Abstract
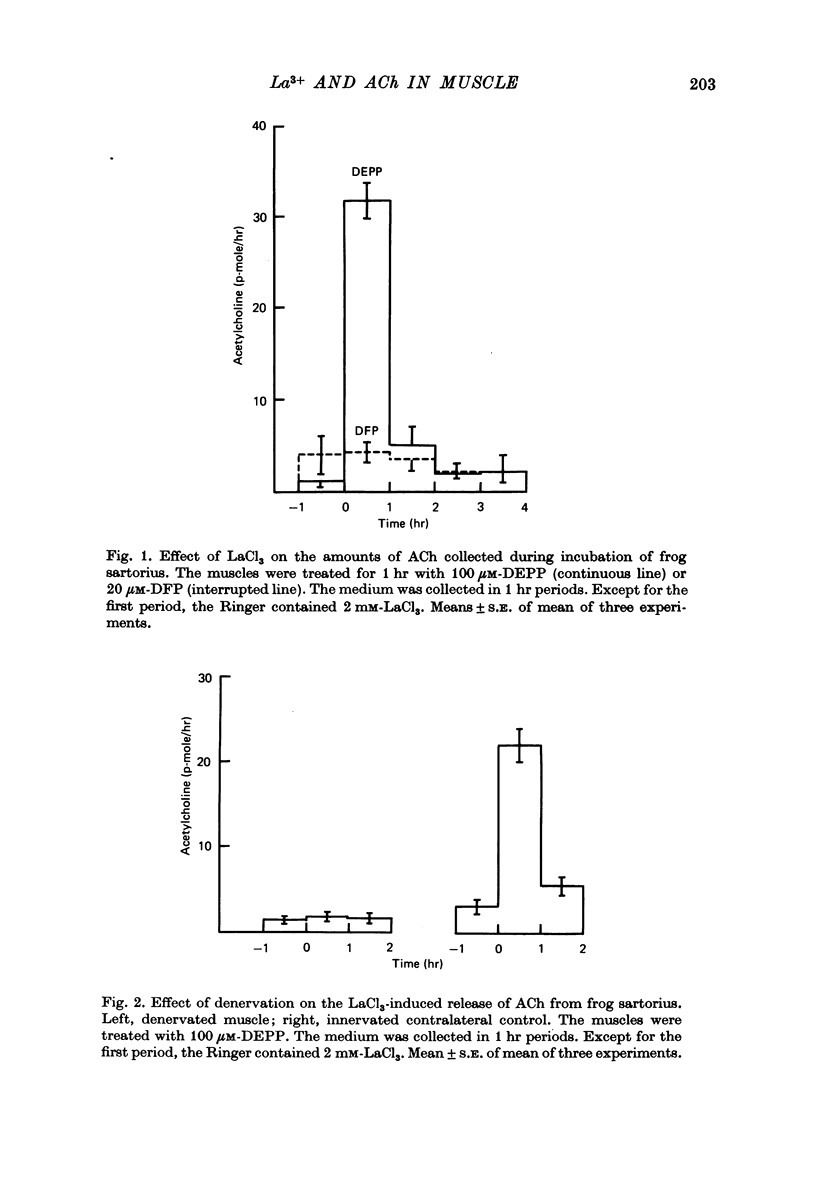
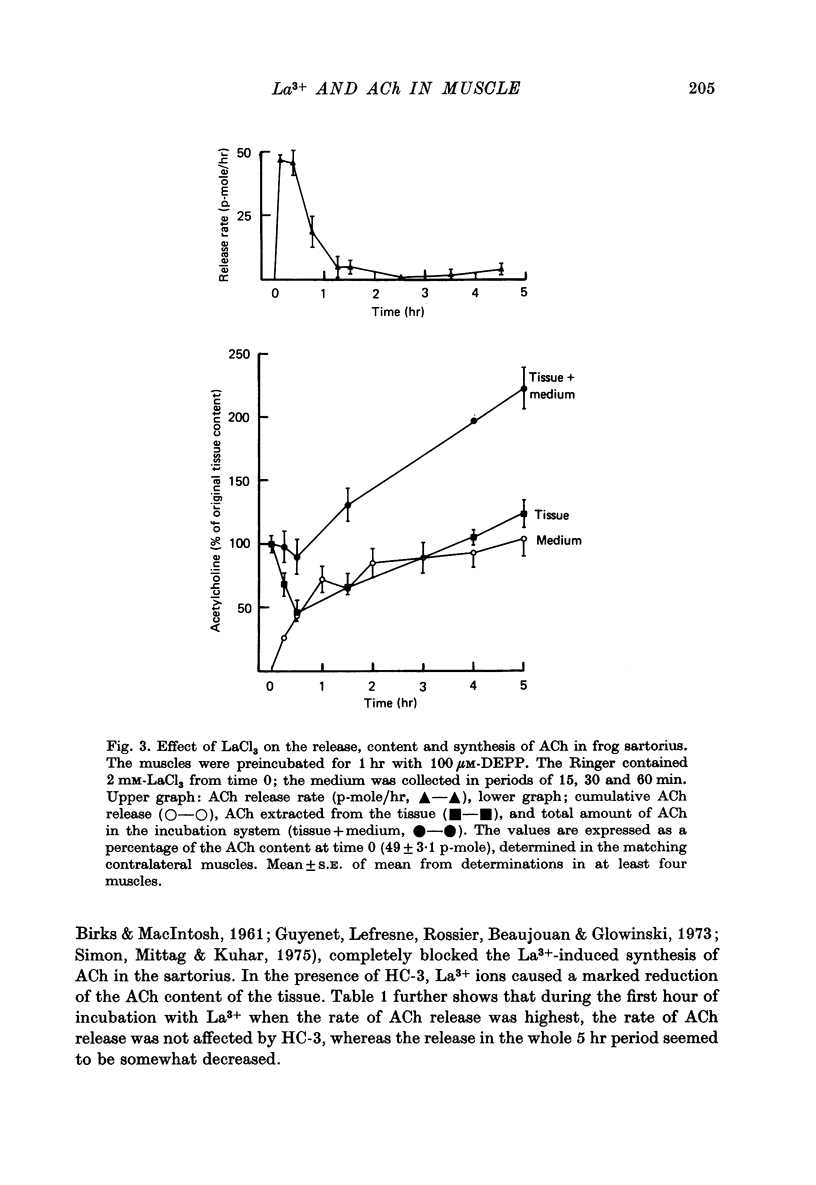
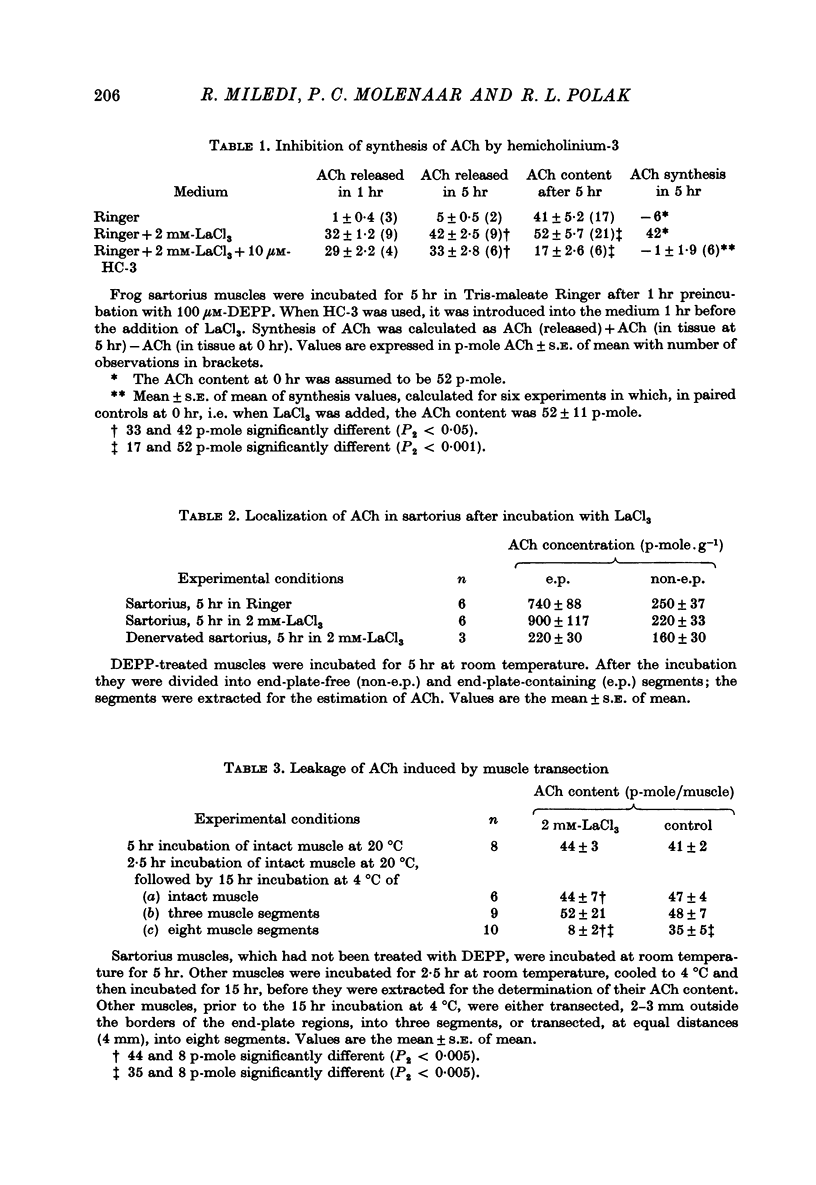
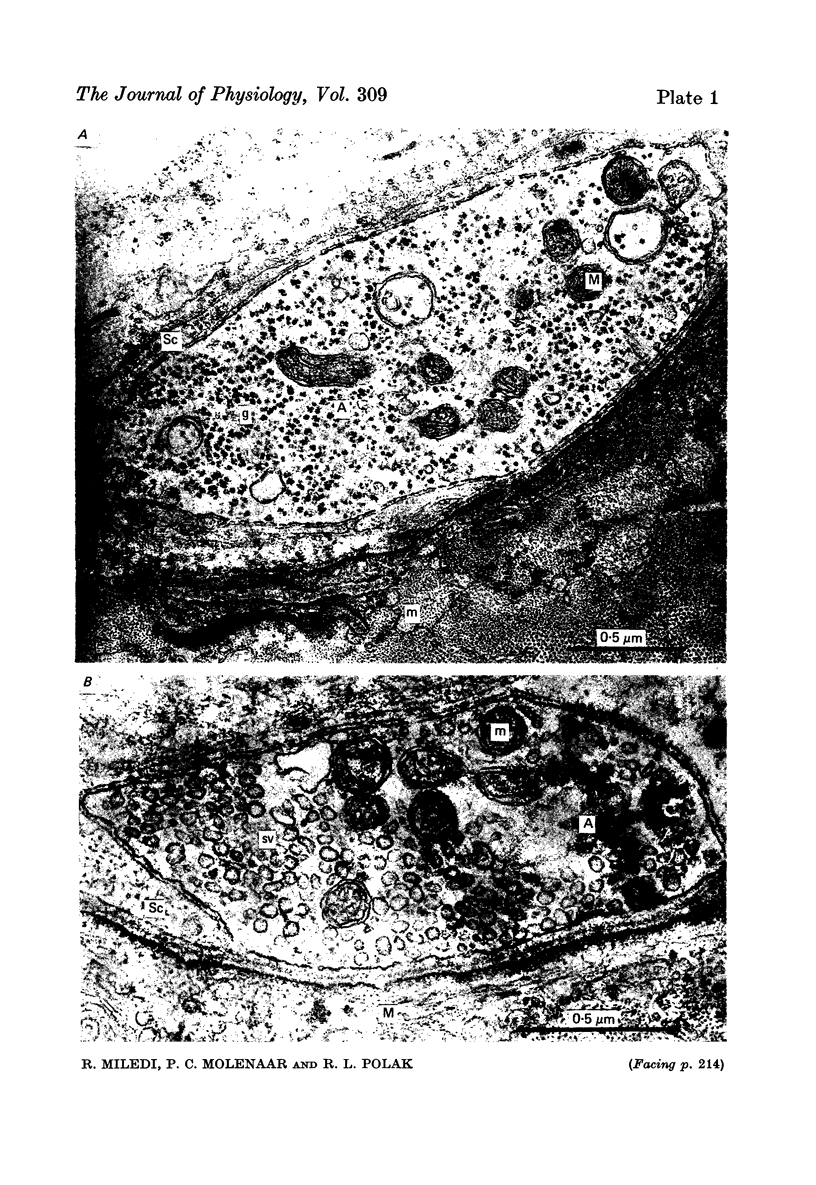
1. Frog sartorius muscles were treated with an irreversible cholinesterase inhibitor and then incubated in Ringer with 2 mM-LaCl3. The amounts of ACh in the tissue and medium were assayed by mass fragmentography, miniature end-plate potentials (min. e.p.p.s) were recorded and the end-plate was investigated by electron microscopy. 2. Addition of La3+ caused in normal, but not in denervated, muscles a discharge of both min. e.p.p.s and chemically detectable ACh. After 30 min both min. e.p.p.s and ACh release decreased. Between 4 and 5 hr after the addition of La3+ min. e.p.p.s had practically ceased and the rate of ACh release was almost back to that in the absence of La3+. 3. La3+ caused a 50% reduction in the ACh content of the tissue within the first 30 min; thereafter ACh gradually increased to 110% by 5 hr. At this time synaptic vesicles were practically absent in most terminals. The ACh was predominantly located in the end-plate regions of the muscles, before as well as after the incubation with La3+. ACh in end-plate free parts of the muscles was unchanged by La3+. 4. Hemicholinium-3 inhibited the synthesis of ACh in the muscles, but it had almost no influence on La3+-induced ACh release. 5. From these and other results, it is concluded that the ACh released by La3+ originates exclusively from the nerve terminals, that most likely this ACh is released via exocytosis from synaptic vesicles, and that the synthesis of ACh following the release of ACh takes place in the nerve terminals. The results further indicate that in freshly excised muscle the greater part (80-90%) of the ACh contained in the nerve terminals is located in the vesicles.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braggaar-Schaap P. A sensitive method for assaying acetylcholine synthesis in human and frog skeletal muscle. J Neurochem. 1979 Jul;33(1):389–392. doi: 10.1111/j.1471-4159.1979.tb11750.x. [DOI] [PubMed] [Google Scholar]
- COUTEAUX R. THE DIFFERENTIATION OF SYNAPTIC AREAS. Proc R Soc Lond B Biol Sci. 1963 Nov 19;158:457–480. doi: 10.1098/rspb.1963.0058. [DOI] [PubMed] [Google Scholar]
- Ceccarelli B., Hurlbut W. P. The effects of prolonged repetitive stimulation in hemicholinium on the frog neuromuscular junction. J Physiol. 1975 May;247(1):163–188. doi: 10.1113/jphysiol.1975.sp010926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A. W., Mauro A., Longenecker H. E., Jr, Hurlbut W. P. Effects of black widow spider venom on the frog neuromuscular junction. Effects on the fine structure of the frog neuromuscular junction. Nature. 1970 Feb 21;225(5234):703–705. doi: 10.1038/225703a0. [DOI] [PubMed] [Google Scholar]
- Fonnum F. A rapid radiochemical method for the determination of choline acetyltransferase. J Neurochem. 1975 Feb;24(2):407–409. doi: 10.1111/j.1471-4159.1975.tb11895.x. [DOI] [PubMed] [Google Scholar]
- Fonnum F. Choline acetyltransferase binding to and release from membranes. Biochem J. 1968 Sep;109(3):389–398. doi: 10.1042/bj1090389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonnum F. The 'compartmentation' of choline acetyltransferase within the synaptosome. Biochem J. 1967 Apr;103(1):262–270. doi: 10.1042/bj1030262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorio A., Hurlbut W. P., Ceccarelli B. Acetylcholine compartments in mouse diaphragm. Comparison of the effects of black widow spider venom, electrical stimulation, and high concentrations of potassium. J Cell Biol. 1978 Sep;78(3):716–733. doi: 10.1083/jcb.78.3.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet P., Lefresne P., Rossier J., Beaujouan J. C., Glowinski J. Inhibition by hemicholinium-3 of (14C)acetylcholine synthesis and (3H)choline high-affinity uptake in rat striatal synaptosomes. Mol Pharmacol. 1973 Sep;9(5):630–639. [PubMed] [Google Scholar]
- Hall Z. W. Multiple forms of acetylcholinesterase and their distribution in endplate and non-endplate regions of rat diaphragm muscle. J Neurobiol. 1973;4(4):343–361. doi: 10.1002/neu.480040404. [DOI] [PubMed] [Google Scholar]
- Heuser J., Lennon A. M. Morphological evidence for exocytosis of acetylcholine during formation of synaptosomes from Torpedo electric organ. J Physiol. 1973 Aug;233(1):39P–41P. [PubMed] [Google Scholar]
- Heuser J., Miledi R. Effects of lanthanum ions on function and structure of frog neuromuscular junctions. Proc R Soc Lond B Biol Sci. 1971 Dec 14;179(1056):247–260. doi: 10.1098/rspb.1971.0096. [DOI] [PubMed] [Google Scholar]
- Johnson C. D., Russell R. L. A rapid, simple radiometric assay for cholinesterase, suitable for multiple determinations. Anal Biochem. 1975 Mar;64(1):229–238. doi: 10.1016/0003-2697(75)90423-6. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. Transmitter leakage from motor nerve endings. Proc R Soc Lond B Biol Sci. 1977 Feb 11;196(1122):59–72. doi: 10.1098/rspb.1977.0029. [DOI] [PubMed] [Google Scholar]
- Liang C. C., Quastel J. H. Uptake of acetylcholine in rat brain cortex slices. Biochem Pharmacol. 1969 May;18(5):1169–1185. doi: 10.1016/0006-2952(69)90120-8. [DOI] [PubMed] [Google Scholar]
- Longenecker H. E., Jr, Hurlbut W. P., Mauro A., Clark A. W. Effects of black widow spider venom on the frog neuromuscular junction. Effects on end-plate potential, miniature end-plate potential and nerve terminal spike. Nature. 1970 Feb 21;225(5234):701–703. doi: 10.1038/225701a0. [DOI] [PubMed] [Google Scholar]
- MILEDI R. The acetylcholine sensitivity of frog muscle fibres after complete or partial devervation. J Physiol. 1960 Apr;151:1–23. [PMC free article] [PubMed] [Google Scholar]
- Marchbanks R. M., Israël M. Aspects of acetylcholine metabolism in the electric organ of Torpedo marmorata. J Neurochem. 1971 Mar;18(3):439–448. doi: 10.1111/j.1471-4159.1971.tb11971.x. [DOI] [PubMed] [Google Scholar]
- Marnay A., Nachmansohn D. Choline esterase in voluntary muscle. J Physiol. 1938 Feb 16;92(1):37–47. doi: 10.1113/jphysiol.1938.sp003582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R. Lanthanum ions abolish the "calcium response" of nerve terminals. Nature. 1971 Feb 5;229(5284):410–411. doi: 10.1038/229410a0. [DOI] [PubMed] [Google Scholar]
- Miledi R., Molenaar P. C., Polak R. L. An analysis of acetylcholine in frog muscle by mass fragmentography. Proc R Soc Lond B Biol Sci. 1977 Jun 15;197(1128):285–297. doi: 10.1098/rspb.1977.0071. [DOI] [PubMed] [Google Scholar]
- Molenaar P. C., Polak R. L. Analysis of the preferential release of newly synthesized acetylcholine by cortical slices from rat brain with the aid of two different labelled precursors. J Neurochem. 1976 Jan;26(1):95–99. [PubMed] [Google Scholar]
- Polak R. L., Meeuws M. M. The influence of atropine on the release and uptake of acetylcholine by the isolated cerebral cortex of the rat. Biochem Pharmacol. 1966 Jul;15(7):989–992. doi: 10.1016/0006-2952(66)90176-6. [DOI] [PubMed] [Google Scholar]
- Polak R. L., Molenaar P. C. A method for determination of acetylcholine by slow pyrolysis combined with mass fragmentography on a packed capillary column. J Neurochem. 1979 Feb;32(2):407–412. doi: 10.1111/j.1471-4159.1979.tb00364.x. [DOI] [PubMed] [Google Scholar]
- Polak R. L., Molenaar P. C. Pitfalls in determination of acetylcholine from brain by pyrolysis-gas chromatography/mass spectrometry. J Neurochem. 1974 Dec;23(6):1295–1297. doi: 10.1111/j.1471-4159.1974.tb12230.x. [DOI] [PubMed] [Google Scholar]
- Polak R. L. The influence of drugs on the uptake of acetylcholine by slices of rat cerebral cortex. Br J Pharmacol. 1969 May;36(1):144–152. doi: 10.1111/j.1476-5381.1969.tb08311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon J. R., Mittag T. W., Kuhar J. M. Inhibition of synaptosomal uptake of choline by various choline analogs. Biochem Pharmacol. 1975 May 15;24(10):1139–1142. doi: 10.1016/0006-2952(75)90208-7. [DOI] [PubMed] [Google Scholar]
- Welsch F., Schmidt D. E., Dettbarn W. D. Acetylcholine, choline acetyltransferase and cholinesterases in motor and sensory nerves of the bull frog. Biochem Pharmacol. 1972 Mar 15;21(6):847–856. doi: 10.1016/0006-2952(72)90128-1. [DOI] [PubMed] [Google Scholar]