Abstract
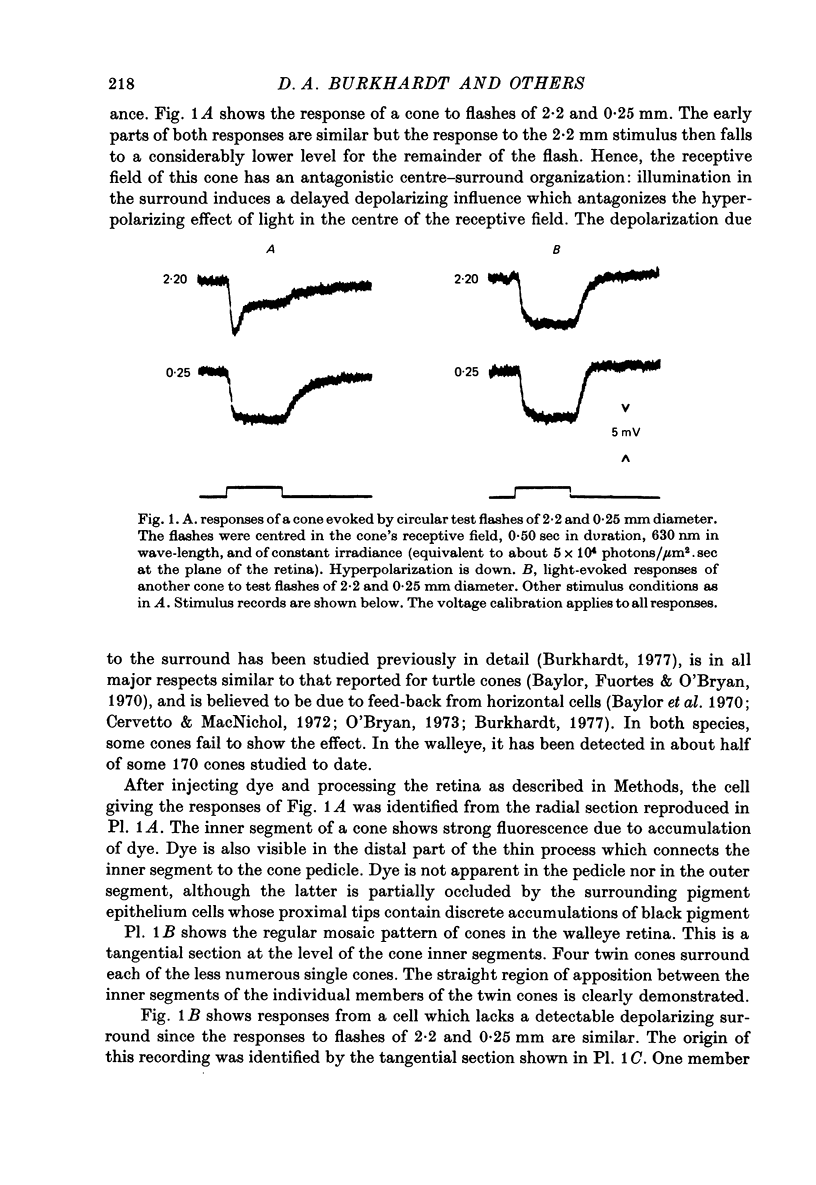
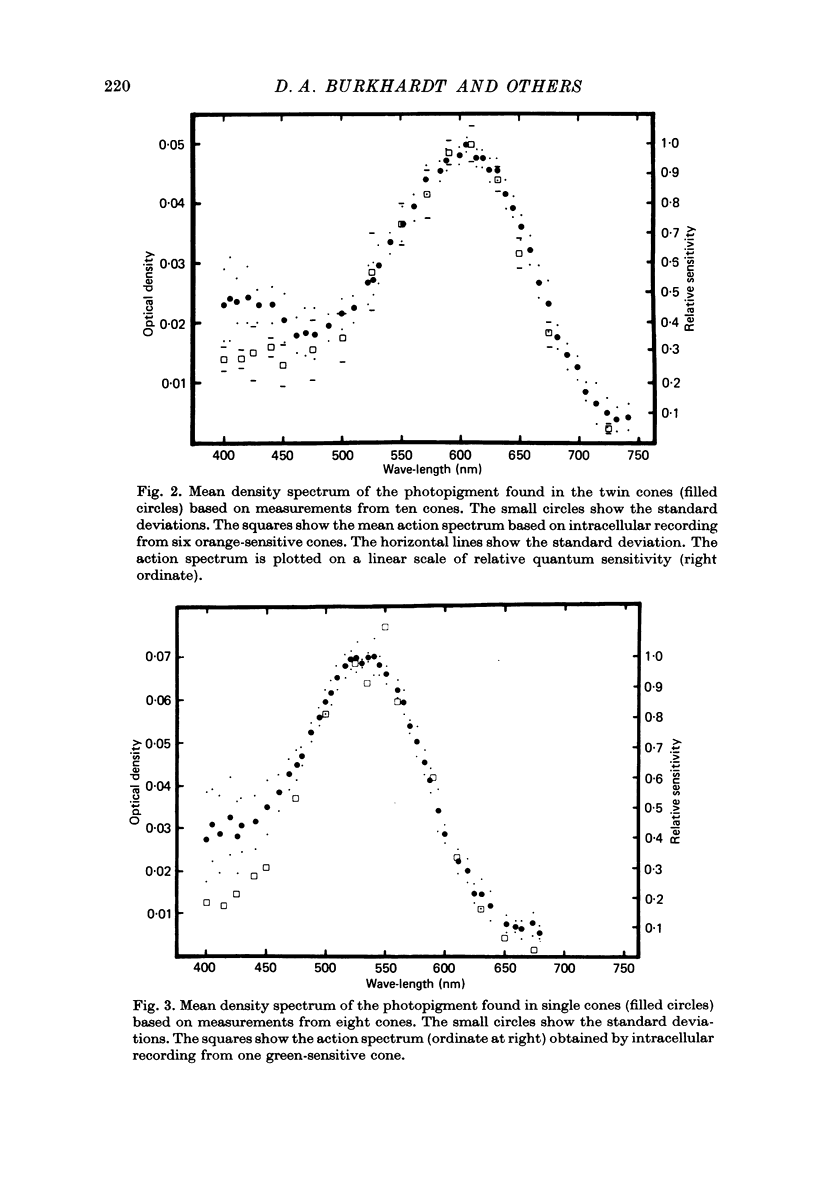
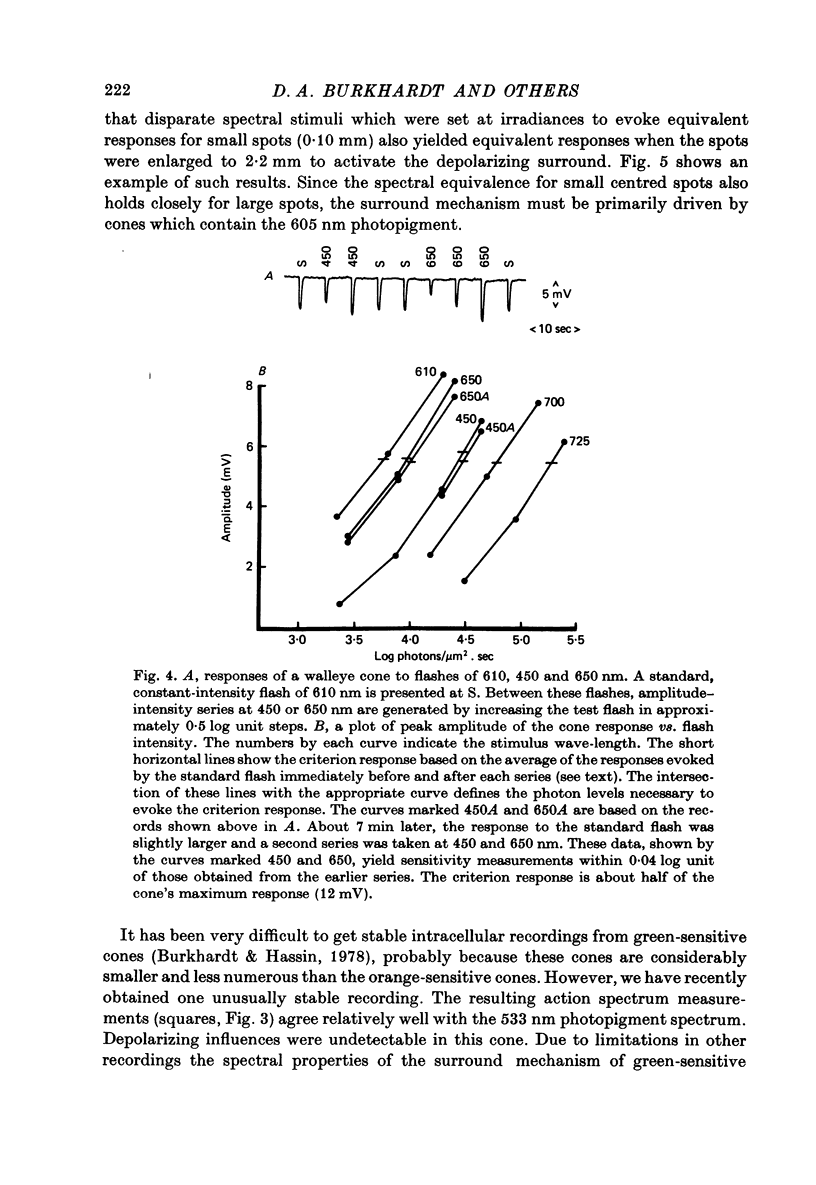
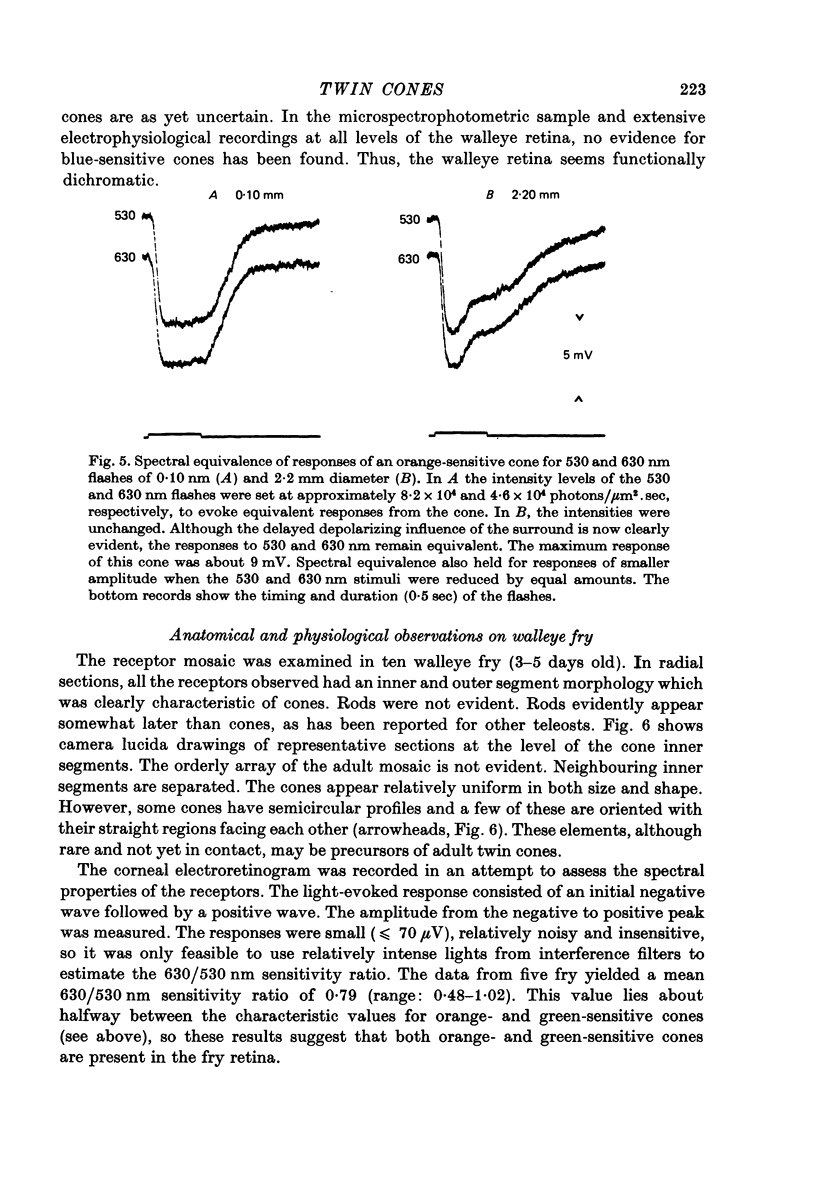
1. The properties of twin and single cones in the retina of the walleye (Stizostedion vitreum vitreum) were studied by intracellular recording, dye injection and microspectrophotometry. 2. Twin cones generate hyperpolarizing responses to central illumination, can receive depolarizing influences (feed-back) from the receptive field surround, and show no detectable dye coupling when injected with Procion yellow. In seventeen of eighteen dye-injected cones, fluorescence was intense in the inner segment and undetectable or weak in the cone pedicle. 3. Both members of the twin cone contain the same photopigment in their outer segments. It absorbs maximally at about 605 nm. 4. A 533 nm green-sensitive photopigment was found in single cones. No blue-sensitive cones have been found. 5. With the exception of a modest discrepancy in the violet, the absorptance spectrum of the 605 nm photopigment of twin cones agrees closely with the action spectrum measured by intracellular recording. 6. The spectral properties established by the twin cone's photopigment are not detectably altered by the hyperpolarizing influences arising from nearby cones or by the depolarizing influences arising from the receptive field surround. 7. The twin cones of the walleye retina are thus "identical twins', both photochemically and physiologically, and seem designed to function as long-wave, spectrally univariant receptor units for colour vision. 8. The available evidence suggests that identical twin cones differ functionally from double cones and non-identical twin cones. 9. Although they outnumber single cones by about three to one in adults, identifiable twin cones were rarely observed in the cone population of retinas examined 3-5 days after birth. If walleye twin cones develop by fusion of single cones this process apparently occurs only for cones containing the 605 nm photopigment.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baylor D. A., Fettiplace R. Light path and photon capture in turtle photoreceptors. J Physiol. 1975 Jun;248(2):433–464. doi: 10.1113/jphysiol.1975.sp010983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Fuortes M. G., O'Bryan P. M. Receptive fields of cones in the retina of the turtle. J Physiol. 1971 Apr;214(2):265–294. doi: 10.1113/jphysiol.1971.sp009432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehlert G. W. Intraspecific evidence for the function of single and double cones in the teleost retina. Science. 1978 Oct 20;202(4365):309–311. doi: 10.1126/science.694534. [DOI] [PubMed] [Google Scholar]
- Bowmaker J. K. The visual pigments, oil droplets and spectral sensitivity of the pigeon. Vision Res. 1977;17(10):1129–1138. doi: 10.1016/0042-6989(77)90147-x. [DOI] [PubMed] [Google Scholar]
- Burkhardt D. A., Hassin G. Influences of cones upon chromatic- and luminosity-type horizontal cells in pikeperch retinas. J Physiol. 1978 Aug;281:125–137. doi: 10.1113/jphysiol.1978.sp012412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhardt D. A. Responses and receptive-field organization of cones in perch retinas. J Neurophysiol. 1977 Jan;40(1):53–62. doi: 10.1152/jn.1977.40.1.53. [DOI] [PubMed] [Google Scholar]
- Byzov A. L. Origin of non-linearity of voltage--current relationships of turtle cones. Vision Res. 1979;19(5):469–477. doi: 10.1016/0042-6989(79)90131-7. [DOI] [PubMed] [Google Scholar]
- Cervetto L., MacNichol E. F., Jr Inactivation of horizontal cells in turtle retina by glutamate and aspartate. Science. 1972 Nov 17;178(4062):767–768. doi: 10.1126/science.178.4062.767. [DOI] [PubMed] [Google Scholar]
- Ebrey T. G., Honig B. New wavelength dependent visual pigment nomograms. Vision Res. 1977;17(1):147–151. doi: 10.1016/0042-6989(77)90213-9. [DOI] [PubMed] [Google Scholar]
- Hassin G. Pikeperch horizontal cells identified by intracellular staining. J Comp Neurol. 1979 Aug 15;186(4):529–540. doi: 10.1002/cne.901860403. [DOI] [PubMed] [Google Scholar]
- Hárosi F. I., MacNichol E. F., Jr Dichroic microspectrophotometer: a computer-assisted, rapid, wavelength-scanning photometer for measuring linear dichroism in single cells. J Opt Soc Am. 1974 Jul;64(7):903–918. doi: 10.1364/josa.64.000903. [DOI] [PubMed] [Google Scholar]
- Hárosi F. I., MacNichol E. F., Jr Visual pigments of goldfish cones. Spectral properties and dichroism. J Gen Physiol. 1974 Mar;63(3):279–304. doi: 10.1085/jgp.63.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hárosi F. I. Spectral relations of cone pigments in goldfish. J Gen Physiol. 1976 Jul;68(1):65–80. doi: 10.1085/jgp.68.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine J. S., MacNichol E. F., Jr, Kraft T., Collins B. A. Intraretinal distribution of cone pigments in certain teleost fishes. Science. 1979 May 4;204(4392):523–526. doi: 10.1126/science.432658. [DOI] [PubMed] [Google Scholar]
- Loew E. R., Lythgoe J. N. The ecology of cone pigments in teleost fishes. Vision Res. 1978;18(6):715–722. doi: 10.1016/0042-6989(78)90150-5. [DOI] [PubMed] [Google Scholar]
- MARKS W. B. VISUAL PIGMENTS OF SINGLE GOLDFISH CONES. J Physiol. 1965 May;178:14–32. doi: 10.1113/jphysiol.1965.sp007611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNichol E. F., Jr, Kunz Y. W., Levine J. S., Hárosi F. I., Collins B. A. Ellipsosomes: organelles containing a cytochrome-like pigment in the retinal cones of certain fishes. Science. 1978 May 5;200(4341):549–552. doi: 10.1126/science.644317. [DOI] [PubMed] [Google Scholar]
- Mariani A. P., Leure-duPree A. E. Photoreceptors and oil droplet colors in the red area of the pigeon retina. J Comp Neurol. 1978 Dec 15;182(4 Pt 2):821–837. doi: 10.1002/cne.901820506. [DOI] [PubMed] [Google Scholar]
- O'Bryan P. M. Properties of the depolarizing synaptic potential evoked by peripheral illumination in cones of the turtle retina. J Physiol. 1973 Nov;235(1):207–223. doi: 10.1113/jphysiol.1973.sp010385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter A., Simon E. J. Electrical responses of double cones in the turtle retina. J Physiol. 1974 Nov;242(3):673–683. doi: 10.1113/jphysiol.1974.sp010730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson I., Rose B., Loewenstein W. R. Size limit of molecules permeating the junctional membrane channels. Science. 1977 Jan 21;195(4275):294–296. doi: 10.1126/science.831276. [DOI] [PubMed] [Google Scholar]
- Stell W. K., Hárosi F. I. Cone structure and visual pigment content in the retina of the goldfish. Vision Res. 1976;16(6):647–657. doi: 10.1016/0042-6989(76)90013-4. [DOI] [PubMed] [Google Scholar]
- Witkovsky P., Burkhardt D. A., Nagy A. R. Synaptic connections linking cones and horizontal cells in the retina of the pikeperch (Stizostedion vitreum). J Comp Neurol. 1979 Aug 15;186(4):541–559. doi: 10.1002/cne.901860404. [DOI] [PubMed] [Google Scholar]



