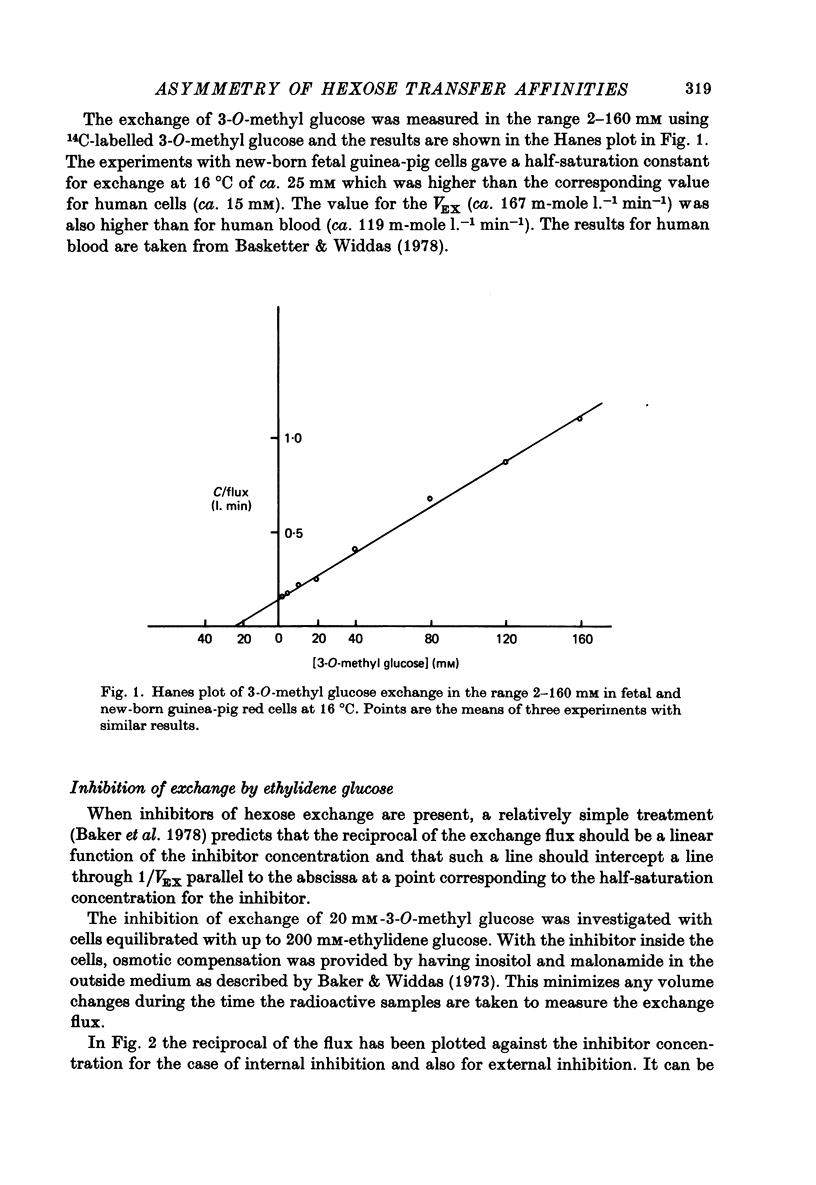
Abstract
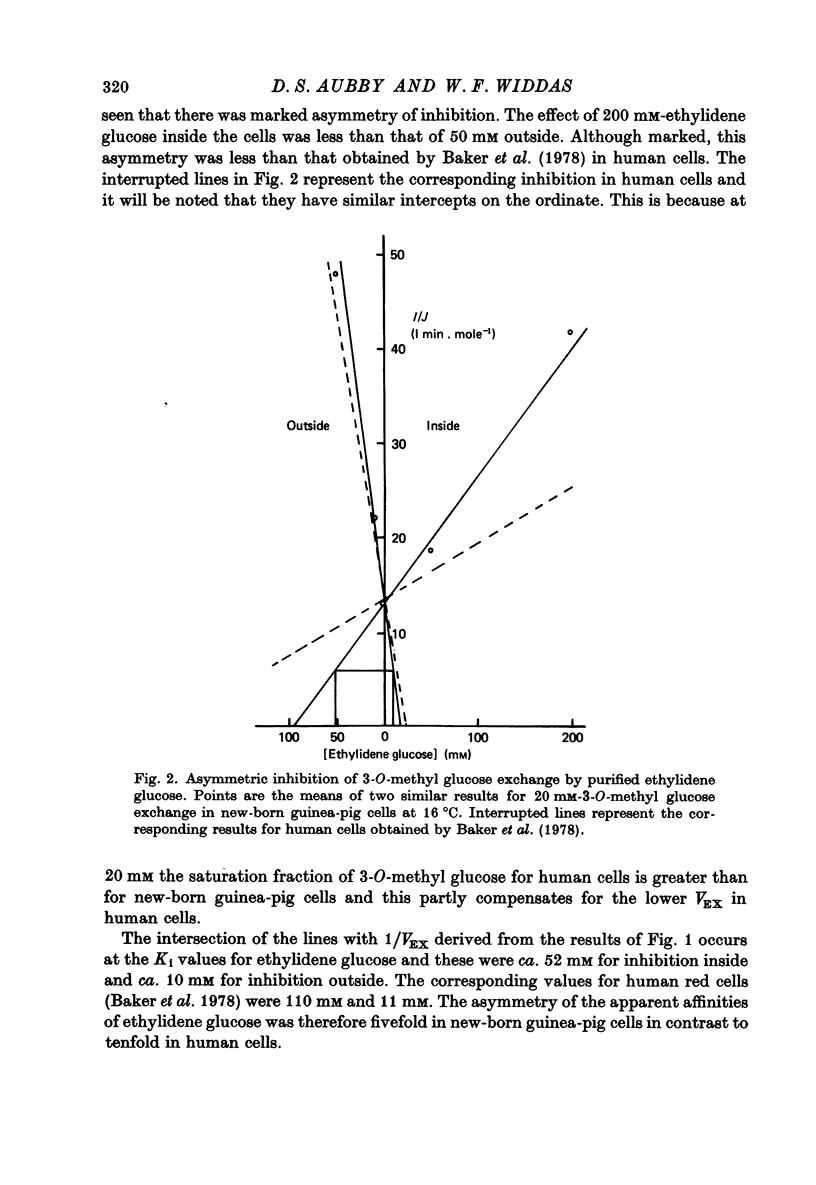
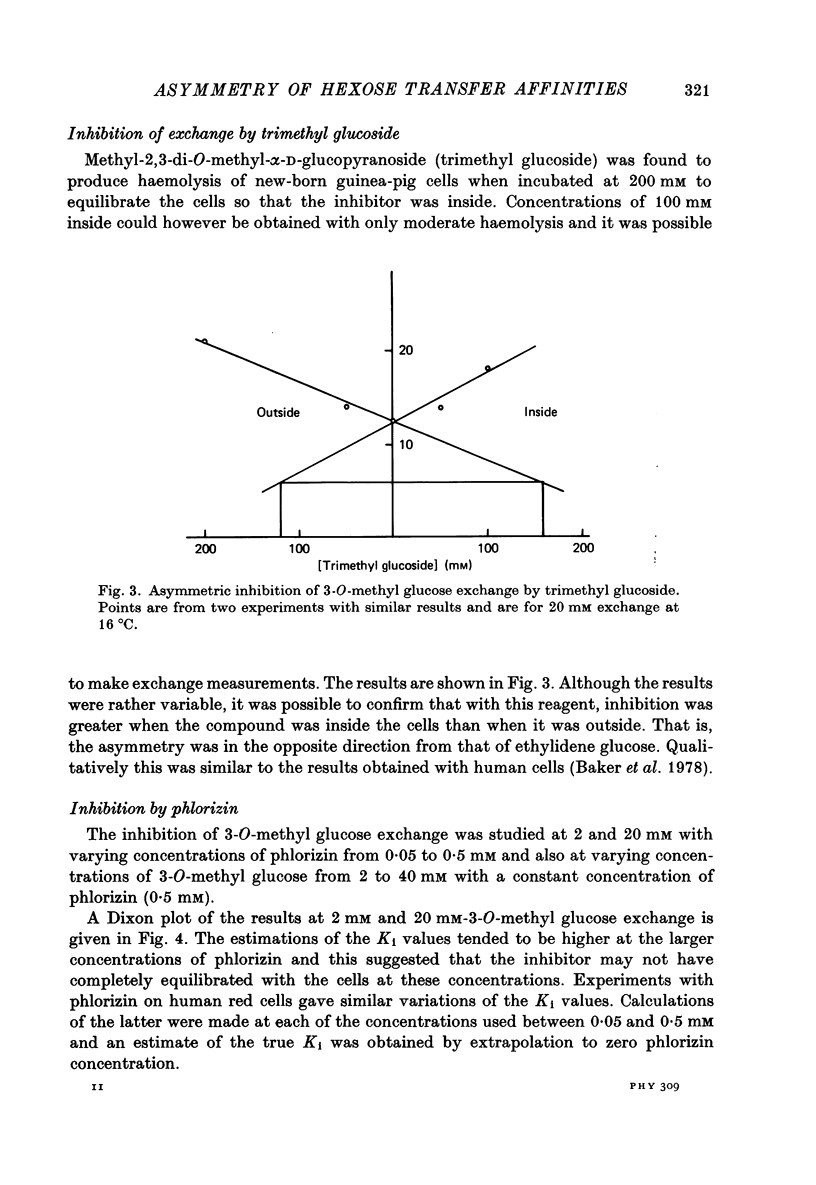
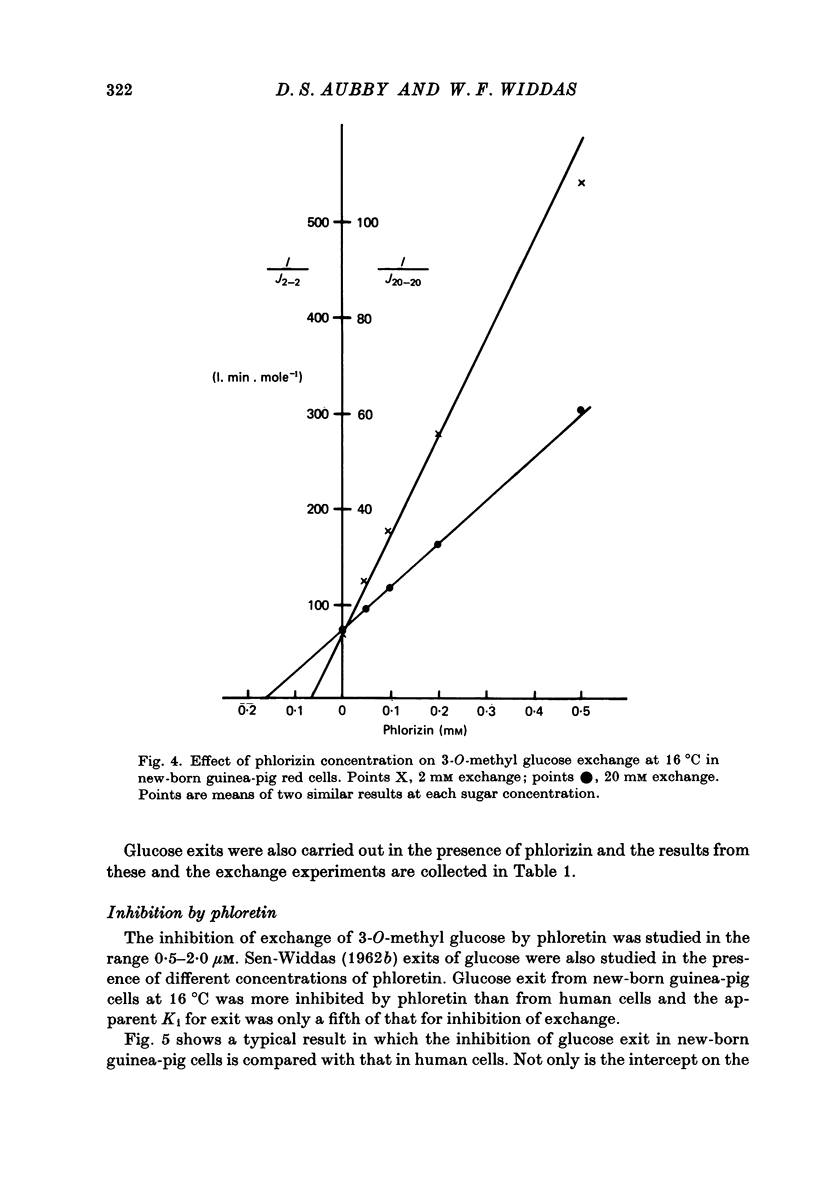
1. The asymmetries of affinities of two non-transportable competitive inhibitors of hexose transfer across fetal and new-born guinea-pig erythrocytes have been studied. 2. At 16 degrees C 4,6-O-ethylidene-alpha-D-glucopyranose (ethylidene glucose) inhibited 3-O-methyl glucose exchange at 20 mM with a K1 oc ca. 52 mM when present inside the cells and with a K1 oc ca. 10 mM when outside. This fivefold asymmetry is qualitatively similar to but smaller than the tenfold asymmetry of human erythrocytes (Baker, Basketter & Widdas, 1978). 3. Methyl-2,3-di-O-methyl-alpha-D-glucopyranoside (trimethyl glucoside) had K1 values of ca. 120 mM and ca. 160mM for inside and outside inhibition respectively. This is also qualitatively similar to the inhibition in human erythrocytes. 4. The inhibition produced by phlorizin, phloretin and Cytochalasin B was also studied in the erythrocytes of new-born guinea-pigs. The results were qualitatively similar to those for human erythrocytes but the inhibitory affinities were different. Thus while phlorizin and phloretin had higher affinities for the inhibition of exchange in new-born guinea-pig cells than human cells, the affinity of Cytochalasin B was less for new-born guinea-pig cells than for human cells. 5. It is concluded that the hexose transfer system in fetal and new-born guinea-pig red cells has asymmetric affinities similar to the system in human red cells but with different values of the inhibitory constants. The differences may represent species variations in a structural protein serving identical functions in the two species. 6. The possibility that fetal red cells with their facilitated transfer system play a role in sugar transport is discussed.
Full text
PDF

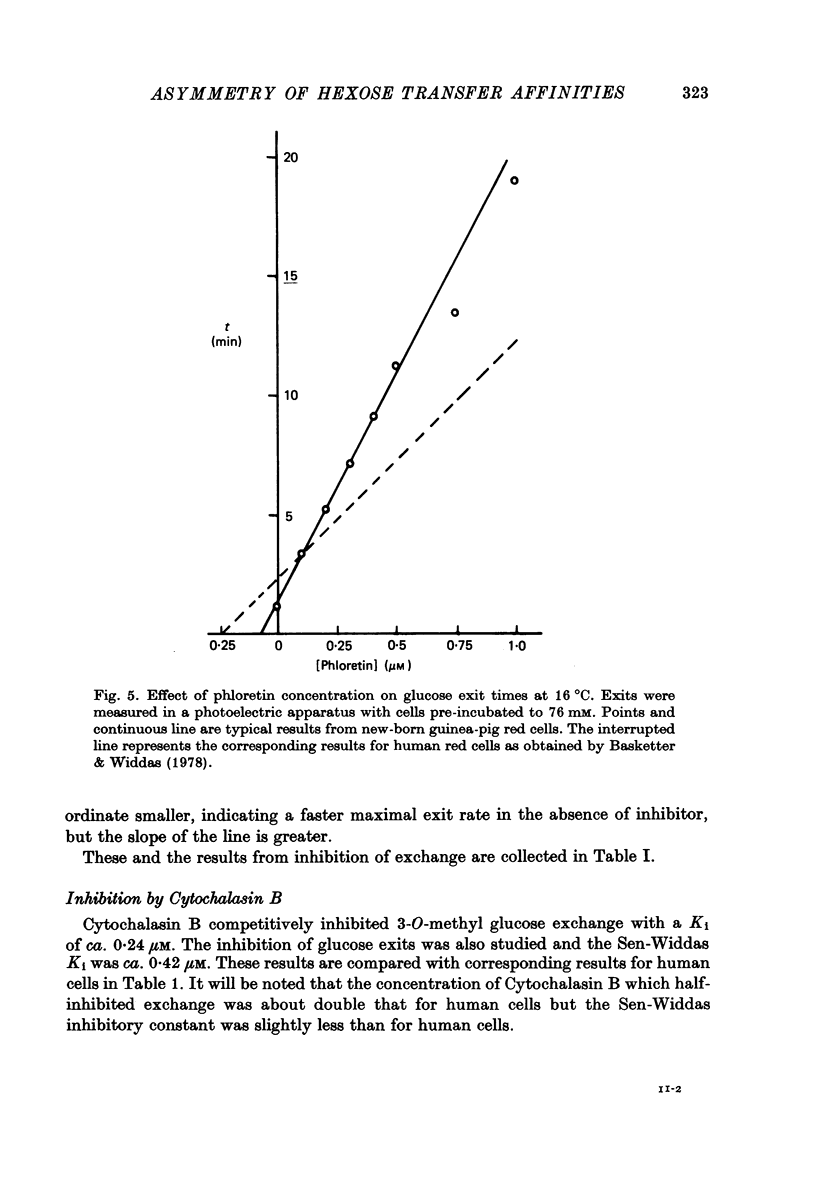

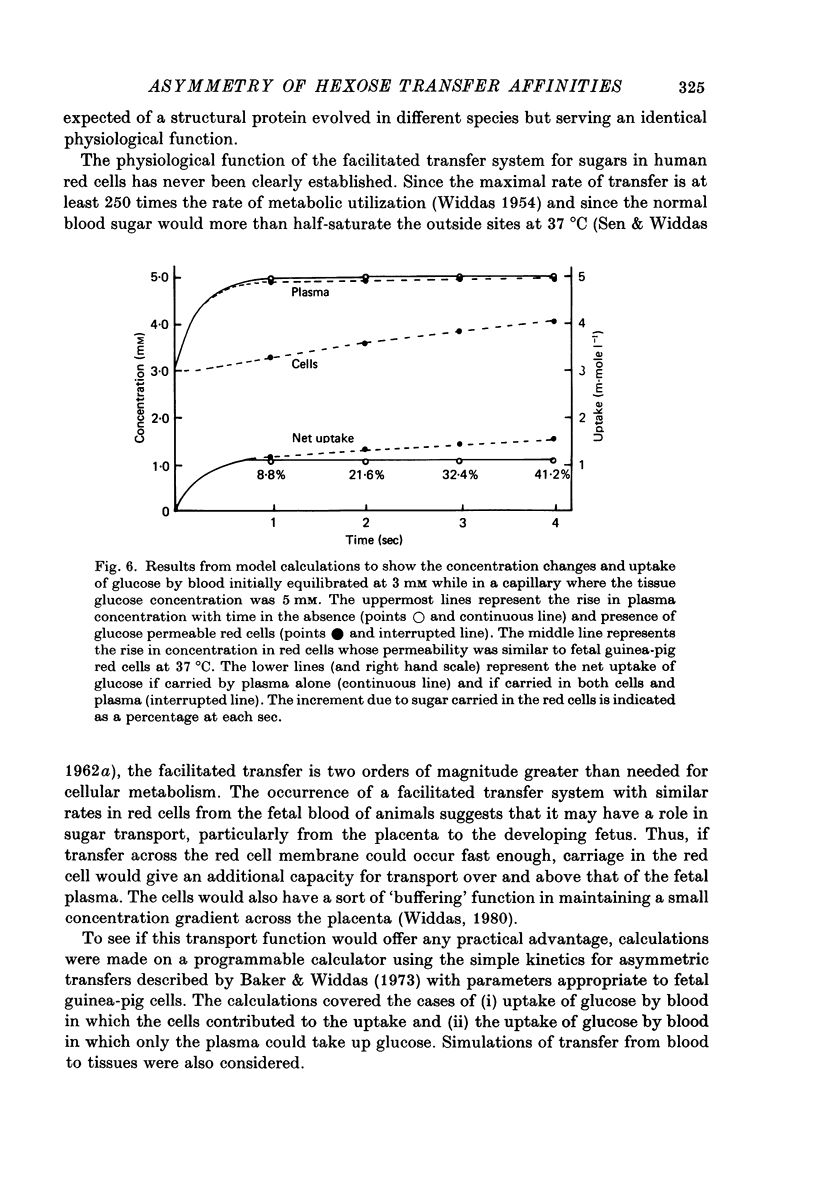


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aubby D. S., Widdas W. F. Asymmetry in the hexose transfer system of erythrocytes from new-born guinea-pigs [proceedings]. J Physiol. 1979 Aug;293:73P–73P. [PubMed] [Google Scholar]
- Augustin H. W., von Rohden L., Häcker M. R. Uber einige Eigenschaften des Monosaccharidtransportsystems in Erythrozyten neugeborener und erwachsener Kaninchen. Acta Biol Med Ger. 1967;19(5):723–735. [PubMed] [Google Scholar]
- Baker G. F., Basketter D. A., Widdas W. F. Asymmetry of the hexose transfer system in human erythrocytes. Experiments with non-transportable inhibitors. J Physiol. 1978 May;278:377–388. doi: 10.1113/jphysiol.1978.sp012310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker G. F., Widdas W. F. The asymmetry of the facilitated transfer system for hexoses in human red cells and the simple kinetics of a two component model. J Physiol. 1973 May;231(1):143–165. doi: 10.1113/jphysiol.1973.sp010225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basketter D. A., Widdas W. F. Asymmetry of the hexose transfer system in human erythrocytes. Comparison of the effects of cytochalasin B, phloretin and maltose as competitive inhibitors. J Physiol. 1978 May;278:389–401. doi: 10.1113/jphysiol.1978.sp012311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWSON A. C., WIDDAS W. F. VARIATIONS WITH TEMPERATURE AND PH OF THE PARAMETERS OF GLUCOSE TRANSFER ACROSS THE ERYTHROCYTE MEMBRANE IN THE FOETAL GUINEA-PIG. J Physiol. 1964 Jul;172:107–122. doi: 10.1113/jphysiol.1964.sp007406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanes C. S. Studies on plant amylases: The effect of starch concentration upon the velocity of hydrolysis by the amylase of germinated barley. Biochem J. 1932;26(5):1406–1421. doi: 10.1042/bj0261406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochachka P. W., Murphy B., Liggins C. G., Zapol W., Crensy R., Snider M., Schneider R., Quist J. Unusual maternal--fetal blood glucose concentrations in Weddell seal. Nature. 1979 Feb 1;277(5695):388–389. doi: 10.1038/277388a0. [DOI] [PubMed] [Google Scholar]
- SEN A. K., WIDDAS W. F. Determination of the temperature and pH dependence of glucose transfer across the human erythrocyte membrane measured by glucose exit. J Physiol. 1962 Mar;160:392–403. doi: 10.1113/jphysiol.1962.sp006854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEN A. K., WIDDAS W. F. Variations of the parameters of glucose transfer across the human erythrocyte membrane in the presence of inhibitors of transfer. J Physiol. 1962 Mar;160:404–416. doi: 10.1113/jphysiol.1962.sp006855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIDDAS W. F. Facilitated transfer of hexoses across the human erythrocyte membrane. J Physiol. 1954 Jul 28;125(1):163–180. doi: 10.1113/jphysiol.1954.sp005148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIDDAS W. F. Hexose permeability of foetal erythrocytes. J Physiol. 1955 Feb 28;127(2):318–327. doi: 10.1113/jphysiol.1955.sp005259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidler R. B., Lee P., Kim H. D. Kinetics of 3-O-methyl glucose transport in red blood cells of newborn pigs. J Gen Physiol. 1976 Jan;67(1):67–80. doi: 10.1085/jgp.67.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]


