Abstract
The combination of an oral artemisinin derivative (usually artesunate) and mefloquine has become standard treatment for multidrug-resistant falciparum malaria in several parts of Southeast Asia. The doses of artesunate used in monotherapy and combination treatment have largely been derived empirically. In order to characterize the in vivo dose-response relationship for artesunate and thus rationalize dosing, 47 adult patients with acute uncomplicated falciparum malaria and parasitemia ≥1% were randomized to receive a single oral dose of artesunate varying between 0 and 250 mg together with a curative dose of oral mefloquine. Acceleration of parasite clearance was used as the pharmacodynamic variable. An inhibitory sigmoidal maximum effect (Emax) pharmacodynamic model typical of a dose-response curve was fitted to the relationship between dose and shortening of parasite clearance time (PCT). The Emax was estimated as 28.6 oral h, and the 50% effective concentration was 1.6 mg/kg of body weight. These results imply that there is no reduction in PCTs with the use of single doses of artesunate higher than 2 mg/kg, and this therefore reflects the average lower limit of the maximally effective dose.
The combination of oral artesunate, a water-soluble artemisinin derivative, and mefloquine is used increasingly in the treatment of uncomplicated multidrug-resistant falciparum malaria infections in Southeast Asia. The addition of artesunate accelerates the therapeutic response, reduces the rate of recrudescence, reduces transmissibility, prevents the emergence of resistance, increases patient compliance, and also provides a treatment that is highly effective in uncomplicated hyperparasitemic patients and in recrudescent infections (8, 13, 14, 15).
The combination of artesunate and mefloquine has recently become the treatment of choice for uncomplicated multidrug-resistant falciparum malaria in several countries in Southeast Asia. The total dose of artesunate used in current recommended regimens usually does not exceed 14 mg/kg of body weight. If used alone, artesunate needs to be given for 7 days for acceptable cure rates. These regimens were adapted from the early Chinese dose-finding studies and are not based on any pharmacokinetic or dynamic evidence.
There has been concern that the artemisinin derivatives induce specific central nervous system toxicity in animals. This has been described in primates, dogs, and rodents when the lipid-soluble artemisinin derivatives, artemether and arteether are given at high doses (>15 mg/kg/day) parenterally (2, 11, 12). The distribution of these pathological lesions is unusual in that it is specific for neurons in the pontine vestibular system. Toxic effects are also demonstrable in vitro in neuronal cell lines (19). Fortunately there has been no evidence of clinically demonstrable neurological damage in practice even in large studies which have enrolled more than 3,000 patients and in studies of brain stem auditory-evoked potentials in patients who have received multiple doses of artemisinin derivatives (18). Use of these drugs is increasing rapidly as it becomes accepted increasingly that artemisinin combinations offer the possibility of providing sustained high efficacy, preventing the emergence of drug resistance, and, in low-transmission areas, reducing malaria incidence (21). The affordability of these drugs is a major determinant of their potential global use. There is therefore an urgent need for dose optimization, both to reduce the potential for toxicity and to limit costs.
MATERIALS AND METHODS
Patients.
The study was performed in Sangklaburi District Hospital, Sangklaburi, Kanchanaburi Province, Thailand, in 1993 and 1994. This is a small 30-bed government hospital on the Thai-Burmese border serving a large population of Mon displaced persons as well as the local town. Malaria transmission and epidemiology are similar to those described further north on the border for the Karen population (7). At the time of the study, Plasmodium falciparum in the area was generally mefloquine sensitive. This study was approved by the Ethical and Scientific Review Subcommittee of the Thai Ministry of Public Health.
Methods.
Patients were enrolled into the study if they had microscopically confirmed infection with asexual forms of P. falciparum in peripheral blood smears (a parasitemia of >1%) but none of the World Health Organization criteria for the diagnosis of severe malaria and gave fully informed consent to the study procedure. Patients with a history of significant quinine (>2-g), artesunate, or mefloquine intake within the past 24 h; those with a history of hypersensitivity to any of the study drugs; pregnant women; and children under the age of 14 years were excluded. A quinine dipstick was used as described previously to exclude quinine intake (16).
Drug and sampling regimens.
On admission a full history was taken, a full examination was performed, and the patient was weighed. A 20-gauge Teflon intravenous cannula was inserted into a forearm vein, and a baseline blood sample drawn for a quantitative parasite count and parasite staging. The parasite counts and axillary temperature were measured at 0, 4, 8, 12, 16, 20, and 24 h posttreatment and then every 6 h until clearance of the parasites (defined as <1 parasite per 200 white blood cell nuclei on a thick film). The hematocrit was measured every 12 h. The microscopist was blinded to the treatment group.
Patients were randomized by use of a computer generated randomization table (in groups of 10) to receive a single oral dose of either 0, 25, 50, 75, 100, 150, 200, or 250 mg of artesunate (Guilin No. 1 Factory, Guangxi, People's Republic of China), i.e., from half a tablet to five tablets. The randomization code was revealed after patients had been admitted to the study, and the analysis was on an intention-to-treat basis. Patients were also given oral paracetamol (15 mg/kg), and after 1 h they were given a single oral dose of mefloquine (15 mg/kg; Mephaquine, Mepha, Basel, Switzerland). After 12 h they were given a further 10-mg/kg dose of mefloquine (total dose, 25 mg of base/kg). A single blood sample for a drug level was taken after 2 h. All patients were followed up for 28 days.
Pharmacodynamic measures of outcome.
The proportional change in parasitemia was plotted against time, and the times taken to clear 50% of the parasitemia (PC50) and PC90 and the parasite clearance time (PCT) were measured graphically. Clinical response was measured by fever clearance, defined as the time for the axillary temperature to remain below 37.5°C. These values were compared between the mefloquine-only groups and the treatment groups, as well as using the drug dosage per kilogram of body weight as a continuous variable. Statistical analysis was performed using Statview 4.1 (1994 release; Abacus Concepts Inc.). Variables were normally distributed except for parasitemia, which was logarithmically transformed before analysis. Modeling of the pharmacodynamic data was done using WinNonlin (version 1.1; Statistical Consultants Inc.) using the inhibitory effect models with baseline effect parameters. The model was chosen a priori as likely to be the most appropriate. The minimization techniques were the same for each variable and the Aikake information criteria are shown for comparison in Table 3.
TABLE 3.
Modeled relationships between outcome measures and drug dose
| Parameters | E0 (h) (SE) | EC50 (SE) (mg/kg) | Emax (h) (SE) | γ (SE) | AICa |
|---|---|---|---|---|---|
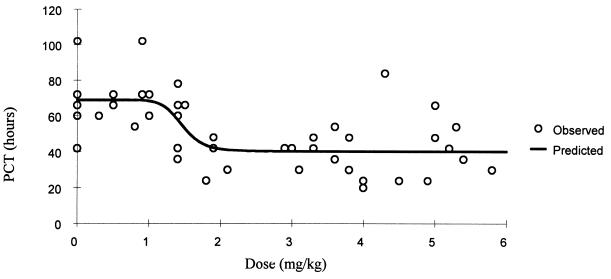
| PCT + dose (mg/kg) | 68.9 (4.2) | 1.46 (0.15) | 28.6 (3.5) | 10.0 (14.3) | 422 |
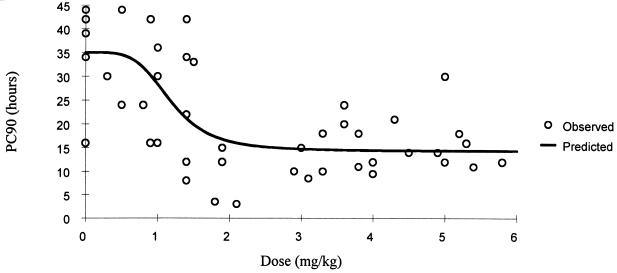
| PC90 + dose (mg/kg) | 35.0 (2.9) | 1.18 (0.21) | 20.7 (2.2) | 4.2 (2.8) | 391 |
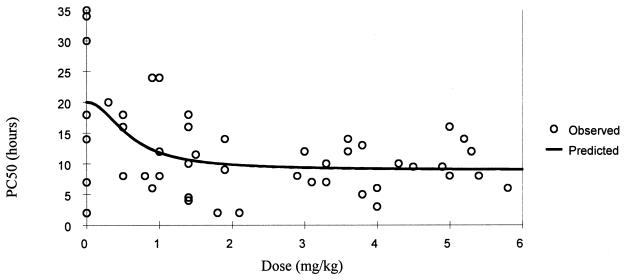
| PC50 + dose (mg/kg) | 20.0 (2.6) | 0.60 (0.34) | 11.0 (2.1) | 2.0 (2.3) | 366 |
Aikake information criterion (model 108).
Drug assays.
Immediately after they were taken, blood samples were centrifuged and the plasma was removed and stored at −50°C for up to a month and then at −80°C for <48 months until assay. Plasma samples were analyzed by high-performance liquid chromatography with electrochemical detection in the reductive mode for separate quantification of artesunate and dihydroartemisinin (DHA) (9). The lower limit of detection of high-performance liquid chromatography-electrochemical detection was 4 ng/ml for both artesunate and DHA, and interassay coefficients of variation were 3.1% for artesunate and 5.9% for DHA at concentrations of 30 and 60 ng/ml, respectively.
RESULTS
Clinical responses.
Forty-seven patients between 15 and 71 years old were enrolled. No patients vomited the tablets or developed severe manifestations of falciparum malaria. All completed the 3 days of medication, and 35 (75%) returned for follow-up at 28 days. There were no treatment failures or recrudescences. All patients completed the drug protocol. Three patients left hospital before completing parasite clearance and are not included in the parasitological outcomes. The clinical and laboratory variables are shown in Table 1.
TABLE 1.
Clinical and laboratory findings on admission
| Finding | Mean (SD) |
|---|---|
| Age (yr) | 23.4 (13.4) |
| Wt (kg) | 42.9 (14.2) |
| Temp (°C) | 38.5 (1.1) |
| Hematocrit (%) | 37.4 (5.6) |
| Parasite count (no./μl) | 103,359a (5,401-598,856)b |
| Total serum bilirubin (mg/dl) | 2.5 (2.8) |
| Serum creatinine (mg/dl) | 1.2 (0.3) |
| Plasma lactate (mmol/liter) | 2.4 (0.9) |
| Serum albumin (g/dl) | 4.0 (0.6) |
Geometric mean.
Range.
Pharmacological responses.
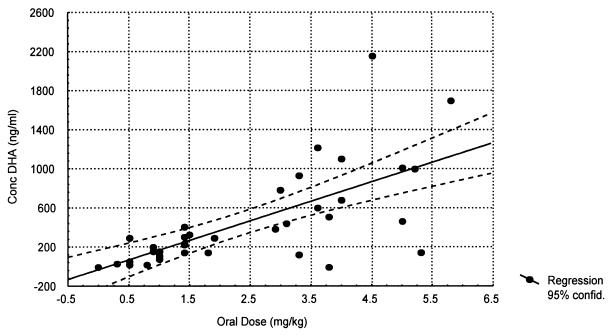
The relationship between dose, measured drug concentrations of artesunate and the biologically active metabolite DHA, and parasite clearance are shown in Table 2 and Fig. 1. A sigmoid inhibitory effect model with the general form E = Emax × Cγ/(Cγ + ECγ50) − E0 where E0 is the baseline effect, C is the dose or concentration, Emax is the maximum effect, γ is the slope coefficient, and EC50 is the dose concentration producing 50% of the maximum effect—was fitted to these individual responses (Table 3). The effect (E) is shortening of parasite clearance. The relationships are shown in Fig. 2 to 4. There was considerable variance in the PC50s and PC90s with mefloquine alone or with low doses of artesunate. A better fit was obtained with the dose-PCT relationship. ED50s for the patient population were 0.7, 1.2, and 1.4 mg/kg for PC50, PC90, and PCT, respectively. However, the extrapolated lowest-dose estimates for Emax were similar for all three variables at approximately 2 mg/kg.
TABLE 2.
Clinical and parasitological outcome measures and drug concentrations
| Dose (mg) | No. of patients | Geometric mean parasitemia/μl (range) | Mean PC50 (SD) h | Mean PC90 (SD) h | Mean PCT (SD) h | Mean FCTa (SD) h | Median 2-h concn (ng/ml) (range) of:
|
|
|---|---|---|---|---|---|---|---|---|
| DHA | Artesunate | |||||||
| 0 | 8 | 87,293 (15,825-215,529) | 20 (13.3) | 35 (9.3) | 68 (19.6) | 53 (12.1) | ||
| 25 | 5 | 162,114 (81,137-597,856) | 17 (5.9) | 35 (8.7) | 66 (4.9) | 40 (29.0) | 49 (292-22) | 0 (12.6-0) |
| 50 | 7 | 162,681 (40,192-288,880) | 9 (7.3) | 20 (12.6) | 61 (24.8) | 37 (18.2) | 158 (303-18) | 0 (38-0) |
| 75 | 6 | 101,485 (47,476-249,818) | 12 (4.8) | 26 (12.4) | 59 (14.9) | 40 (24.5) | 230 (407-121) | 3.0 (18.0-0) |
| 100 | 7 | 93,692 (5,400-575,876) | 9 (3.9) | 10 (4.4) | 42 (9.2) | 21 (19.5) | 385 (1,012-147) | 0 (214-0) |
| 150 | 5 | 184,919 (106,19-628,000) | 12 (3.2) | 18.6 (7.4) | 47 (11.5) | 25 (16.9) | 784 (1,212-0) | 0 (112-0) |
| 200 | 6 | 87,586 (13,941-362,984) | 8.5 (3.6) | 15 (5.5) | 39 (22.9) | 25 (19.1) | 640 (2,155-512) | 21 (52-0) |
| 250 | 3 | 83,272 (53,882-135,648) | 9 (4.6) | 14 (3.5) | 32 (9.2) | 24 (0) | 1,098 (1,698-1,000) | 0 (0-0) |
FCT, fever clearance time.
FIG.1.
Relationship of oral artesunate dose to the 2-h concentration of DHA in plasma (r = 0.70).
FIG. 2.
Relationship of oral artesunate dose to PC50.
FIG. 4.
Relationship of oral artesunate dose to PCT.
DISCUSSION
Drug dosage regimens are usually based on studies of their pharmacokinetic and pharmacodynamic properties. The objective of treatment in uncomplicated malaria is to provide a rapid resolution of symptoms and cure of the infection (i.e., prevention of recrudescence) with a minimum of adverse effects. The artemisinin derivatives are absorbed and eliminated very rapidly (1, 10). Terminal elimination half-lives of the orally administered drugs are usually less than 2 h. This compares with an asexual life cycle of the human malaria parasites of 2 days (or 3 days in the case of Plasmodium malariae). Previous studies have shown that once-daily administration with the artemisinin derivatives provides equivalent cure rates to more-frequent administration (4, 6). This suggests that exposure of the infecting parasite population to therapeutic concentrations of drug twice each life cycle is sufficient for maximum effect (20). Despite the excellent intrinsic activity of these compounds, which produce reductions in asexual parasite biomass of up to 10,000-fold per cycle, these drugs still need to be present in therapeutic concentrations during four asexual life cycles, or 8 days. Shorter courses of treatment are associated with higher recrudescence rates. However, in order to protect these compounds from resistance and to optimize antimalarial therapy, they are often used in combination with a slower-acting antimalarial drug—usually mefloquine (21). In the initial dose-finding studies conducted in China a single dose-response relationship was not determined, as both dose and duration of treatment were varied at the same time. In this study a single dose-response relationship was determined in which the effect parameter was acceleration of parasite clearance. The artemisinin derivatives affect the circulating ring stages of the malaria parasites and produce a consistent acceleration in circulating parasite clearance through removal of drug-affected circulating parasites (5). This translates into a more rapid reduction in parasitemia. Thus, the shortening of parasite clearance relative to a second antimalarial drug, in this case mefloquine, provides a measure of antimalarial activity.
In an individual patient several factors determine the profile of parasite clearance. The stage and synchronicity of the infecting parasite population and the multiplication rate of sequestered mature stages (schizonts) determine the initial parasitemia time profile after drug administration (20). If the patient is admitted to hospital at a time when a significant proportion of the parasites are undergoing schizogony, then parasitemia will rise following antimalarial drug treatment as schizonts rupture and release merozoites, which then invade new erythrocytes to produce circulating ring stages. These stages can be seen by the microscopist and counted, whereas the sequestered stages are not seen and therefore are not counted. On the other hand, if the majority of parasites are approaching the latter half of the asexual cycle, then, in the case of P. falciparum, they will be expressing red blood cell surface adhesins and sequestering in the microcirculation. Parasitemia will fall. If the majority of the parasites in the blood are young rings, and a drug which has little effect on the ring stage is used in treatment (such as mefloquine, quinine, or sulfadoxine-pyrimethamine), then parasitemia will plateau and fall later as a result of sequestration (17, 20). The overall individual parasitemia profile thus represents a hybrid of these different processes, and a population of patients provides a series of very different parasitemia profiles. As a result there is considerable interindividual variance in parasite clearance profiles, although it is reasonable to assume that the distribution of parasite stages should be random in a patient population as there is no particular reason why a patient should present to hospital at one time in parasite development compared with another. Thus, even before attempting to assess the effect of the drug in vivo, there is considerable interpatient variance in the pharmacodynamic variables.
The effects of the antimalarial drug will be determined by absorption, distribution, and elimination, which also vary considerably between individuals, and also to some extent the susceptibility profile of the infecting malaria parasite population (or populations). In fact, in vitro profiles of sensitivity to artesunate and DHA for P. falciparum in Thailand show a relatively narrow range of sensitivities, and this is unlikely to be a significant source of variance (3). It is not known which pharmacokinetic parameter is the most important determinant of pharmacodynamic effect for the artemisinin compounds, i.e., whether peak concentration, area under the concentration-time curve, or time above a putative MIC is the most important. Further studies will be needed to characterize the concentration-effect relationships in vivo with these compounds.
In these studies a classic sigmoid dose-response relationship was evident with maximum effects using the three prospectively defined parameters at a dosage of 2 mg of artesunate/kg or more. The PC50 and PC90 times corresponded to a value less than or equal to the dosage interval, whereas PCTs were longer than the dosage interval and therefore potentially confounded by the subsequent mefloquine treatment. Nevertheless, the profiles produced with the three parameters were similar. This suggests that a dosage of 2 mg/kg would be the lowest to give maximum effects in an “average” patient. However, taking into account the considerable variance between individuals in both pharmacokinetics and pharmacodynamics, a larger dose would be required to ensure that a maximum effect was obtained in all patients. This is particularly important for the prevention of the emergence of resistance. The artemisinin compounds have been extremely well tolerated, and to date there is no convincing evidence that they have significant toxicity. The current practice in combination treatments is to use a dose of 4 mg of artesunate/kg daily for 3 days (13). These data suggest that this dose offers a satisfactory margin of safety and would be unlikely to produce a submaximal antimalarial effect. Further studies should now be conducted to characterize the relationship between pharmacokinetic parameters and shortening of parasite clearance as an effect measure.
FIG. 3.
Relationship of oral artesunate dose to PC90.
Acknowledgments
This study was part of the Wellcome Trust Mahidol University Oxford Tropical Medicine Research Programme funded by the Wellcome Trust of Great Britain.
We are grateful to the Dean of the Faculty of Tropical Medicine at the time of this study, Tan Chongsuphajaisiddhi, for his support to and the medical and nursing staff of Sangklaburi Hospital for their help with this study. We also thank Impone Vangkonevilay, Jamie and Sarah Craig, and Juanita de Lope for their help with the clinical work. We are very grateful to V. Navaratnam and S. Mansor for conducting the drug assays and to Melba Gomes (WHO TDR) for supporting this.
REFERENCES
- 1.Batty, K. T., L. T. Thu, T. M. E. Davis, K. F. Ilett, T. X. Mai, N. C. Hung, N. P. Tien, S. M. Powell, H. V. Thien, T. Q. Binh, and N. V. Kim. 1998. A pharmacokinetic and pharmacodynamic study of intravenous vs oral artesunate in uncomplicated falciparum malaria. Br. J. Clin. Pharmacol. 45:123-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brewer, T. G., J. O. Peggins, S. J. Grate, J. M. Petras, B. S. Levine, P. J. Weina, J. Swearengen, M. H. Heiffer, and B. G. Schuster. 1994. Neurotoxicity in animals due to arteether and artemether. Trans. R. Soc. Trop. Med. Hyg. 88(Suppl. 1):S33-S36. [DOI] [PubMed] [Google Scholar]
- 3.Brockman, A., R. N. Price, M. van Vugt, D. G. Heppner, D. Walsh, P. Sookto, T. Wimonwattrawatee, S. Looareesuwan, N. J. White, and F. Nosten. 2000. Plasmodium falciparum antimalarial drug susceptibility on the northwestern border of Thailand during five years of extensive artesunate-mefloquine use. Trans. R. Soc. Trop. Med. Hyg. 94:537-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bunnag, D., C. Viravan, S. Looareesuwan, J. Karbwang, and T. Harinasuta. 1991. Double blind randomised clinical trial of oral artesunate at once or twice daily dose in falciparum malaria. Southeast Asian J. Trop. Med. Public Health 22:539-543. [PubMed] [Google Scholar]
- 5.Chotivanich, K., R. Udomsangpetch, A. Dondorp, T. Williams, B. Angus, J. A. Simpson, S. Pukrittayakamee, S. Looareesuwan, C. I. Newbold, and N. J. White. 2000. The mechanisms of parasite clearance after antimalarial treatment of Plasmodium falciparum malaria. J. Infect. Dis. 182:629-633. [DOI] [PubMed] [Google Scholar]
- 6.Luxemburger, C., F. O. ter Kuile, F. Nosten, G. Dolan, J. H. Bradol, L. Phaipun, T. Chongsuphajaisiddhi, and N. J. White. 1994. Single day mefloquine-artesunate combination in the treatment of multi-drug resistant falciparum malaria. Trans. R. Soc. Trop. Med. Hyg. 88:213-217. [DOI] [PubMed] [Google Scholar]
- 7.Luxemburger, C., K. L., Thwai, N. J. White, H. K. Webster, D. E. Kyle, L. Maelankirri, T. Chongsuphajaisiddhi, and F. Nosten. 1996. The epidemiology of malaria in a Karen population on the western border of Thailand. Trans. R. Soc. Trop. Med. Hyg. 90:105-111. [DOI] [PubMed] [Google Scholar]
- 8.Luxemburger, C., F. Nosten, S. D. Raimond, T. Chongsuphajaisiddhi, and N. J. White. 1995. Oral artesunate in the treatment of uncomplicated hyperparasitemic falciparum malaria. Am. J. Trop. Med. Hyg. 53:522-525. [DOI] [PubMed] [Google Scholar]
- 9.Navaratnam, V., M. N. Mordi, and S. M. Mansor. 1997. Simultaneous determination of artesunic acid and dihydroartemisinin in blood plasma by high performance liquid chromatography for application in clinical pharmacological studies. J. Chromatogr. B 69:157-162. [DOI] [PubMed] [Google Scholar]
- 10.Newton, P., Y. Suputtamongkol, P. Teja-Isavadharm, S. Pukrittayakamee, V. Navaratnam, I. Bates, and N. White. 2000. Antimalarial bioavailability and disposition of artesunate in acute falciparum malaria. Antimicrob. Agents Chemother. 44:972-977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nontprasert, A., M. Nosten-Bertrand, S. Pukrittayakamee, S. Vanijanonta, B. J. Angus, and N. J. White. 1998. Assessment of the neurotoxicity of parenteral artemisinin derivatives in mice. Am. J. Trop. Med. Hyg. 59:519-522. [DOI] [PubMed] [Google Scholar]
- 12.Nontprasert, A., S. Pukrittayakamee, M. Nosten-Bertrand, S. Vanijanonta, and N. J. White. 2000. Studies of the neurotoxicity of oral artemisinin derivatives in mice. Am. J. Trop. Med. Hyg. 62:409-412. [DOI] [PubMed] [Google Scholar]
- 13.Nosten, F., C. Luxemburger, F. O. ter Kuile, C. Woodrow, J. P. Eh, T. Chongsuphajaisiddhi, and N. J. White. 1994. Treatment of multidrug-resistant Plasmodium falciparum malaria with 3-day artesunate-mefloquine combination. J. Infect. Dis. 170:971-977. [DOI] [PubMed] [Google Scholar]
- 14.Nosten, F., M. van Vugt, R. Price, C. Luxemburger, K. L. Thway, A. Brockman, R. McGready, F. ter Kuile, S. Looareesuwan, and N. J. White. 2000. Effects of artesunate-mefloquine combination on incidence of Plasmodium falciparum malaria and mefloquine resistance in western Thailand: a prospective study. Lancet 356:297-302. [DOI] [PubMed] [Google Scholar]
- 15.Price, R. N., F. Nosten, C. Luxemburger, A. Kham, A. Brockman, T. Chongsuphajaisiddhi, and N. J. White. 1995. Artesunate versus artemether in combination with mefloquine for the treatment of multidrug-resistant falciparum malaria. Trans. R. Soc. Trop. Med. Hyg. 89:523-527. [DOI] [PubMed] [Google Scholar]
- 16.Silamut, K., R. Hough, T. Eggelte, S. Pukrittayakamee, B. Angus, and N. J. White. 1995. A simple method for assessing quinine pre-treatment in acute malaria. Trans. R. Soc. Trop. Med. Hyg. 89:665-667. [DOI] [PubMed] [Google Scholar]
- 17.ter Kuile, F., N. J. White, P. Holloway, G. Pasvol, and S. Krishna. 1993. Plasmodium falciparum: in vitro studies of the pharmacodynamic properties of drugs used for the treatment of severe malaria. Exp. Parasitol. 76:85-95. [DOI] [PubMed] [Google Scholar]
- 18.Van Vugt, M., B. J. Angus, R. N. Price, C. Mann, J. A. Simpson, C. Poletto, S. E. Htoo, S. Looareesuwan, N. J. White, and F. Nosten. 2000. A case-control auditory evaluation of patients treated with artemisinin derivatives for multidrug-resistant Plasmodium falciparum malaria. Am. J. Trop. Med. Hyg. 62:65-69. [DOI] [PubMed] [Google Scholar]
- 19.Wesche, D. L., M. A. DeCoster, F. C. Tortella, and T. G. Brewer. 1994. Neurotoxicity of artemisinin analogs in vitro. Antimicrob. Agents Chemother. 38:1813-1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.White, N. J. 1997. Assessment of the pharmacodynamic properties of antimalarial drugs in vivo. Antimicrob. Agents Chemother. 41:1413-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.White, N. J., F. Nosten, S. Looareesuwan, W. M. Watkins, K. Marsh, R. W. Snow, G. Kokwaro, J. Ouma, T. T. Hien, M. E. Molyneux, T. Taylor, C. I. Newbold, T. K. Ruebush II, M. Danis, B. M. Greenwood, R. M. Anderson, and P. Olliaro. 1999. Averting a malaria disaster. Lancet 353:1965-1967. [DOI] [PubMed] [Google Scholar]