Abstract
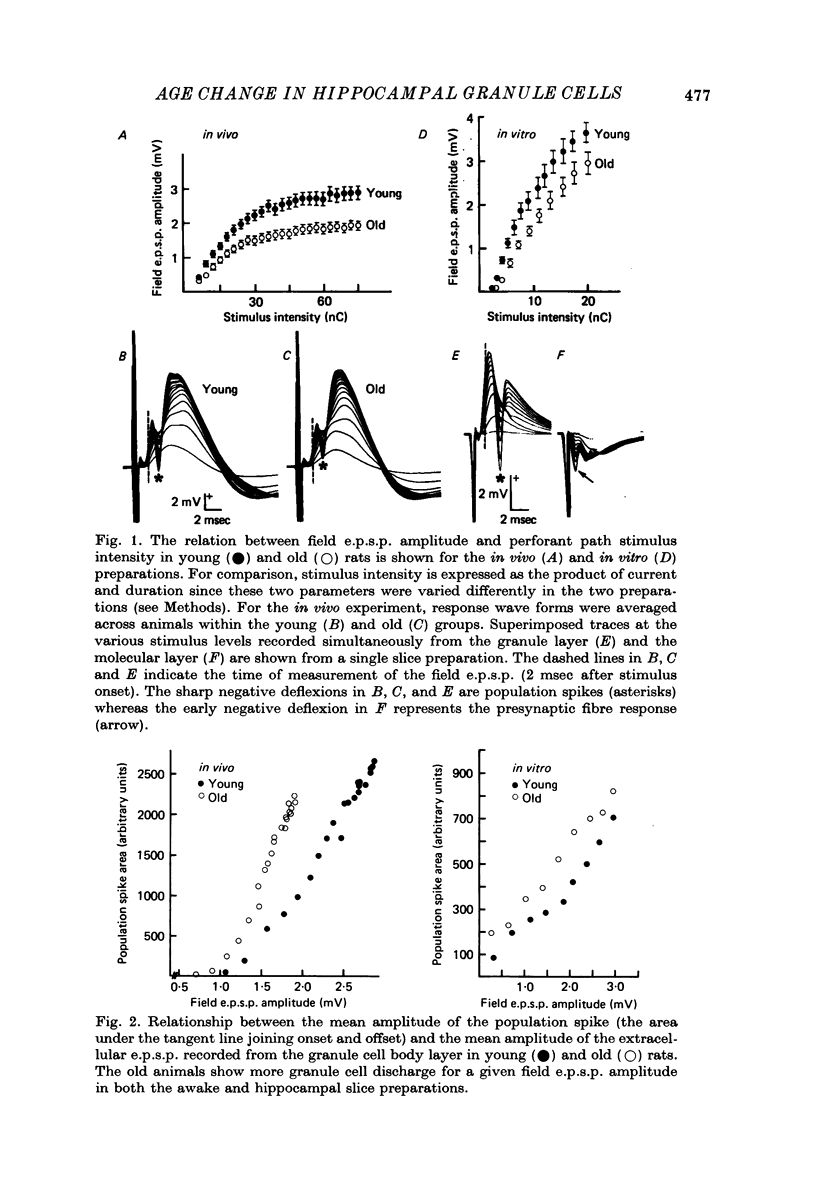
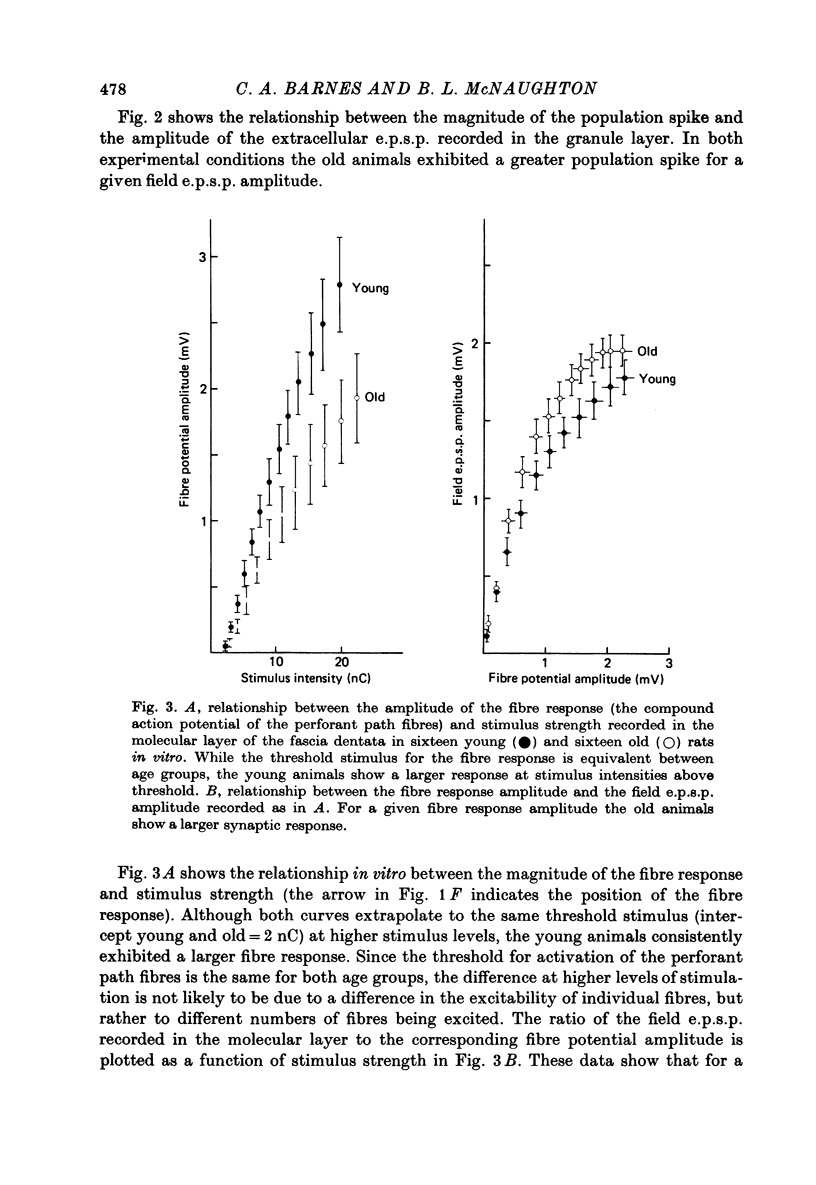
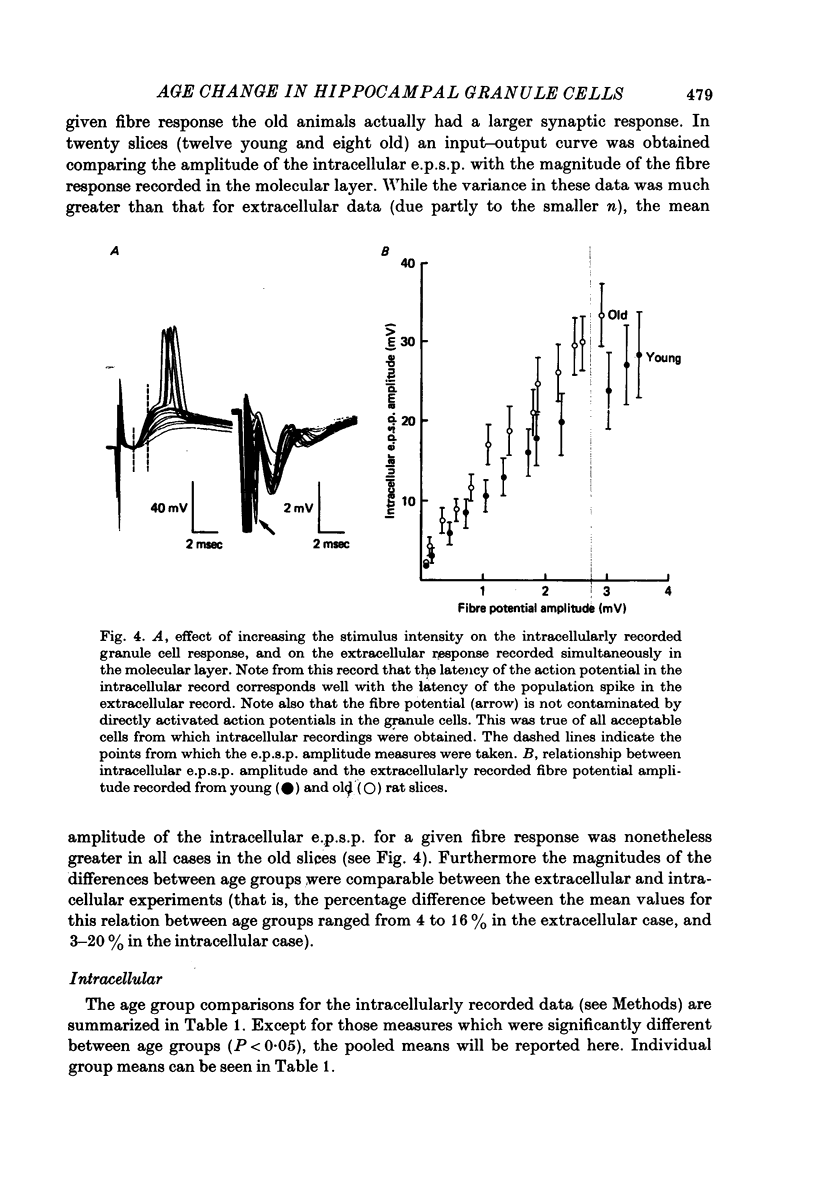
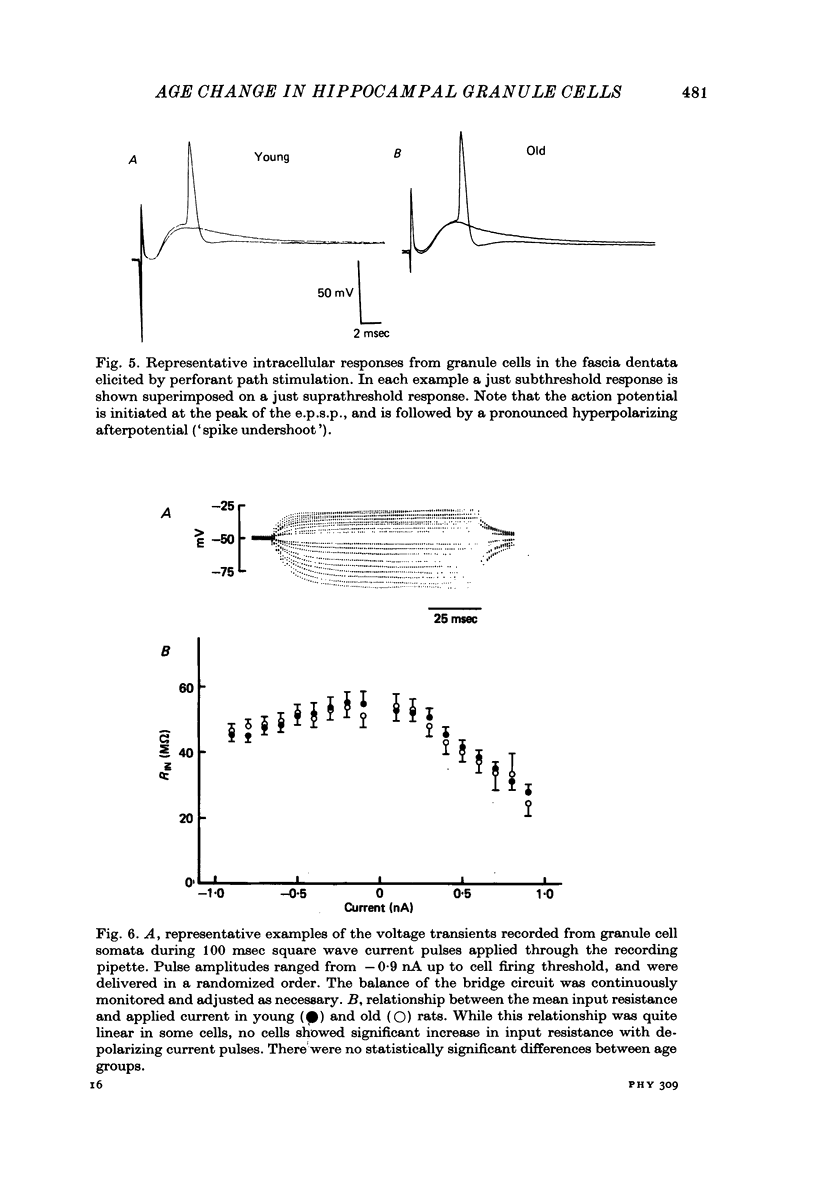
1. The effects of senescence on the input-output characteristics of the perforant path projection to granule cells of the fascia dentata were studied in rats using extracellular techniques in vivo, and both extra- and intracellular recording in vitro. 2. Senescent animals exhibited a significant reduction in the perforant path excitatory synaptic field potential at all stimulus intensities tested. This was associated with a reduction in the size of the afferent fibre response, although there was no apparent change in the threshold for fibre activation. These data support the anatomical literature which indicates a loss of afferent synapses with advanced age. 3. For a given magnitude of afferent fibre response, however, the old animals exhibited a larger synaptic field potential, suggesting that the remaining synapses were in fact more powerful. Furthermore, the magnitude of the extracellular population spike, an index of the number of discharging granule cells, was greater in the old animals when plotted as a function of extracellular e.p.s.p. amplitude. 4. Intracellular recording from a total of 190 granule cells in the transverse hippocampal slice preparation revealed a 17% reduction in the voltage threshold for synaptically elicited granule cell discharge, and a 13% reduction in the latency of the action potential in old compared to young rats. Resting potentials, action potential amplitudes, whole neurone time constants, the relations between applied current and input resistance, and the discharge threshold following depolarizing current pulses, were not different between age groups. 5. These data indicate that granule cells could partly compensate for a loss of synapses during senescence by an increase in their electrical responsiveness to synaptic activation and possibly by an increase in synaptic efficacy.
Full text
PDF



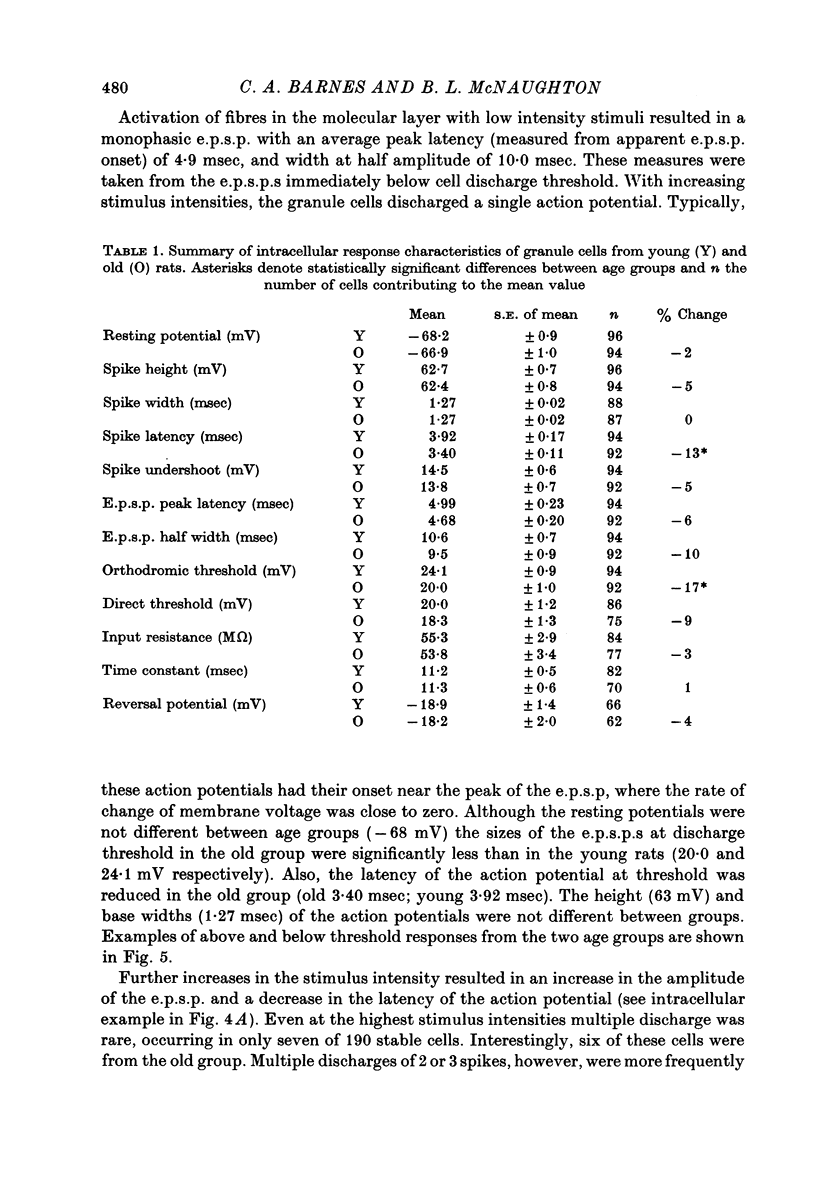




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen P., Bliss T. V., Skrede K. K. Unit analysis of hippocampal polulation spikes. Exp Brain Res. 1971;13(2):208–221. doi: 10.1007/BF00234086. [DOI] [PubMed] [Google Scholar]
- Andersen P., Holmqvist B., Voorhoeve P. E. Entorhinal activation of dentate granule cells. Acta Physiol Scand. 1966 Apr;66(4):448–460. doi: 10.1111/j.1748-1716.1966.tb03223.x. [DOI] [PubMed] [Google Scholar]
- Assaf S. Y., Kelly J. S. On the nature of depolarizing after-potentials in granule cells of the rat dentate gyrus maintained in vitro [proceedings]. J Physiol. 1979 Nov;296:68P–68P. [PubMed] [Google Scholar]
- Barnes C. A., McNaughton B. L. Neurophysiological comparison of dendritic cable properties in adolescent, middle-aged, and senescent rats. Exp Aging Res. 1979 Jun;5(3):195–206. doi: 10.1080/03610737908257198. [DOI] [PubMed] [Google Scholar]
- Barnes C. A. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J Comp Physiol Psychol. 1979 Feb;93(1):74–104. doi: 10.1037/h0077579. [DOI] [PubMed] [Google Scholar]
- Bondareff W., Geinisman Y. Loss of synapses in the dentate gyrus of the senescent rat. Am J Anat. 1976 Jan;145(1):129–136. doi: 10.1002/aja.1001450110. [DOI] [PubMed] [Google Scholar]
- Buell S. J., Coleman P. D. Dendritic growth in the aged human brain and failure of growth in senile dementia. Science. 1979 Nov 16;206(4420):854–856. doi: 10.1126/science.493989. [DOI] [PubMed] [Google Scholar]
- Dudek F. E., Deadwyler S. A., Cotman C. W., Lynch G. Intracellular responses from granule cell layer in slices of rat hippocampus: perforant path synapse. J Neurophysiol. 1976 Mar;39(2):384–393. doi: 10.1152/jn.1976.39.2.384. [DOI] [PubMed] [Google Scholar]
- Geinisman Y., Bondareff W., Dodge J. T. Dendritic atrophy in the dentate gyrus of the senescent rat. Am J Anat. 1978 Jul;152(3):321–329. doi: 10.1002/aja.1001520305. [DOI] [PubMed] [Google Scholar]
- Geinisman Y., Bondareff W., Dodge J. T. Partial deafferentation of neurons in the dentate gyrus of the senescent rat. Brain Res. 1977 Oct 14;134(3):541–545. doi: 10.1016/0006-8993(77)90828-9. [DOI] [PubMed] [Google Scholar]
- Genisman Y., Bondareff W. Decrease in the number of synapses in the senescent brain: a quantitative electron microscopic analysis of the dentate gyrus molecular layer in the rat. Mech Ageing Dev. 1976 Jan-Feb;5(1):11–23. doi: 10.1016/0047-6374(76)90003-8. [DOI] [PubMed] [Google Scholar]
- Hotson J. R., Prince D. A., Schwartzkroin P. A. Anomalous inward rectification in hippocampal neurons. J Neurophysiol. 1979 May;42(3):889–895. doi: 10.1152/jn.1979.42.3.889. [DOI] [PubMed] [Google Scholar]
- Jefferys J. G. Initiation and spread of action potentials in granule cells maintained in vitro in slices of guinea-pig hippocampus. J Physiol. 1979 Apr;289:375–388. doi: 10.1113/jphysiol.1979.sp012742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KANDEL E. R., SPENCER W. A. Electrophysiology of hippocampal neurons. II. After-potentials and repetitive firing. J Neurophysiol. 1961 May;24:243–259. doi: 10.1152/jn.1961.24.3.243. [DOI] [PubMed] [Google Scholar]
- Landfield P. W., Rose G., Sandles L., Wohlstadter T. C., Lynch G. Patterns of astroglial hypertrophy and neuronal degeneration in the hippocampus of ages, memory-deficient rats. J Gerontol. 1977 Jan;32(1):3–12. doi: 10.1093/geronj/32.1.3. [DOI] [PubMed] [Google Scholar]
- Lomo T. Patterns of activation in a monosynaptic cortical pathway: the perforant path input to the dentate area of the hippocampal formation. Exp Brain Res. 1971;12(1):18–45. [PubMed] [Google Scholar]
- McNaughton B. L., Barnes C. A. Physiological identification and analysis of dentate granule cell responses to stimulation of the medial and lateral perforant pathways in the rat. J Comp Neurol. 1977 Oct 15;175(4):439–454. doi: 10.1002/cne.901750404. [DOI] [PubMed] [Google Scholar]
- Scheibel M. E., Lindsay R. D., Tomiyasu U., Scheibel A. B. Progressive dendritic changes in the aging human limbic system. Exp Neurol. 1976 Nov;53(2):420–430. doi: 10.1016/0014-4886(76)90082-0. [DOI] [PubMed] [Google Scholar]
- Skrede K. K., Westgaard R. H. The transverse hippocampal slice: a well-defined cortical structure maintained in vitro. Brain Res. 1971 Dec 24;35(2):589–593. doi: 10.1016/0006-8993(71)90508-7. [DOI] [PubMed] [Google Scholar]


