Abstract
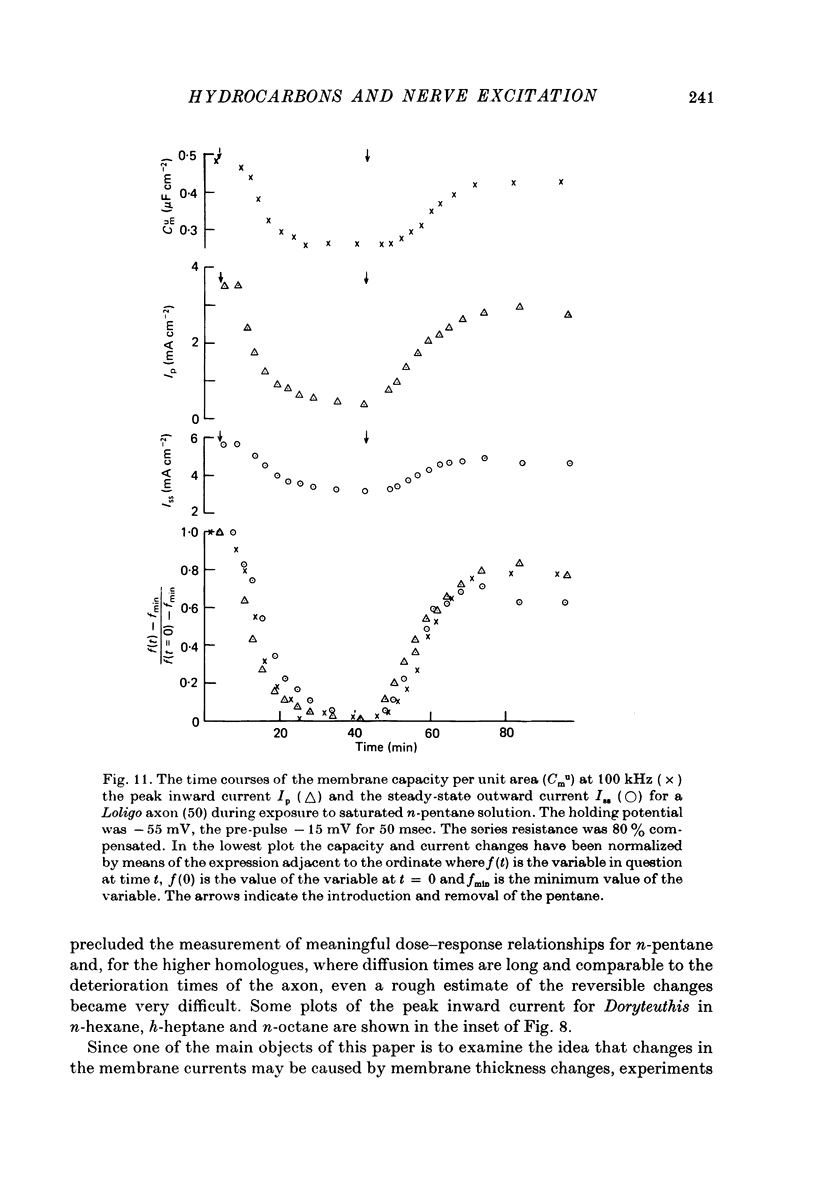
1. The electrical properties of squid giant axons were examined by means of admittance bridges at frequencies from 0.5 to 300 kHz. A simple equivalent circuit was used to estimate the membrane capacity. 2. The calculated membrane capacities decreased monotonically over the whole frequency range. 3. At 100 kHz and higher frequencies the membrane capacity was independent of potential. 4. At frequencies greater than 20 kHz, exposure of the axons to saturated or 0.9 saturated solutions of n-pentane (275-306 micrometer) reduced the capacity per unit area by 0.1-0.15 micro F cm-2. 5. At 1 kHz the effect of the saturated pentane solutions depended on the membrane potential. In axons having potentials between -60 and zero mV the pentane solutions lowered the capacity, whereas for potentials between -160 and -60 mV they produced little or no change. 6. Saturated solutions of n-hexane, n-heptane and n-octane exhibited qualitatively similar, but quantitatively smaller influences on the membrane capacity, the changes declining as the chain length increased. 7. Under voltage clamp, the peak inward and steady-state outward currents were partially suppressed by the hydrocarbons. Saturated solutions of n-pentane usually reduced the former (reversibly) by 60-80% and the latter by 20-40%. Solutions of n-hexane, n-heptane and n-octane appeared to have successively less effect. Except in deteriorating axons, none of the hydrocarbons produced any consistent changes in the passive membrane resistance, the resting potential or in the reversal potential of the transient inward current. 8. Both the changes in the clamp currents and in the membrane capacity were largely, though not usually completely, reversible. In the hydrocarbon solution the axons deteriorated more rapidly than normal. 9. The responses of axons of Doryteuthis plei to the hydrocarbons were very similar to those of Loligo forbesi with the exception that for the former all observed changes were some five times faster. 10. The time courses of the peak inward and steady-state outward currents on exposure of the axons to n-pentane resembled the time course of the change in membrane capacity at 100 kHz. 11. The simplest interpretation of the high frequency capacity results is suggested to be that, as for lipid bilayers, the membranes become thicker through adsorption of the hydrocarbon.
Full text
PDF



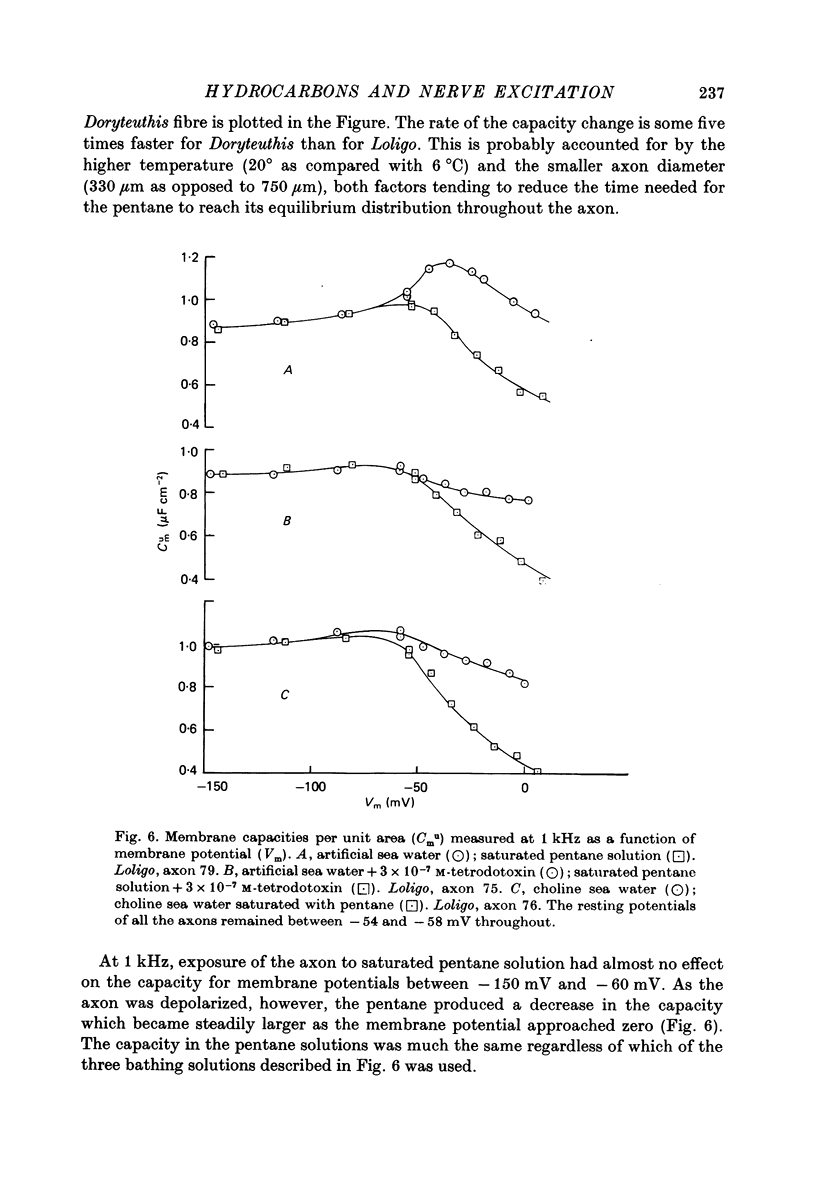




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
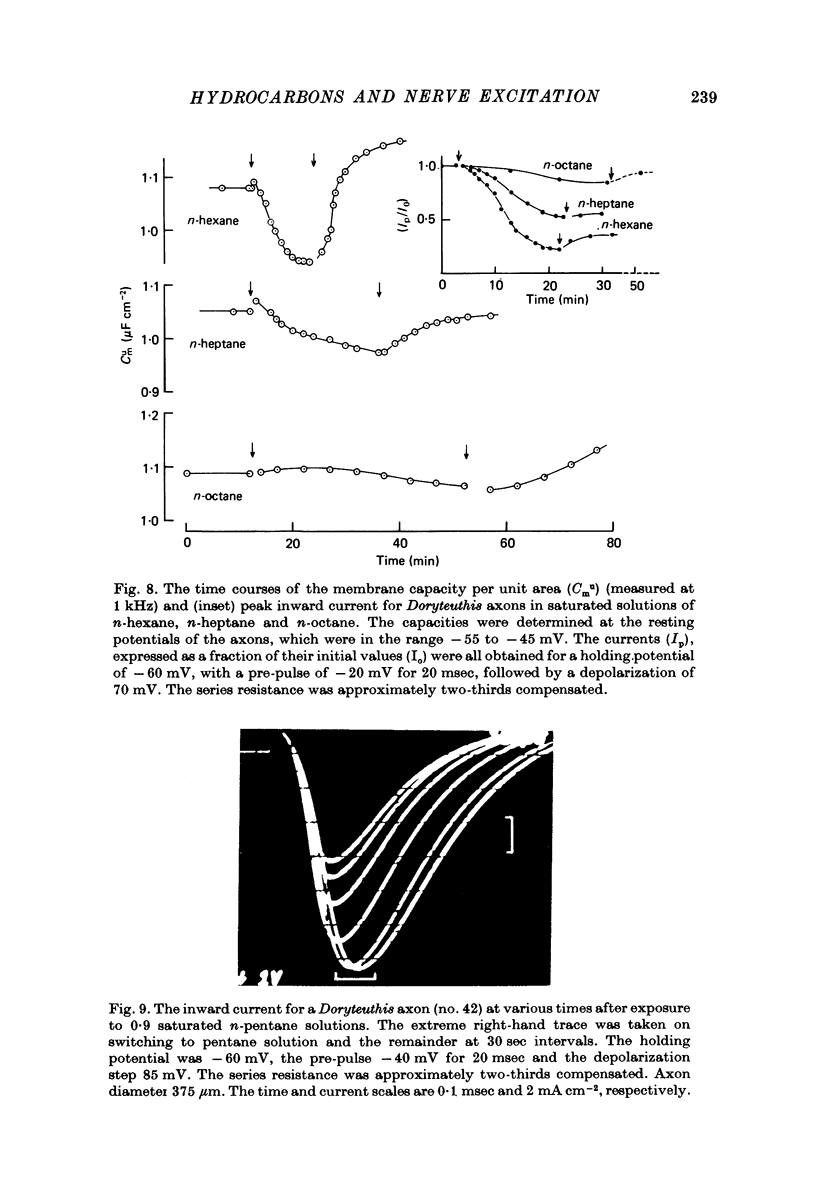
- Ashcroft R. G., Coster H. G., Smith J. R. Local anaesthetic benzyl alcohol increases membrane thickness. Nature. 1977 Oct 27;269(5631):819–820. doi: 10.1038/269819a0. [DOI] [PubMed] [Google Scholar]
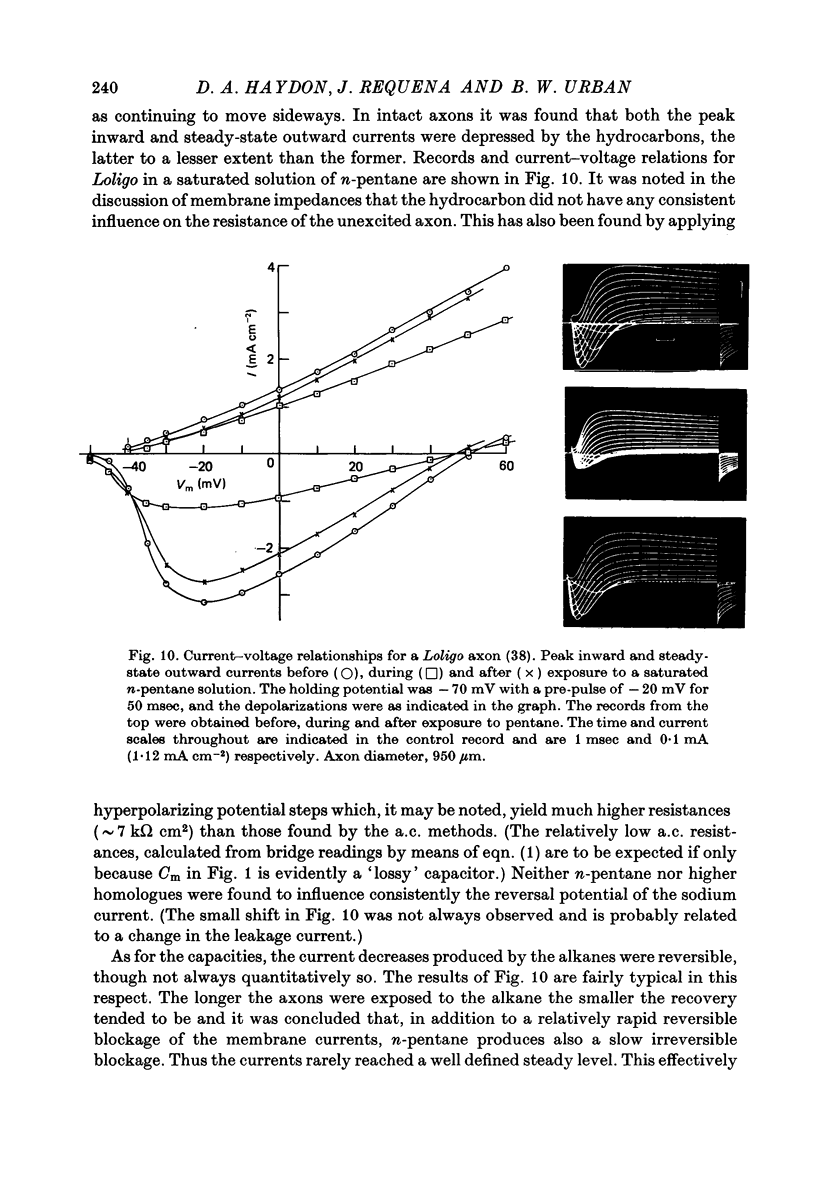
- Ashcroft R. G., Coster H. G., Smith J. R. The molecular organisation of bimolecular lipid membranes. The effect of benzyl alcohol on the structure. Biochim Biophys Acta. 1977 Aug 15;469(1):13–22. doi: 10.1016/0005-2736(77)90321-2. [DOI] [PubMed] [Google Scholar]
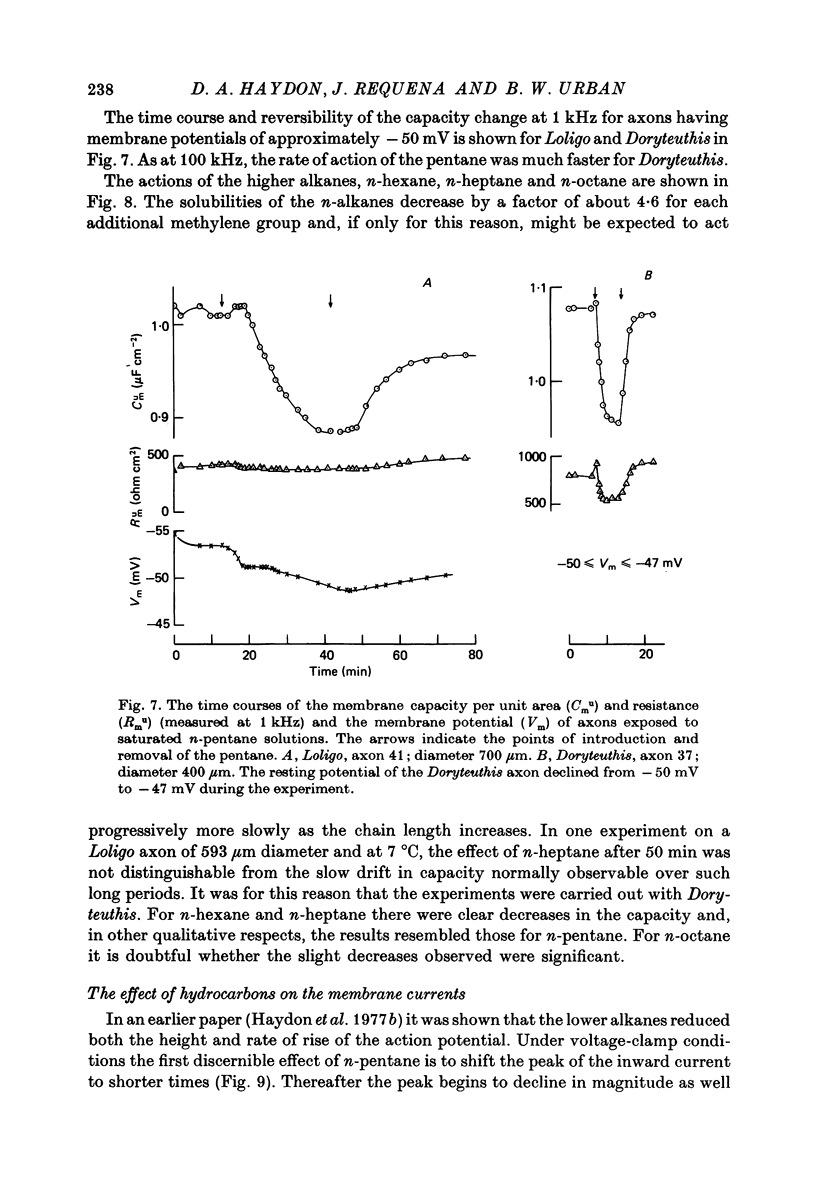
- Benz R., Fröhlich O., Läuger P., Montal M. Electrical capacity of black lipid films and of lipid bilayers made from monolayers. Biochim Biophys Acta. 1975 Jul 3;394(3):323–334. doi: 10.1016/0005-2736(75)90287-4. [DOI] [PubMed] [Google Scholar]
- Camejo G., Villegas G. M., Barnola F. V., Villegas R. Characterization of two different membrane fractions isolated from the first stellar nerves of the squid Dosidicus gigas. Biochim Biophys Acta. 1969;193(2):247–259. doi: 10.1016/0005-2736(69)90186-2. [DOI] [PubMed] [Google Scholar]
- Chacko G. K., Villegas G. M., Barnola F. V., Villegas R., Goldman D. E. The polypeptide and the phospholipid components of axon plasma membranes. Biochim Biophys Acta. 1976 Aug 4;443(1):19–32. doi: 10.1016/0005-2736(76)90488-0. [DOI] [PubMed] [Google Scholar]
- Cole K. S. Electrical properties of the squid axon sheath. Biophys J. 1976 Feb;16(2 Pt 1):137–142. doi: 10.1016/s0006-3495(76)85670-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKENHAEUSER B., HODGKIN A. L. The after-effects of impulses in the giant nerve fibres of Loligo. J Physiol. 1956 Feb 28;131(2):341–376. doi: 10.1113/jphysiol.1956.sp005467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman H. M., Macey R. I. The N-shaped current-potential characteristic in frog skin. I. Time development during step voltage clamp. Biophys J. 1969 Feb;9(2):127–139. doi: 10.1016/S0006-3495(69)86374-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F., KATZ B. Measurement of current-voltage relations in the membrane of the giant axon of Loligo. J Physiol. 1952 Apr;116(4):424–448. doi: 10.1113/jphysiol.1952.sp004716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon D. A. Functions of the lipid in bilayer ion permeability. Ann N Y Acad Sci. 1975 Dec 30;264:2–16. doi: 10.1111/j.1749-6632.1975.tb31472.x. [DOI] [PubMed] [Google Scholar]
- Haydon D. A., Hendry B. M., Levinson S. R., Requena J. The molecular mechanisms of anaesthesia. Nature. 1977 Jul 28;268(5618):356–358. doi: 10.1038/268356a0. [DOI] [PubMed] [Google Scholar]
- Haydon D. A., Kimura J., Requena J. Some effects of n-pentane on the capacity and ionic currents of the squid giant axon membrane [proceedings]. J Physiol. 1979 Feb;287:38P–39P. [PubMed] [Google Scholar]
- Hendry B. M., Urban B. W., Haydon D. A. The blockage of the electrical conductance in a pore-containing membrane by the n-alkanes. Biochim Biophys Acta. 1978 Oct 19;513(1):106–116. doi: 10.1016/0005-2736(78)90116-5. [DOI] [PubMed] [Google Scholar]
- Kimura J. E., Meves H. The effect of temperature on the asymmetrical charge movement in squid giant axons. J Physiol. 1979 Apr;289:479–500. doi: 10.1113/jphysiol.1979.sp012748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh T. J., Simon S. A., MacDonald R. C. The organization of n-alkanes in lipid bilayers. Biochim Biophys Acta. 1980 Apr 24;597(3):445–463. doi: 10.1016/0005-2736(80)90219-9. [DOI] [PubMed] [Google Scholar]
- Padron R., Mateu L., Requena J. A dynamic x-ray diffraction study of anesthesia action. Thickening of the myelin membrane by n-pentane. Biochim Biophys Acta. 1979 Apr 19;552(3):535–539. doi: 10.1016/0005-2736(79)90198-6. [DOI] [PubMed] [Google Scholar]
- Seeman P. The membrane actions of anesthetics and tranquilizers. Pharmacol Rev. 1972 Dec;24(4):583–655. [PubMed] [Google Scholar]
- Takashima S. Membrane capacity of squid giant axon during hyper- and depolarizations. J Membr Biol. 1976 Jun 9;27(1-2):21–39. doi: 10.1007/BF01869127. [DOI] [PubMed] [Google Scholar]
- Takashima S., Schwan H. P. Passive electrical properties of squid axon membrane. J Membr Biol. 1974;17(1):51–68. doi: 10.1007/BF01870172. [DOI] [PubMed] [Google Scholar]
- Takashima S., Yantorno R., Novack R. Dipole moment changes and voltage dependent membrane capacity of squid axon. Biochim Biophys Acta. 1977 Aug 15;469(1):74–88. doi: 10.1016/0005-2736(77)90327-3. [DOI] [PubMed] [Google Scholar]


