Abstract
As part of the SENTRY antimicrobial surveillance program, we examined the prevalence rates, types, and antibiograms of oxacillin-resistant Staphylococcus aureus from hospitalized patients from 17 institutions in eight countries in Asia-Pacific and South Africa (APAC). From April 1998 to December 1999, a total of 1,711 isolates of S. aureus (814 from blood, 392 from the respiratory tract, 467 from skin and skin structures, and 38 from urine) were collected from hospitalized patients within the APAC region. Multidrug-resistant oxacillin-resistant S. aureus (MORSA) isolates, defined as strains with three or more resistances to drug classes other than β-lactams, were the most common type of oxacillin-resistant S. aureus (ORSA). They were the most frequently identified pathogen in wound infections and were common in bloodstream and lower respiratory tract infections. In all contributing institutions combined, more than 45% (range, 4 to 74%) of S. aureus isolates were oxacillin resistant, and in six institutions, this rate exceeded 60%. MORSA accounted for 91.2% of all oxacillin-resistant isolates. Distinct resistance patterns predominated at various sites within the APAC region, suggesting the local evolution of resistant clones. Non-multidrug-resistant strains were frequent in one part of Australia. No vancomycin-intermediate strains were detected, and no strains were resistant to linezolid or quinupristin-dalfopristin. MORSA strains are a very common cause of infection in hospitalized patients in the APAC region.
Methicillin-resistant Staphylococcus aureus (MRSA) was first identified in the United Kingdom in 1961 (8). Since that time, MRSA (hereafter called oxacillin-resistant S. aureus [ORSA]) strains have assumed increasing importance internationally as a cause of both nosocomial and community-acquired infections (2). The relative virulence of ORSA is generally considered to be comparable to that of oxacillin-sensitive S. aureus (OSSA). However, a recent United States study found that primary nosocomial bacteremia due to ORSA resulted in an approximately threefold increase in direct hospitalization costs when compared with those due to OSSA (1).
Although the presence of ORSA in the Asia-Pacific and South Africa (APAC) region has been well recorded, data on the true prevalence of ORSA in the region are limited. Longitudinal data from Australia have been published and show that in hospital isolates from the Eastern Seaboard, the percentage of S. aureus strains that were ORSA remained relatively constant at approximately 30% from 1986 to 1994 (14). Until recently, most ORSA strains internationally were multidrug resistant, nosocomially acquired, and prone to become endemic in hospitals. Community-acquired strains of ORSA, with few or no additional resistances apart from β-lactams, have been described quite recently from a number of countries, including Australia, New Zealand, Canada, the United States, and Saudi Arabia (3, 5, 6, 9).
SENTRY is an international antimicrobial surveillance program that documents resistance patterns in bacteria isolated from predominantly hospitalized patients (12). Participating sentinel institutions from 1997 include medical centers in the United States, Canada, Latin America, and Europe. In 1998, the APAC region was added to SENTRY, covering 17 institutions in eight countries (Australia, mainland China, Hong Kong, Japan, Philippines, Singapore, South Africa, and Taiwan). The monitored indicators included bloodstream infection, pneumonia, wound infection, and urinary tract infection in hospitalized patients. Each participating institution submitted isolates from the monitored indicators, according to a predetermined schedule, to the coordinating study center (Women's and Children's Hospital, Adelaide, Australia).
Susceptibility testing was conducted at the coordinating center by using broth microdilution according to the National Committee for Clinical Laboratory Standards (NCCLS) (10). The breakpoints for resistance were those recommended by the NCCLS (11). For linezolid, a breakpoint of ≤4 μg/ml was used to define susceptibility. The following antibiotics were tested: penicillin, oxacillin, erythromycin, clindamycin, tetracycline, chloramphenicol, ciprofloxacin, rifampin, gentamicin, trimethoprim-sulfamethoxazole, vancomycin, teicoplanin, nitrofurantoin, quinupristin-dalfopristin, and linezolid.
Multidrug resistance (MORSA) was defined as resistance to penicillin and oxacillin plus three or more of the following agents: erythromycin, clindamycin, rifampin, ciprofloxacin, gentamicin, trimethoprim-sulfamethoxazole, and chloramphenicol. In the case of trimethoprim-sulfamethoxazole, the highest concentration tested was 1 dilution lower than the current NCCLS breakpoint. For the purposes of this analysis, organisms for which the MICs were above 1/19 μg/ml for this combination were classified as resistant. Potentially glycopeptide-intermediate strains for which vancomycin MICs were ≥4 μg/ml or the teicoplanin MICs were ≥8 μg/ml on broth microdilution testing were confirmed by Etest MIC testing and growth on brain-heart infusion agar containing 6 μg of vancomycin per ml.
Between April 1998 and December 1999, a total of 1,711 S. aureus isolates were referred by SENTRY participating hospitals in the APAC region: 814 were from blood, 392 were from the respiratory tract, 467 were from skin and skin structures, and 38 were from urine. Oxacillin resistance was detected in 43.1% of blood isolates, 56.9% of respiratory tract isolates, 40.5% of wound isolates, and 57% of urine isolates, the majority of which were MORSA. MORSA were the most frequently identified pathogen of any type in wound infections (15.0%) and were common in bloodstream and lower respiratory tract infections (8.3 and 10.5%, respectively). MORSA was less commonly isolated in urine (1.7% of all pathogens). The proportions of ORSA among S. aureus isolates by country overall and isolated from blood are shown in Table 1. The percentages of ORSA varied from 5% in the Philippines to 69% in Japan and Hong Kong, China.
TABLE 1.
Prevalence of ORSA in different countries and sites
| Country | Institution no. | Total no. of S. aureus isolates | % Oxacillin resistant
|
|
|---|---|---|---|---|
| All sites | Blooda | |||
| Australia | 200 | 192 | 34.4 | 29.1 |
| 201 | 187 | 25.7 | 19.1 | |
| 203 | 181 | 23.8 | 30.2 | |
| 202 | 122 | 4.1 | 0.0 | |
| All | 682 | 23.8 | 22.4 | |
| Japan | 207 | 184 | 74.5 | 74.0 |
| 206 | 88 | 64.8 | 56.0 | |
| 205 | 151 | 66.2 | 63.4 | |
| All | 423 | 69.5 | 66.8 | |
| Taiwan | 214 | 77 | 57.1 | 34.5 |
| 215 | 4 | 75.0 | 75.0 | |
| 216 | 13 | 69.2 | 66.7 | |
| All | 94 | 59.6 | 46.7 | |
| Mainland China | 209 | 25 | 16.0 | 22.2 |
| 208 | 17 | 23.5 | 20.0 | |
| 210 | 12 | 58.3 | 100 | |
| All | 54 | 27.8 | 26.9 | |
| Hong Kong | 204 | 202 | 69.8 | 58.2 |
| Philippines | 211 | 40 | 5.0 | 11.8 |
| Singapore | 212 | 122 | 62.3 | 60.6 |
| South Africa | 213 | 94 | 41.5 | 40.4 |
Percentage of all S. aureus strains from blood cultures that were oxacillin resistant.
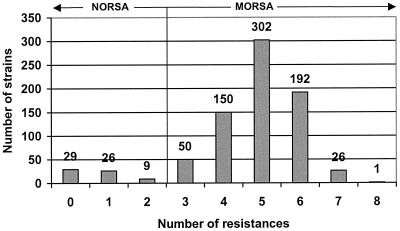
Figure 1 plots the number of resistances harbored by all ORSA. The majority of strains (91.8%) harbored three or more resistances in addition to penicillin and oxacillin. A small number of strains, mostly from Western Australia, were non-multidrug-resistant (NORSA), a pattern characteristic of strains in that locale (13).
FIG. 1.
Numbers of instances of resistance to classes of antimicrobial agents other than β-lactams.
There was considerable variation in resistance profiles between countries was observed in ORSA strains. The predominant antibiogram for Australia, Singapore, and Taiwan included resistance to erythromycin, clindamycin, ciprofloxacin, gentamicin, tetracycline, and sulfamethoxazole-trimethoprim. The most common profile for Japan, Hong Kong, and mainland China included resistance to erythromycin, clindamycin, ciprofloxacin, tetracycline, and gentamicin for Hong Kong and China, as well as a proportion of Japanese strains (56.5%). The different antibiograms in Japan were not confined to individual institutions.
The most marked geographic variations in resistance profiles were in nonsusceptibility to sulfamethoxazole-trimethoprim, which was common in isolates from Australia, Singapore, and Taiwan, but uncommon or nonexistent in isolates from other countries, and resistance to rifampin, which was common in South African strains, but less common in other countries.
Overall, MORSA isolates in the region demonstrated very little resistance to several antibiotics: glycopeptides, nitrofurantoin, and the investigational agents quinupristin-dalfopristin and linezolid. No strains with reduced susceptibility to teicoplanin were identified. Despite a previous Japanese report of ORSA strains with reduced susceptibility to vancomycin, vancomycin-intermediate S. aureus (VISA) strains were not identified in any of the three sites in Japan (7). Resistance to nitrofurantoin was rare (0.8% of strains overall). For two isolates, quinupristin-dalfopristin MICs were 2 μg/ml, but for none of the isolates were the linezolid MICs above 4 μg/ml.
During the survey period, S. aureus was found to be the most common cause of bloodstream infection, skin and soft tissue infection, and pneumonia in all participating countries in the APAC region. This concords with previously published reports of SENTRY surveillance data (1997 to 1999), where S. aureus was found to be the most common or second most common cause of bloodstream infection, skin and soft tissue infection, and pneumonia in the United States, Canada, Europe, and Latin America (4).
ORSA is an important cause of infection in hospitalized patients throughout the APAC region. The proportion of S. aureus strains resistant to oxacillin in the APAC region (45.9%) was high (ranging from 5.0% in the Philippines to 79.5% in Hong Kong). This proportion was higher overall than those reported by other geographic regions contributing to SENTRY over the same time period: Latin America (34.9%), United States (34.2%), Europe (26.3%), and Canada (5.7%) (4). This finding must be interpreted with caution, however, due to the small number of contributing institutions within the region. For example, data from Hong Kong came from a single contributing institution. The levels of oxacillin resistance identified in SENTRY data from Hong Kong may thus reflect the patient mix and antibiotic prescription practices of a single institution rather than for Hong Kong as a whole. Similarly, data from the Philippines were also from a single institution, which only contributed 40 isolates of S. aureus in total.
Within the APAC region, notable variations in ORSA resistance patterns between and within countries were identified. This is consistent with the establishment and gradual spread within countries of distinct ORSA clones, perhaps favored by the relative geographic isolation of some contributors.
Of interest is the significant proportion of ORSA isolates from Australia that were NORSA. Almost all (10 of 12 [83%]) of these isolates were found in a single Western Australian institution. NORSA strains emerged from the remote Kimberly region of Western Australia in the mid-1980s. Since that time, the epidemiology of these strains has been further characterized. They are most commonly found in remote Central and Western Australian Aboriginal communities, where skin colonization rates can be as high as 42% and skin sepsis is the most frequent clinical manifestation. While they typically cause community-acquired infections, more recently, they have been responsible for hospital outbreaks (13). Clusters of community-acquired NORSA strains have since been reported in the APAC region, previously in Australia and New Zealand, but also in Canada, the United States, and Saudi Arabia.
Strains of MRSA with reduced susceptibility to vancomycin (VISA) were isolated in Japan in 1997 and have since been described in the United States, France, Hong Kong, China, and Korea. No strains of VISA were detected in this study, despite having three sites in Japan, consistent with the suggestion that these strains are still relatively rare.
Acknowledgments
This study was sponsored by research and educational grants from Bristol-Myers Squibb.
We thank Celia Cooper for assistance with preparation of the manuscript.
APPENDIX
The participants in the SENTRY antimicrobial surveillance program are as follows: Australia, Graeme Nimmo and Jacqueline Schooneveldt, Princess Alexandra Hospital, Brisbane; Irene Lim and Ming Xiao, Royal Adelaide Hospital, Adelaide; Keryn Christiansen and Geoffrey Coombs, Royal Perth Hospital, Perth; and John Turnidge and Jan Bell, Women's and Children Hospital, Adelaide; Japan, Matsuhisa Inoue, Kitasato University Hospital, Kitasato; Shigeru Kohno, Yoichi Hirakata, and Yoshitsuga Miyazaki, Nagasaki University Hospital, Nagasaki; and Yasuo Ono, Teikyo University Hospital, Tokyo; Taiwan, Leu Hsieh-Shong, Chang Gung Memorial Hospital, Taoyuan; Hsueh Po-Ren, National Taiwan University Hospital, Taipei; and Yu Kwok-Yoon, Veterans General Hospital, Taipei; China, Li Jia-Tai, Beijing Medical University, Beijing; and Zhong Nang-Shan, First Municipal Peoples Hospital Of Guangzhou and Guangzhou Medical College First Affiliated Hospital, Guangzhou; Hong Kong, Seto Wing-Hong and Raymond Leung, Queen Mary Hospital; Philippines, Thelma Tupasi, Makati Medical Center, Manila; Singapore, Ling Moi-Lin, Singapore General Hospital; and South Africa, Adrian Brink, Drs. du Buisson, Brunette and Partners, Johannesburg.
REFERENCES
- 1.Abramson, M. A. 1999. Nosocomial methicillin-resistant and methicillin-susceptible Staphylococcus aureus primary bacteraemia: at what costs? Infect. Control. Hosp. Epidemiol. 20:408-411. [DOI] [PubMed] [Google Scholar]
- 2.Ayliffe, G. A. J. 1997. The progressive intercontinental spread of methicillin-resistant Staphylococcus aureus. Clin. Infect. Dis. 24:S74-S79. [DOI] [PubMed] [Google Scholar]
- 3.Bukharie, H. A., M. S. Abdelhadi, I. A. Saeed, A. M. Rubaish, and E. B. Larbi. 2001. Emergence of methicillin-resistant Staphylococcus aureus as community pathogens. Diagn. Microbiol. Infect. Dis. 40:1-4. [DOI] [PubMed] [Google Scholar]
- 4.Diekema, D. J., M. A. Pfaller, F. J. Schmitz, J. Smayevski, J. Bell, R. N. Jones, and M. Beach. 2001. Survey of infections due to Staphylococcus species: frequency of occurrence, antimicrobial susceptibility and resistance trends of isolates collected in the united states, Canada, Latin America, Europe and the Western Pacific for the sentry antimicrobial surveillance program, 1997-1999. Clin. Infect. Dis. 32(Suppl. 2):S114-S132. [DOI] [PubMed] [Google Scholar]
- 5.Embil, J., K. Ramotar, L. Romance et al. 1994. Methicillin-resistant Staphylococcus aureus in tertiary care institutions on the Canadian prairies 1990-1992. Infect. Control Hosp. Epidemiol. 15:646-651. [DOI] [PubMed] [Google Scholar]
- 6.Herold, B. C., L. C. Immergluck, M. C. Maranan, D. S. Lauderdale, R. E. Gaskin, S. Boyle-Vavra, C. D. Leitch, and R. S. Daum. 1998. Community-acquired methicillin-resistant Staphylococcus aureus in children with no identified predisposing risk. JAMA 279:593-598. [DOI] [PubMed] [Google Scholar]
- 7.Hiramatsu, K., H. Hanaki, T. Ino, K. Yabuta, T. Oguri, and F. C. Tenover. 1997. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. J. Antimicrob. Chemother. 40:135-136. [DOI] [PubMed] [Google Scholar]
- 8.Jevons, M. P. 1961. “Celbenin”-resistant staphylococci. Br. Med. J. 1:124-125. [Google Scholar]
- 9.Mitchell, J. M., D. MacCulloch, and A. J. Morris. 1996. MRSA in the community. N. Z. Med. J. 109:411.. [PubMed] [Google Scholar]
- 10.National Committee for Clinical Laboratory Standards. 2000. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 5th ed. Approved standard M7-A5. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 11.National Committee for Clinical Laboratory Standards. 2000. Performance standards for antimicrobial susceptibility testing. Tenth informational supplement. M100-S10. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 12.Pfaller, M. A., R. N. Jones, G. V. Doern, K. Kugler, and the SENTRY Participants Group. 1998. Bacterial pathogens isolated from patients with bloodstream infection: frequencies of occurrence and antimicrobial susceptibility patterns from the SENTRY antimicrobial surveillance program (United States and Canada, 1997). Antimicrob. Agents Chemother. 42:1762-1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turnidge, J. D., and J. M. Bell. 2000. MRSA evolution in Australia over 35 years. Microb. Drug Resist. 6:223-239. [DOI] [PubMed] [Google Scholar]
- 14.Turnidge, J. D., G. R. Nimmo, and G. Francis. 1996. Evolution of resistance in Staphylococcus aureus in Australian teaching hospitals. Med. J. Aust. 164:68-71. [PubMed] [Google Scholar]