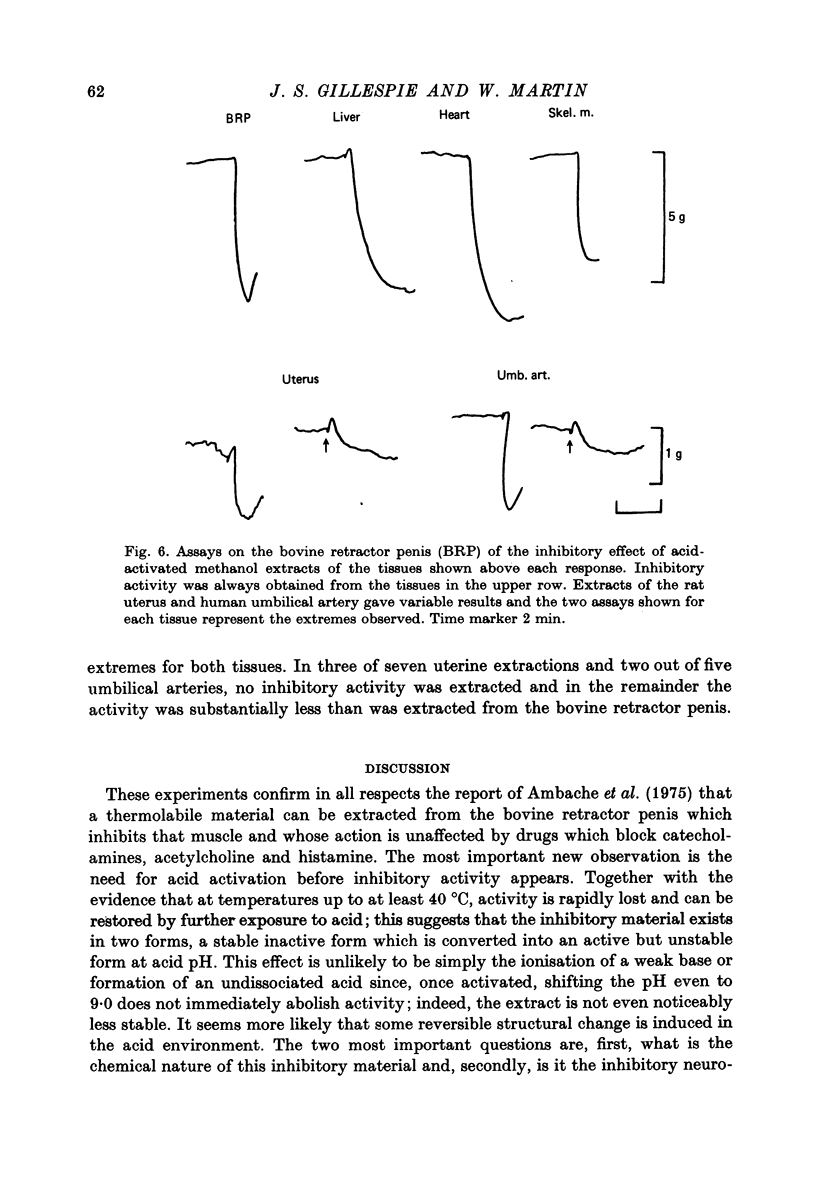
- Ambache N., Killick S. W., Aboo Aar M. Extraction from ox retractor penis of an inhibitory substance which mimics its atropine-resistant neurogenic relaxation. Br J Pharmacol. 1975 Jul;54(3):409–410. doi: 10.1111/j.1476-5381.1975.tb07585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman A., Gillespie J. S., Martin W. The inhibitory material in extracts from the bovine retractor penis muscle is not an adenine nucleotide. Br J Pharmacol. 1979 Nov;67(3):327–328. doi: 10.1111/j.1476-5381.1979.tb08683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G., Cocks T., Crowe R. Evidence for purinergic innervation of the anococcygeus muscle. Br J Pharmacol. 1978 Sep;64(1):13–20. doi: 10.1111/j.1476-5381.1978.tb08635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G., Wong H. Comparison of the effects of ultraviolet light and purinergic nerve stimulation on the guinea-pig taenia coli. Br J Pharmacol. 1978 Feb;62(2):293–302. doi: 10.1111/j.1476-5381.1978.tb08459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creed K. E., Gillespie J. S., McCaffery H. The rabbit anococcygeus muscle and its response to field stimulation and to some drugs. J Physiol. 1977 Dec;273(1):121–135. doi: 10.1113/jphysiol.1977.sp012085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FURCHGOTT R. F., EHRREICH S. J., GREENBLATT E. The photoactivated relaxation of smooth muscle of rabbit aorta. J Gen Physiol. 1961 Jan;44:499–519. doi: 10.1085/jgp.44.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J. S., Martin W. A smooth muscle inhibitory material extracted from the bovine retractor penis and rat anococcygeus muscles [proceedings]. J Physiol. 1978 Jul;280:45P–46P. [PubMed] [Google Scholar]
- Gillespie J. S. The rat anococcygeus muscle and its response to nerve stimulation and to some drugs. Br J Pharmacol. 1972 Jul;45(3):404–416. doi: 10.1111/j.1476-5381.1972.tb08097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris H. R., Piper P. J., Taylor G. W., Tippins J. R. Comparative studies on immunologically and non-immunologically produced slow-reacting substances from man, guinea-pig and rat. Br J Pharmacol. 1979 Oct;67(2):179–184. doi: 10.1111/j.1476-5381.1979.tb08664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


