Abstract
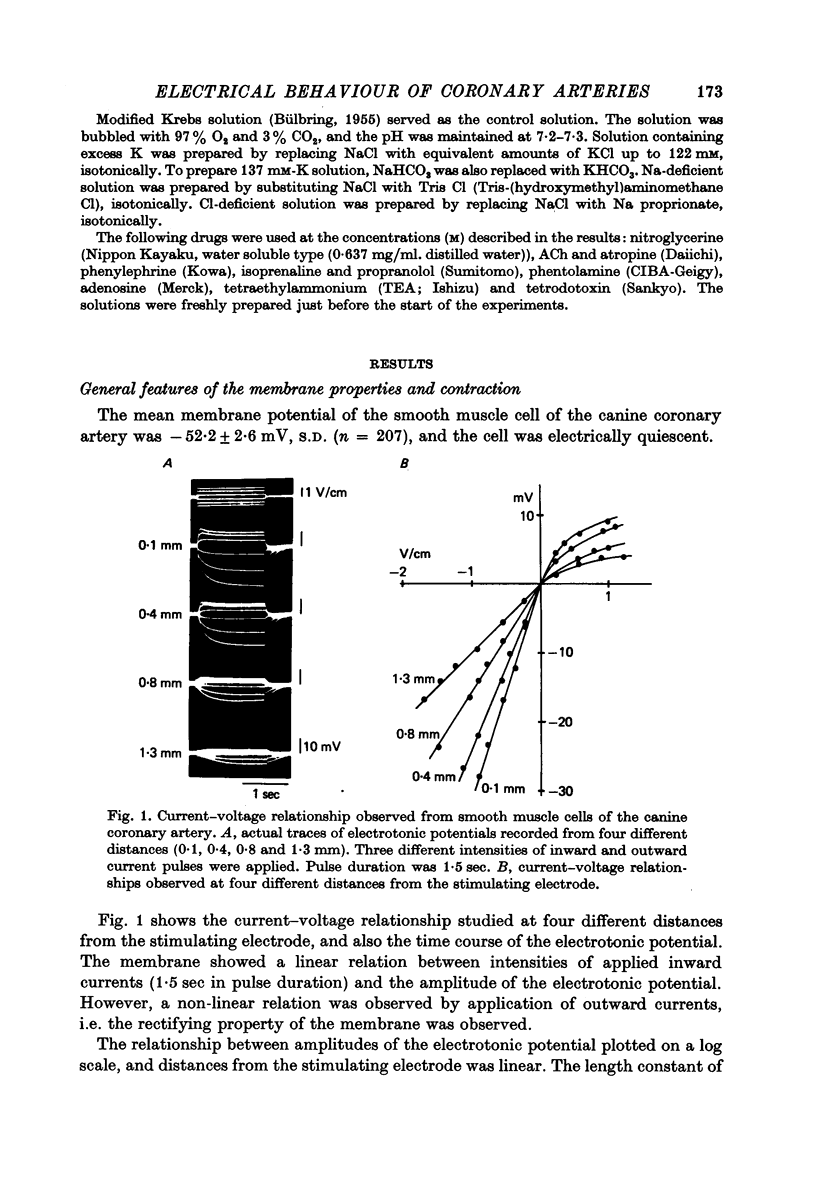
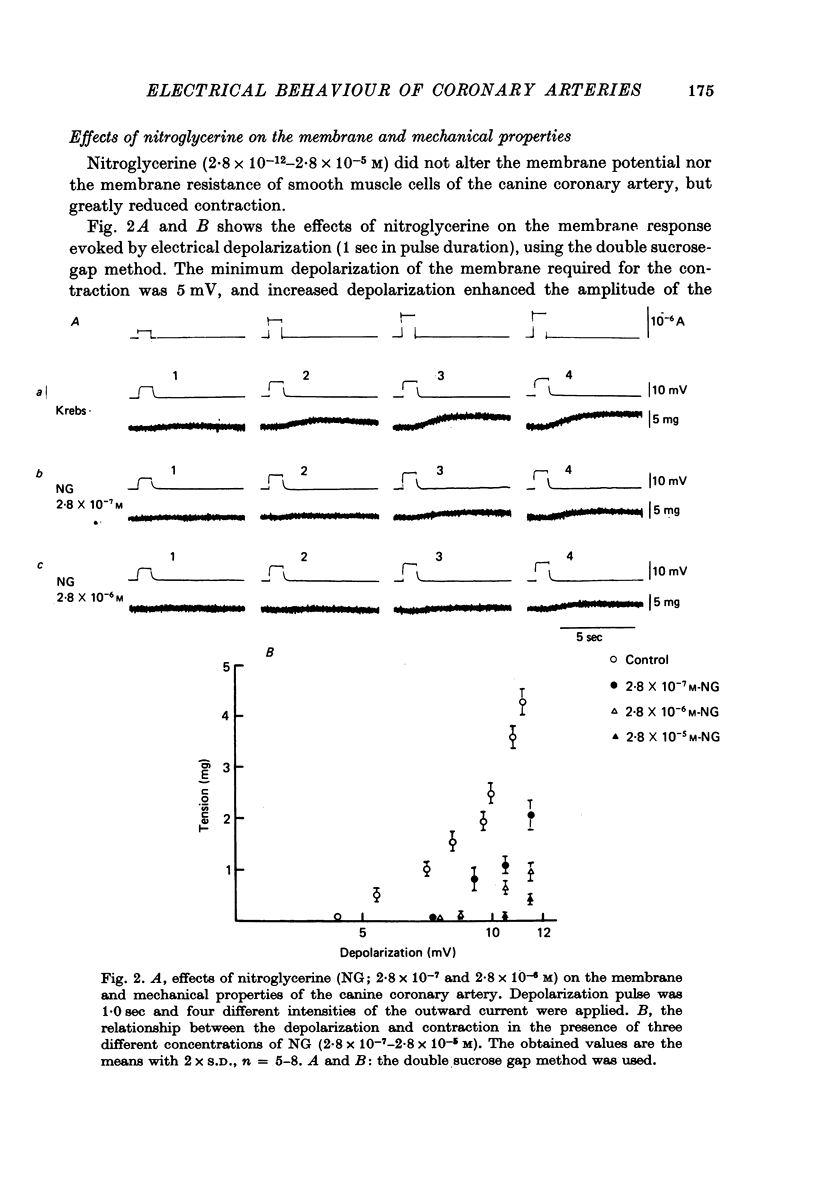
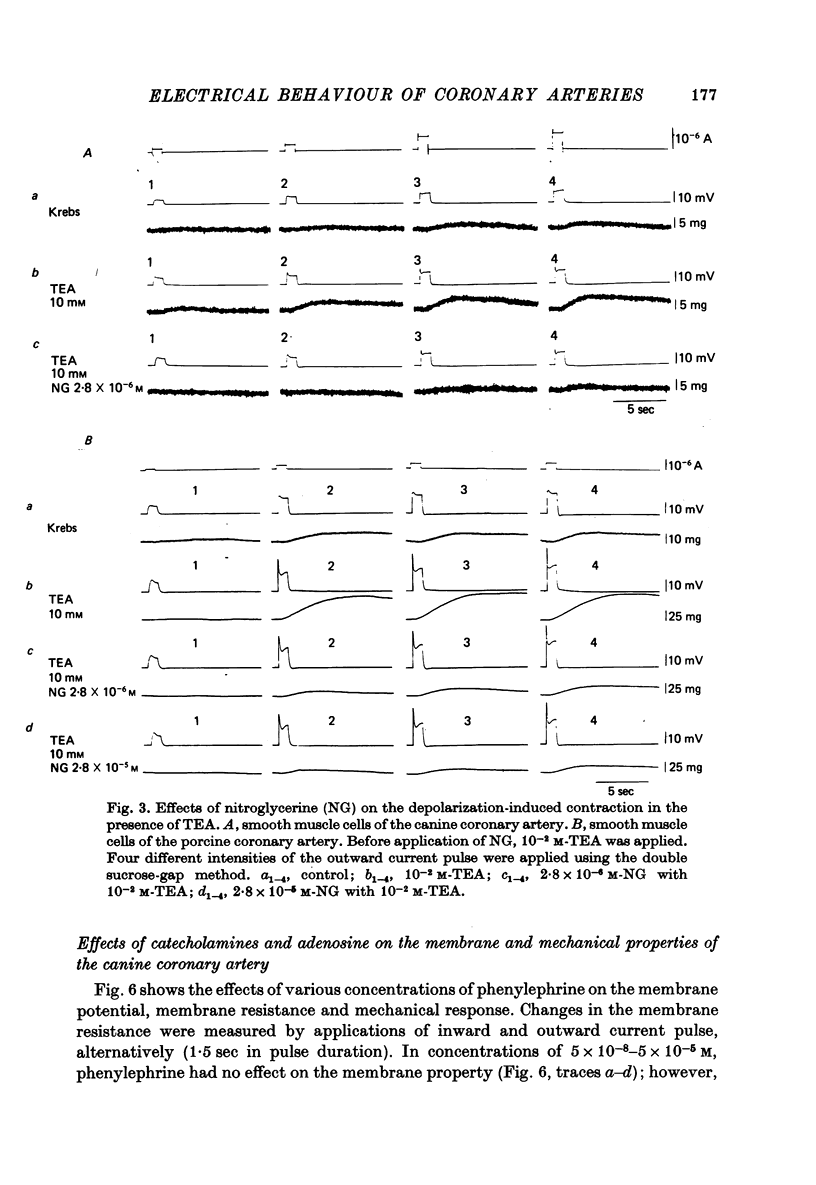
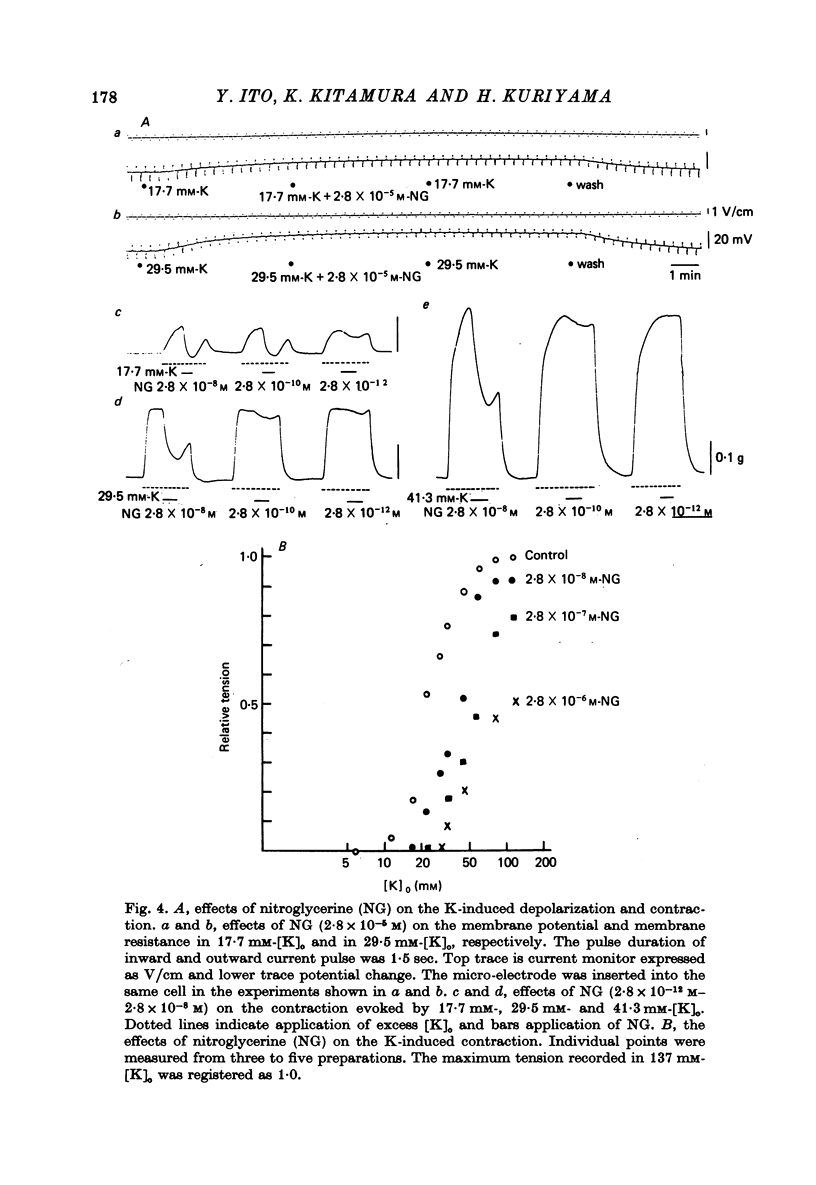
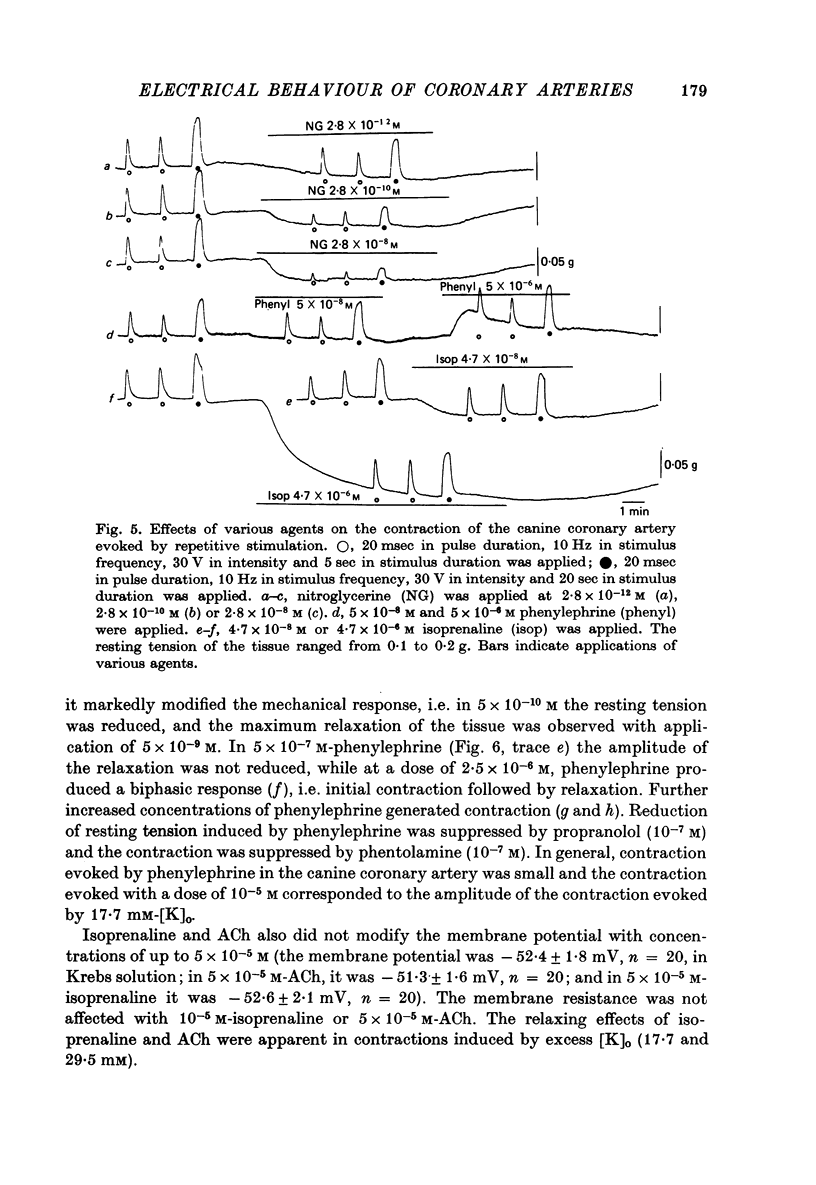
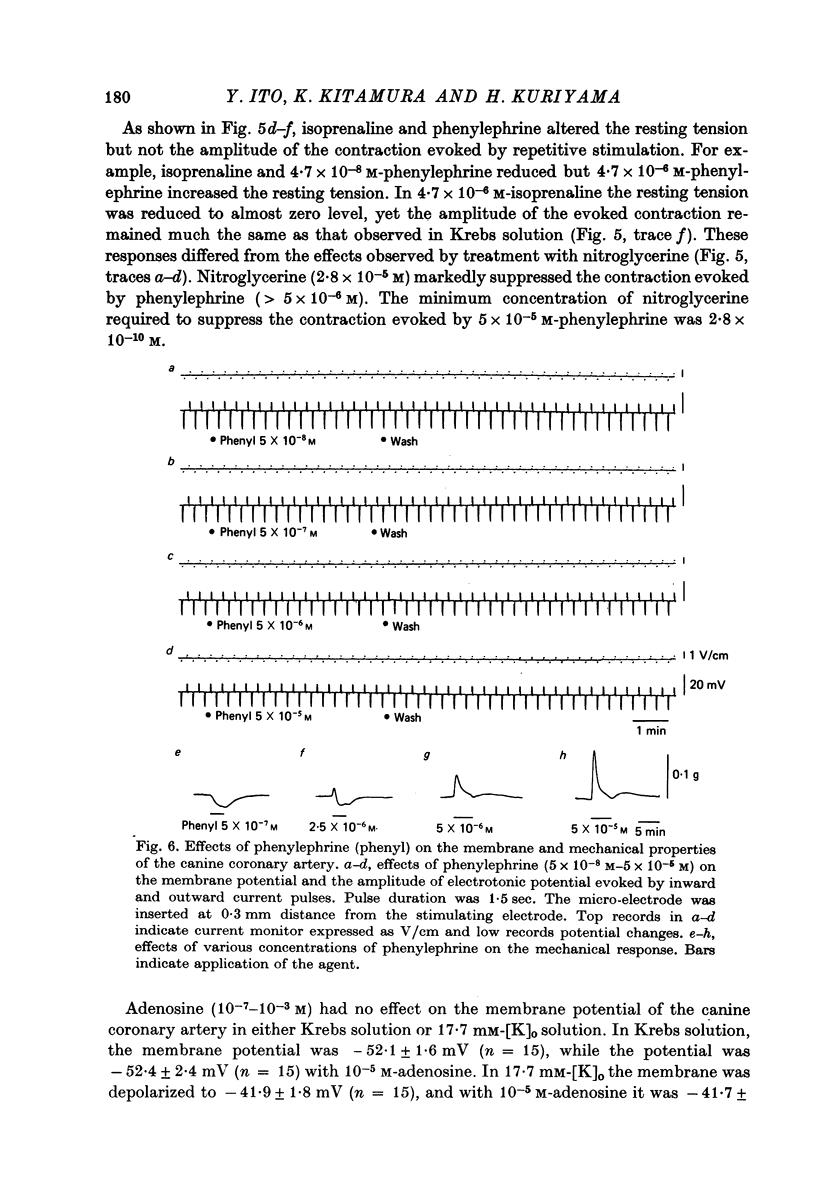
1. The membrane potential of smooth muscle cells of the canine coronary artery was - 52 mV, and cells were electrically quiescent. The length and time constant of the membrane were 0.83 mm and 410 msec, respectively. 2. TEA (> 10(-3) M) depolarized the membrane, increased the membrane resistance and suppressed the rectifying property of the membrane. In 10(-2) M-TEA, an outward current pulse evoked a small active response in the canine coronary artery and a large response in the porcine coronary artery. 3. In the canine coronary artery, the minimum [K]o required for contraction was 11.8 mM (-48 mV), and the maximum amplitude of the contraction was evoked in 89 mM-[K]o (-13 mV). The maximum depolarization produced by a tenfold increase in [K]o was 49 mV. 4. In the canine coronary artery, isoprenaline and a low concentration of phenylephrine reduced the resting tension, and a high concentration of phenylephrine (> 5 x 10(-5) M) produced a contraction without affecting the membrane properties. 5. Nitroglycerine reduced resting tension, suppressed the amplitude and raised the threshold of contraction evoked by excess [K]o, electrical depolarization or phenylephrine. In the canine coronary artery, the minimum concentration of nitroglycerine required to suppress K-induced contraction (17.7 mM) was 2.8 x 10(-12) M nitroglycerine. However, resting membrane properties were not affected by 2.8 x 10(-5) M nitroglycerine. TEA induced electrical response was only a little depressed by 2.8 x 10(-5) M nitroglycerine in the porcine coronary artery, while the mechanical response was markedly suppressed. 6. In the canine coronary artery, adenosine (> 10(-5) M) relaxed the tissue in the presence of 17.7 M-[K]o without affecting the membrane property. ACh (> 5 x 10(-5) M) had much the same effects as those observed by treatment with adenosine.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe Y., Tomita T. Cable properties of smooth muscle. J Physiol. 1968 May;196(1):87–100. doi: 10.1113/jphysiol.1968.sp008496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BULBRING E. Correlation between membrane potential, spike discharge and tension in smooth muscle. J Physiol. 1955 Apr 28;128(1):200–221. doi: 10.1113/jphysiol.1955.sp005299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R., Kitamura K., Kuriyama H., Suzuki H. The membrane properties of the smooth muscle cells of the rabbit main pulmonary artery. J Physiol. 1977 Sep;271(1):41–61. doi: 10.1113/jphysiol.1977.sp011989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droogmans G., Raeymaekers L., Casteels R. Electro- and pharmacomechanical coupling in the smooth muscle cells of the rabbit ear artery. J Gen Physiol. 1977 Aug;70(2):129–148. doi: 10.1085/jgp.70.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder D. R., Belardinelli L., Sperelakis N., Rubio R., Berne R. M. Differential effects of adenosine and nitroglycerin on the action potentials of large and small coronary arteries. Circ Res. 1979 Feb;44(2):176–182. doi: 10.1161/01.res.44.2.176. [DOI] [PubMed] [Google Scholar]
- Harder D. R., Sperelakis N. Action potentials induced in guinea pig arterial smooth muscle by tetraethylammonium. Am J Physiol. 1979 Jul;237(1):C75–C80. doi: 10.1152/ajpcell.1979.237.1.C75. [DOI] [PubMed] [Google Scholar]
- Ito Y., Kitamura K., Kuriyama H. Effects of acetylcholine and catecholamines on the smooth muscle cell of the porcine coronary artery. J Physiol. 1979 Sep;294:595–611. doi: 10.1113/jphysiol.1979.sp012948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keatinge W. R. Electrical and mechanical responses of vascular smooth muscle to vasodilator agents and vasoactive polypeptides. Circ Res. 1966 Jun;18(6):641–649. doi: 10.1161/01.res.18.6.641. [DOI] [PubMed] [Google Scholar]
- Kitamura K., Kuriyama H. Effects of acetylcholine on the smooth muscle cell of isolated main coronary artery of the guinea-pig. J Physiol. 1979 Aug;293:119–133. doi: 10.1113/jphysiol.1979.sp012881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekata F., Niu H. Biophysical effects of adrenaline on the smooth muscle of the rabbit common carotid artery. J Gen Physiol. 1972 Jan;59(1):92–102. doi: 10.1085/jgp.59.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]


