Abstract
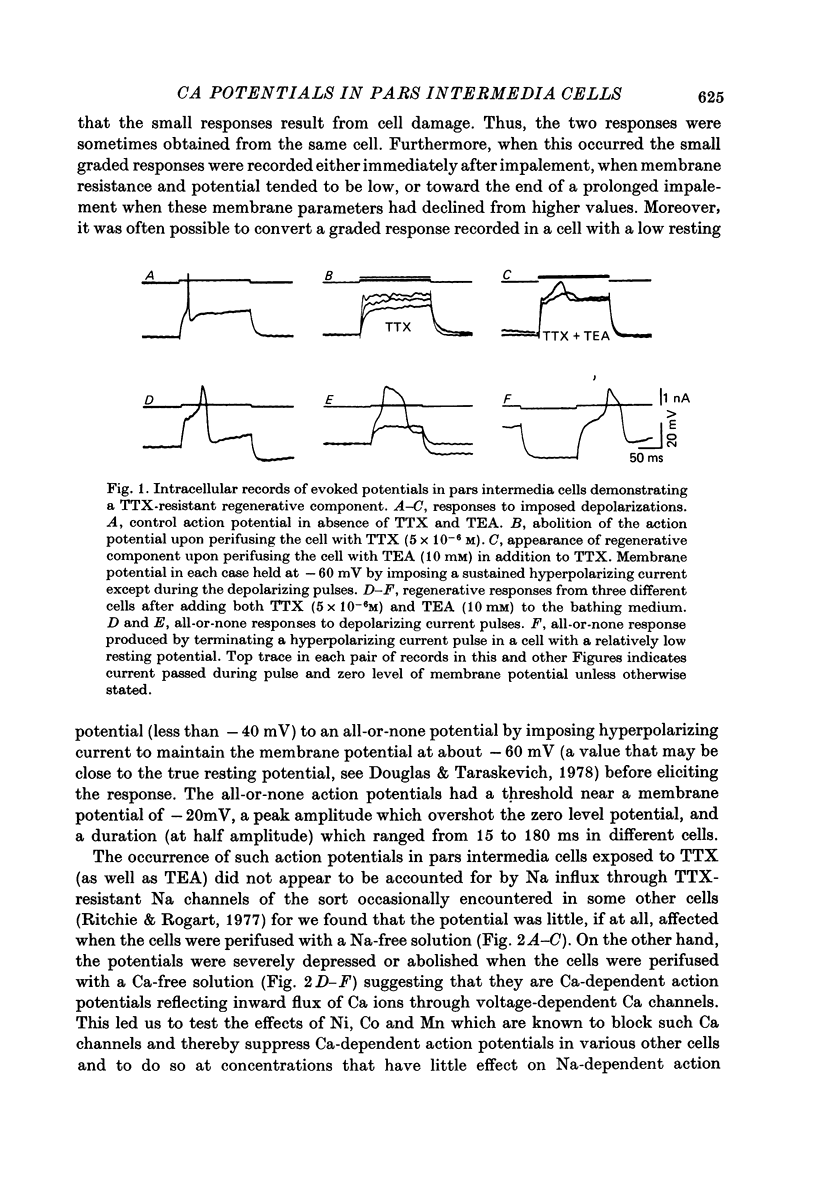
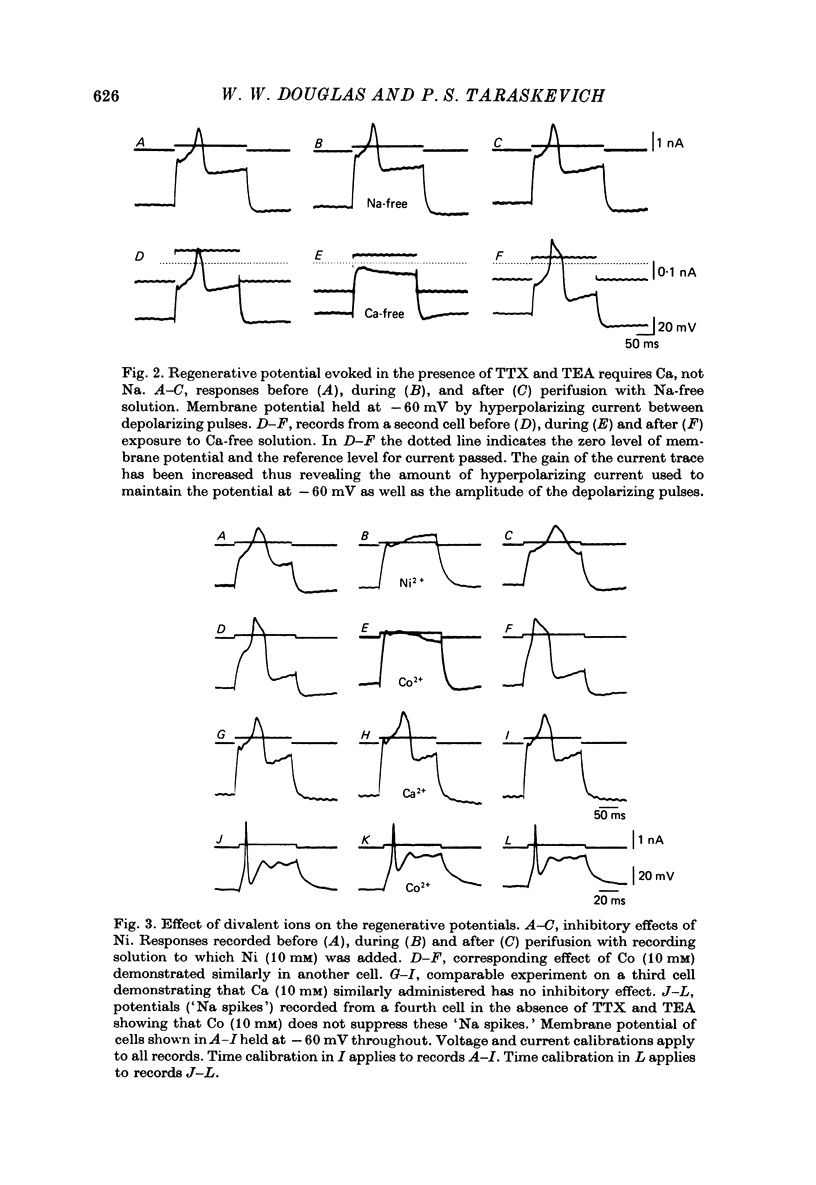
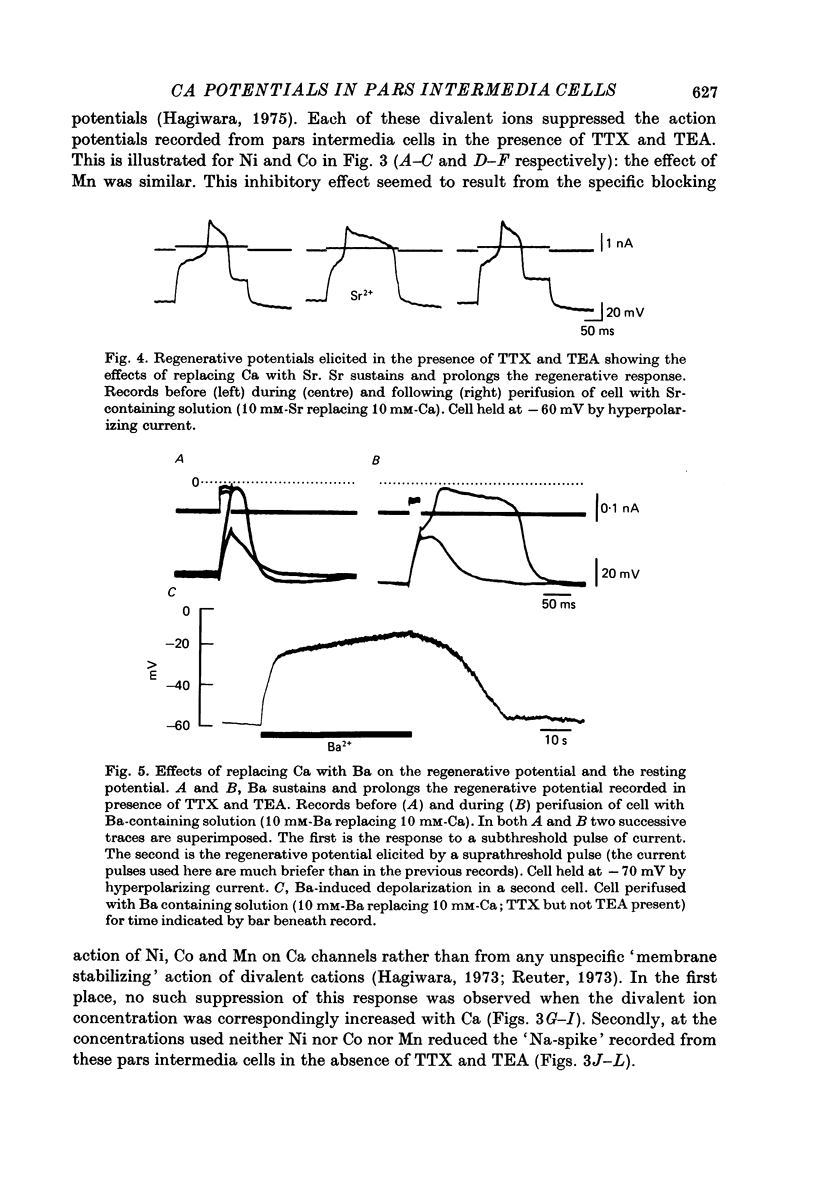
1. The ionic dependence of the action potential of rat pars intermedia cells was investigated by using intracellular recording techniques. 2. In the presence of tetrodotoxin (TTX, 5 x 10(-6)M), the action potentials evoked by passing depolarizing current through the recording electrode were abolished, confirming that they are mainly dependent on Na; however, when tetraethylammonium (TEA, 10 mM) was added to the TTX-containing solution the imposed depolarizations triggered all-or-none regenerative potentials indicative of involvement of another ion. 3. There TTX-insensitive regenerative potentials persisted when the cells were perifused with Na-free solution but were severely reduced or abolished by Ca-free solution. This suggests that the ion producing these potentials is Ca. 4. These Ca action potentials were suppressed by Ni, Co and Mn in concentrations that did not suppress the "Na spikes' recorded in the absence of TTX and TEA. 5. Sr and Ba could substitute for Ca in maintaining the action potentials recorded in the presence of TTX. These ions also prolonged the duration of these action potentials. 6. The demonstration of a Ca component to the predominantly Na-dependent action potentials of pars intermedia cells heightens the possibility that these action potentials participate in the regulation of secretion.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. Mn2+ ions pass through Ca2+ channels in myoepithelial cells. J Exp Biol. 1979 Oct;82:227–238. doi: 10.1242/jeb.82.1.227. [DOI] [PubMed] [Google Scholar]
- Biales B., Dichter M. A., Tischler A. Sodium and calcium action potential in pituitary cells. Nature. 1977 May 12;267(5607):172–174. doi: 10.1038/267172a0. [DOI] [PubMed] [Google Scholar]
- Bicknell R. J., Schofield J. G. Mechanism of action of somatostatin: inhibition of ionophore A23187-induced release of growth hormone from dispersed bovine pituitary cells. FEBS Lett. 1976 Sep 15;68(1):23–26. doi: 10.1016/0014-5793(76)80395-x. [DOI] [PubMed] [Google Scholar]
- Bower A., Hadley M. E. Ionic requirements for melanophore-stimulating hormone (MSH) release. Gen Comp Endocrinol. 1972 Aug;19(1):147–158. doi: 10.1016/0016-6480(72)90015-9. [DOI] [PubMed] [Google Scholar]
- Brandt B. L., Hagiwara S., Kidokoro Y., Miyazaki S. Action potentials in the rat chromaffin cell and effects of acetylcholine. J Physiol. 1976 Dec;263(3):417–439. doi: 10.1113/jphysiol.1976.sp011638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. D., Hadley M. E. Pars intermedia electrical potentials: changes in spike frequency induced by regulatory factors of melanocyte stimulating hormone (MSH) secretion. Neuroendocrinology. 1978;26(5):277–282. doi: 10.1159/000122783. [DOI] [PubMed] [Google Scholar]
- Douglas W. W. Stimulus-secretion coupling: the concept and clues from chromaffin and other cells. Br J Pharmacol. 1968 Nov;34(3):451–474. doi: 10.1111/j.1476-5381.1968.tb08474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W. Stimulus-secretion coupling: variations on the theme of calcium-activated exocytosis involving cellular and extracellular sources of calcium. Ciba Found Symp. 1978;(54):61–90. doi: 10.1002/9780470720356.ch4. [DOI] [PubMed] [Google Scholar]
- Douglas W. W., Taraskevich P. S. Action potentials in gland cells of rat pituitary pars intermedia: inhibition by dopamine, an inhibitor of MSH secretion. J Physiol. 1978 Dec;285:171–184. doi: 10.1113/jphysiol.1978.sp012565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman A. L., Hermann A. Internal effects of divalent cations on potassium permeability in molluscan neurones. J Physiol. 1979 Nov;296:393–410. doi: 10.1113/jphysiol.1979.sp013012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S. Ca spike. Adv Biophys. 1973;4:71–102. [PubMed] [Google Scholar]
- Hagiwara S. Ca-dependent action potential. Membranes. 1975;3:359–381. [PubMed] [Google Scholar]
- Hagiwara S. Differentiation of Na and Ca channels during early development. Soc Gen Physiol Ser. 1979;33:189–197. [PubMed] [Google Scholar]
- Katz B., Miledi R. Tetrodotoxin-resistant electric activity in presynaptic terminals. J Physiol. 1969 Aug;203(2):459–487. doi: 10.1113/jphysiol.1969.sp008875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidokoro Y. Spontaneous calcium action potentials in a clonal pituitary cell line and their relationship to prolactin secretion. Nature. 1975 Dec 25;258(5537):741–742. doi: 10.1038/258741a0. [DOI] [PubMed] [Google Scholar]
- Ozawa S., Miyazaki S. I. Electrical excitability in the rat clonal pituitary cell and its relation to hormone secretion. Jpn J Physiol. 1979;29(4):411–426. doi: 10.2170/jjphysiol.29.411. [DOI] [PubMed] [Google Scholar]
- Ozawa S., Sand O. Electric activity of rat anterior pituitary cells in vitro. Acta Physiol Scand. 1978 Mar;102(3):330–341. doi: 10.1111/j.1748-1716.1978.tb06080.x. [DOI] [PubMed] [Google Scholar]
- Reuter H. Divalent cations as charge carriers in excitable membranes. Prog Biophys Mol Biol. 1973;26:1–43. doi: 10.1016/0079-6107(73)90016-3. [DOI] [PubMed] [Google Scholar]
- Ritchie J. M., Rogart R. B. The binding of saxitoxin and tetrodotoxin to excitable tissue. Rev Physiol Biochem Pharmacol. 1977;79:1–50. doi: 10.1007/BFb0037088. [DOI] [PubMed] [Google Scholar]
- Taraskevich P. S., Douglas W. W. Action potentials occur in cells of the normal anterior pituitary gland and are stimulated by the hypophysiotropic peptide thyrotropin-releasing hormone. Proc Natl Acad Sci U S A. 1977 Sep;74(9):4064–4067. doi: 10.1073/pnas.74.9.4064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taraskevich P. S., Douglas W. W. Catecholamines of supposed inhibitory hypophysiotrophic function suppress action potentials in prolactin cells. Nature. 1978 Dec 21;276(5690):832–834. doi: 10.1038/276832a0. [DOI] [PubMed] [Google Scholar]
- Taraskevich P. S., Douglas W. W. Electrical behaviour in a line of anterior pituitary cells (GH cells) and the influence of the hypothalamic peptide, thyrotrophin releasing factor. Neuroscience. 1980;5(2):421–431. doi: 10.1016/0306-4522(80)90117-7. [DOI] [PubMed] [Google Scholar]
- Taraskevich P. S., Douglas W. W. Stimulant effect of 5-hydroxytryptamine on action potential activity in pars intermedia cells of the lizard Anolis carolinensis: contrasting effects in pars intermedia of rat and rostral pars distalis of fish (Alosa pseudoharengus). Brain Res. 1979 Dec 14;178(2-3):584–588. doi: 10.1016/0006-8993(79)90719-4. [DOI] [PubMed] [Google Scholar]
- Vale W., Rivier C., Brown M. Regulatory peptides of the hypothalamus. Annu Rev Physiol. 1977;39:473–527. doi: 10.1146/annurev.ph.39.030177.002353. [DOI] [PubMed] [Google Scholar]


