Abstract
The pharmacokinetics, excretion, and mass balance of liposomal amphotericin B (AmBisome) (liposomal AMB) and the conventional formulation, AMB deoxycholate (AMB-DOC), were compared in a phase IV, open-label, parallel study in healthy volunteers. After a single 2-h infusion of 2 mg of liposomal AMB/kg of body weight or 0.6 mg of AMB-DOC/kg, plasma, urine, and feces were collected for 168 h. The concentrations of AMB were determined by liquid chromatography tandem mass spectrometry (plasma, urine, feces) or high-performance liquid chromatography (HPLC) (plasma). Infusion-related side effects similar to those reported in patients, including nausea and back pain, were observed in both groups. Both formulations had triphasic plasma profiles with long terminal half-lives (liposomal AMB, 152 ± 116 h; AMB-DOC, 127 ± 30 h), but plasma concentrations were higher (P < 0.01) after administration of liposomal AMB (maximum concentration of drug in serum [Cmax], 22.9 ± 10 μg/ml) than those of AMB-DOC (Cmax, 1.4 ± 0.2 μg/ml). Liposomal AMB had a central compartment volume close to that of plasma (50 ± 19 ml/kg) and a volume of distribution at steady state (Vss) (774 ± 550 ml/kg) smaller than the Vss of AMB-DOC (1,807 ± 239 ml/kg) (P < 0.01). Total clearances were similar (approximately 10 ml hr−1 kg−1), but renal and fecal clearances of liposomal AMB were 10-fold lower than those of AMB-DOC (P < 0.01). Two-thirds of the AMB-DOC was excreted unchanged in the urine (20.6%) and feces (42.5%) with >90% accounted for in mass balance calculations at 1 week, suggesting that metabolism plays at most a minor role in AMB elimination. In contrast, <10% of the liposomal AMB was excreted unchanged. No metabolites were observed by HPLC or mass spectrometry. In comparison to AMB-DOC, liposomal AMB produced higher plasma exposures and lower volumes of distribution and markedly decreased the excretion of unchanged drug in urine and feces. Thus, liposomal AMB significantly alters the excretion and mass balance of AMB. The ability of liposomes to sequester drugs in circulating liposomes and within deep tissue compartments may account for these differences.
The treatment of systemic fungal infections remains challenging, especially in neutropenic patients. Recent advances in drug delivery technology have resulted in several lipid-based formulations of amphotericin B, an effective but toxic fungicidal drug (5). Liposomal amphotericin B (AmBisome) is a liposomal formulation consisting of amphotericin B in small, unilamellar vesicles. Liposomal amphotericin B is safer than conventional amphotericin B (amphotericin B deoxycholate) (6, 12, 33) and is currently indicated for the treatment of disseminated fungal infections and visceral leishmaniasis and for empirical therapy for febrile neutropenia. The unique therapeutic properties of liposomal amphotericin B may result from its ability to alter the disposition of amphotericin B in the body (5). Studies in animals and humans have shown that liposomal amphotericin B produces higher drug levels in plasma than other formulations as well as high levels of amphotericin B in tissue (7, 9, 11, 25, 32). This is thought to result from the unique composition of the liposomal amphotericin B liposomes, which contain rigid, charged phospholipids and cholesterol to retain amphotericin B within the bilayer membranes of the circulating liposomes (1). However, the amphotericin B in liposomal amphotericin B and other lipid amphotericin B formulations does reach fungal cells (1), lipoproteins (35), and other sites within the body. To understand the therapeutic properties of liposomal amphotericin B, and evaluate potential differences between it and other amphotericin B formulations, it is important to determine how liposomal amphotericin B alters the disposition of amphotericin B.
Although the plasma pharmacokinetics of conventional amphotericin B (amphotericin B deoxycholate) and liposomal amphotericin B have been studied, the tissue distribution, metabolism, and excretion of these formulations remain poorly characterized in humans. We therefore conducted a phase IV study in healthy human volunteers to define and compare the disposition of liposomal amphotericin B and amphotericin B deoxycholate. In this report, the plasma pharmacokinetics, urinary excretion, fecal excretion, and mass balance of liposomal amphotericin B are compared to those of amphotericin B deoxycholate.
MATERIALS AND METHODS
Subjects and protocol.
This was a single-dose, open-label, randomized parallel comparison of liposomal amphotericin B and amphotericin B deoxycholate given by intravenous infusion. The study was conducted at Harris Laboratories, Lincoln, Nebr., after approval by the Institutional Review Board. Informed consent was given by all participants. Subjects were healthy, nonsmoking adults, who were within 15% of their ideal body weights and without medical abnormalities. Pregnant or lactating females were excluded. Subjects underwent a prestudy screening evaluation, which included medical history, physical examination, 12-lead electrocardiogram, clinical chemistry and hematology, urinalysis, pregnancy test, drug screen, and vital signs. Subjects were confined to the study site overnight prior to dosing and for 96 h after dosing and were not allowed to consume alcohol or caffeine or to take any medication during the study. Subjects were fed a standardized low-fat breakfast at 0700 h, approximately 1 h prior to the start of drug infusion. Prior to administration, subjects were premedicated with acetaminophen ER (650 mg) and diphenhydramine HCl (50 mg). Liposomal amphotericin B-treated subjects received [14C]cholesterol liposomal amphotericin B (NeXstar Pharmaceuticals, San Dimas, Calif.) as a single 2-h infusion (2 mg of amphotericin B/kg of body weight, 1 μCi of 14C/kg). Amphotericin B deoxycholate-treated subjects received amphotericin B (Fungizone; Apothecon, Princeton, N.J.) as a single 2-h infusion (0.6 mg of amphotericin B/kg). Amphotericin B deoxycholate-treated subjects also received an intravenous test dose of amphotericin B (1 mg) 1 day prior to the study dose with premedication as described above. The disposition of the [14C]cholesterol component and the pharmacokinetics of unbound amphotericin B are reported separately (8; I. Bekersky, D. Dressler, R. M. Fielding, D. N. Buell, and T. J. Walsh, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 856, p. 26, 2000).
Sampling of plasma, urine, and feces.
Venous blood was collected in EDTA tubes prior to and at 0.5, 1, 2, 2.25, 2.5, 3, 4, 6, 8, 10, 18, 24, 36, 60, 84, 96, 120, 144, and 168 h after the start of the 2-h infusion. Plasma was separated by centrifugation and stored frozen at −20°C until analyzed. Urine and feces were collected at 24-h intervals throughout the 1-week study (urine was also collected at 4, 8, and 12 h on day 1). At the end of each collection period, the total volume of urine was recorded, and aliquots were frozen at −20°C for analysis. At the end of each period, collected feces were weighed and homogenized in water. Aliquots of fecal homogenate were frozen at −20°C for analysis. Total amphotericin B in urine and feces and in plasma samples with concentrations of >2 μg/ml was measured by liquid chromatography tandem mass spectrometry (LC/MS/MS). Plasma amphotericin B concentrations of <2 μg/ml were measured by high-performance liquid chromatography (HPLC).
LC/MS/MS assays.
Analysis of amphotericin B was performed using an LC/MS/MS method as previously described (26). All samples for LC/MS/MS analysis were spiked with an internal standard (natamycin; USP). Urine and fecal homogenates were diluted with an equal volume of blank human plasma prior to analysis. All samples were then treated with 16% dimethyl sulfoxide in methanol to release liposomal amphotericin B. Treated plasma was diluted in water and injected into the LC/MS/MS system. Treated urine and fecal samples were diluted in water, placed onto a solid-phase extraction column (Isolute C2 EC; Jones Chromatography, Lakewood, Colo.), washed with 10% methanol, and eluted with 100% methanol. Eluents were dried, reconstituted in methanol, and injected into the LC/MS/MS system. Samples were separated on a C18 reverse-phase column (5-μm particle size, 3-mm internal diameter, 150 mm long; Symmetry; Waters Corp., Milford, Mass.) by using a mobile phase of methanol-water-acetic acid (69:29:2) and detected by using a PE Sciex API tandem mass spectrometer with a turbo ion spray interface in positive-ion mode. The analyte and internal standard molecular ions (m/z, 924 and 666, respectively) were fragmented by collision-induced dissociation in the second quadrupole to produce ions (m/z, 906 and 503, respectively) which were selected in the third quadrupole. The validated LC/MS/MS assay ranges were 2 to 150 μg/ml for plasma, 0.05 to 30 μg/ml for urine, and 0.4 to 40 μg/ml for feces. Interday and intraday accuracies were within ±8% for plasma, ±20% for urine, and ±14% for feces. Interday and intraday precisions were <6% for plasma, <10% for urine, and <18% for feces. Recoveries from the blank matrix were 101 to 102% for plasma, 34 to 42% for urine, and 57 to 87% for feces over the range of the assay.
HPLC assay.
Plasma samples containing <2 μg of amphotericin B/ml (all samples from amphotericin B deoxycholate-treated subjects and samples from liposomal amphotericin B-treated subjects obtained after 10 to 36 h) were analyzed by a modified HPLC method (2). Plasma (0.2 ml) was deproteinized with methanol (0.3 ml), and samples of the supernatant were separated on a reverse-phase column (3-μm particle size, 4.6-mm internal diameter, by 150 mm long; Supelcosil ABZ+; Supelco, Inc., Bellefonte, Pa.) with a mobile phase of acetonitrile-0.05 N sodium acetate (34:66) delivered at a rate of 1.0 ml/min. Detection was done by measuring UV absorbance at 405 nm. Interday and intraday accuracies were within ±6.5% over the validated range of the assay (0.1 to 15 μg/ml). Interday and intraday precisions were ≤9% and <2.5%, and recovery from blank plasma was 66 to 77% over the assay range.
Pharmacokinetic and statistical analysis.
Pharmacokinetic parameters for total amphotericin B were determined by noncompartmental methods as previously described (32). In addition, postinfusion plasma concentrations were subjected to polyexponential curve fitting with a nonlinear least-squares technique (RSTRIP; MicroMath, Salt Lake City, Utah) to determine the number of phases present and their half-lives (half-life at α phase [t1/2α], β phase [t1/2β], and γ phase [t1/2γ]). The Akaike information criteria were used in fitting as a goodness-of-fit parameter. The volume of the central compartment (V1) was calculated as dose/C0, where C0 (the concentration at time zero after a bolus dose) was estimated from the intercepts of the postinfusion curve fit by the method of Loo and Riegelman (27). The volume of distribution during elimination (V) was calculated as CL/kel where CL is the plasma clearance and kel is the terminal elimination rate constant. The steady-state volume of distribution (Vss) was calculated as (dose × AUMC)/AUC2 where AUMC is the area under the first moment of the plasma curve and AUC is the area under the plasma concentration-versus-time curve over the collection interval. Renal clearance (CLR) was determined as Ae/AUC, where Ae was the amount excreted in the urine. The fraction of drug excreted unchanged in urine (Furine) was calculated as CLR/CL, where CL is the plasma clearance (dose/AUC0-∞). Fecal clearance (CLF) and the fraction of drug excreted unchanged in feces (Ffeces) were calculated similarly. The statistical significance of differences between the pharmacokinetic parameters of liposomal amphotericin B and amphotericin B deoxycholate was evaluated by using the unpaired two-sample t test with a significance level of 0.05 (two tailed). The t test for unequal variances was used in cases where the F test for variances showed group variances to be unequal. Due to the small number of females enrolled, data were analyzed without regard to gender.
RESULTS
Subjects.
Eleven subjects (8 males, 3 females) were enrolled in the study. One subject experienced syncope 10 min into the infusion and was withdrawn from the study prior to receiving a complete dose of liposomal amphotericin B. The remaining 10 subjects completed one of the two treatments and were evaluated (Table 1). While liposomal amphotericin B-treated subjects tended to be older, the treatment groups were matched with respect to gender, height, and weight. Infusion-related effects typical of those reported in patients receiving amphotericin B were observed in 5 of 5 subjects receiving 2 mg of liposomal amphotericin B/kg and in 3 of 5 subjects receiving 0.6 mg of amphotericin B deoxycholate/kg. These events, which included nausea, vomiting, flushing, lightheadedness, and somatic discomfort, were nonserious and self limiting.
TABLE 1.
Subject demographic characteristics by treatment group
| Characteristic | Value for group
|
|
|---|---|---|
| Liposomal amphotericin B | Amphotericin B deoxycholate | |
| Dose (mg/kg) | 2.0 | 0.6 |
| Male/female | 4/1 | 4/1 |
| Mean age ± SD (yr) | 49 ± 16 | 30 ± 5 |
| Mean height ± SD (cm) | 171 ± 10 | 175 ± 6 |
| Mean weight ± SD (kg) | 77 ± 9 | 79 ± 11 |
Plasma pharmacokinetics.
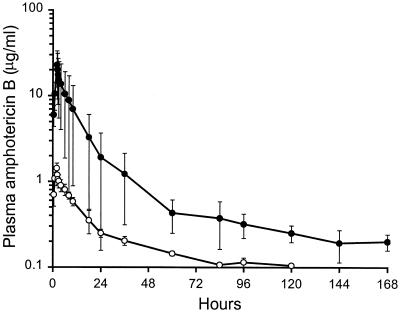
All subjects displayed triphasic plasma amphotericin B concentration profiles, with similar half-lives for the two formulations (Fig. 1). However, the pharmacokinetics of liposomal amphotericin B differed significantly from those of amphotericin B deoxycholate (Table 2). Although liposomal amphotericin B was administered at a dose 3.3-fold higher than that of amphotericin B deoxycholate, plasma concentrations during the first 24 h were 8- to 16-fold higher in liposomal amphotericin B-treated subjects (P < 0.01 for the maximum concentration of drug in serum [Cmax]) with plasma AUCs similarly increased (not statistically significant). The levels in plasma remained above 2 μg/ml for the entire study in liposomal amphotericin B-treated subjects but fell below the limit of detection (0.1 μg/ml) by 96 to 144 h in the amphotericin B deoxycholate-treated subjects. Although the two formulations had similar half-lives, the terminal elimination phase contained 80% of the total AUC for amphotericin B deoxycholate but only 47% of the total AUC for liposomal amphotericin B. Volumes of distribution for liposomal amphotericin B were smaller than those for amphotericin B deoxycholate (P < 0.05 for V1 and Vss). Liposomal amphotericin B had a central compartment volume (V1) similar to the plasma volume, the smallest compartment into which liposomes could distribute. The total clearance of liposomal amphotericin B was 26% less than the clearance of amphotericin B deoxycholate (not statistically significant).
FIG. 1.
Concentrations of amphotericin B in plasma after a 2-h infusion of 2 mg of liposomal amphotericin B/kg (closed circles) or 0.6 mg of amphotericin B deoxycholate/kg (open circles) in healthy subjects. Values are means ± standard deviations.
TABLE 2.
Clinical pharmacokinetic parameters of amphotericin B after a 2-h infusion of liposomal amphotericin B (2 mg/kg) or amphotericin B deoxycholate (0.6 mg/kg)
| Parameter (unit)b | Result fora:
|
Pc | |
|---|---|---|---|
| Liposomal amphoter-icin B (2 mg/kg) | Amphotericin B deoxy-cholate (0.6 mg/kg) | ||
| Cmax (μg/ml) | 22.9 ± 10 | 1.43 ± 0.2 | <0.01 |
| C24h (μg/ml) | 1.9 ± 1.8 | 0.25 ± 0.03 | NS |
| AUC0-24 (μgh ml−1) | 171 ± 126 | 13.9 ± 2.0 | NS |
| AUC0-∞ (μgh ml−1) | 288 ± 209 | 46.6 ± 7.2 | NS |
| t1/2α (h) | 0.56 ± 0.48 | 0.17 ± 0.14 | NS |
| t1/2β (h) | 6.0 ± 2.1 | 6.8 ± 1.6 | NS |
| t1/2γ (h) | 152 ± 116 | 127 ± 30 | NS |
| V1 (ml/kg) | 50.1 ± 19 | 136 ± 60 | <0.05 |
| V (ml/kg) | 1,628 ± 876 | 2,340 ± 202 | NS |
| Vss (ml/kg) | 774 ± 550 | 1,807 ± 239 | <0.01 |
| CL (ml h−1 kg−1) | 9.7 ± 5.4 | 13.1 ± 2.0 | NS |
| CLR (ml h−1 kg−1) | 0.495 ± 0.25 | 4.1 ± 0.68 | <0.01 |
| CLF (ml h−1 kg−1) | 0.488 ± 0.46 | 5.4 ± 0.91d | <0.01 |
| Furine | 0.053 ± 0.006 | 0.32 ± 0.06 | <0.01 |
| Ffeces | 0.047 ± 0.04 | 0.43 ± 0.11d | <0.01 |
Values are means ± standard deviations (n = 5 subjects per treatment).
C24h, concentration of drug in plasma at 24 h.
NS, not significant (P > 0.05).
n = 4 (incomplete fecal collection in one subject).
Excretion and mass balance.
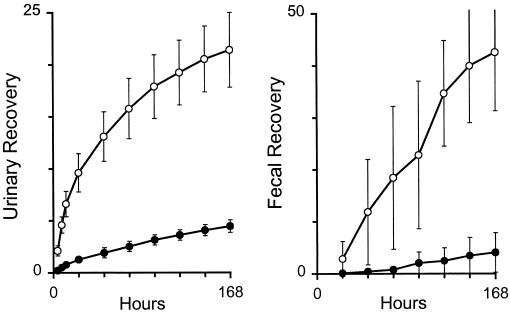
By the end of the 1-week study, the mean cumulative urinary recovery of unchanged amphotericin B was 20.6% in amphotericin B deoxycholate-treated subjects and 4.5% in liposomal amphotericin B-treated subjects (Fig. 2). In consequence, the renal clearance of amphotericin B deoxycholate was nearly a third of its total clearance (i.e., Furine = 0.32) while the renal clearance of liposomal amphotericin B was only about 5% of its total clearance (Table 2). Cumulative fecal recovery was 42.5% in amphotericin B deoxycholate-treated subjects but only 4% in liposomal amphotericin B-treated subjects, so that fecal clearance accounted for 43% of the total clearance of amphotericin B deoxycholate but only 5% of the total clearance of liposomal amphotericin B.
FIG. 2.
Cumulative recoveries of amphotericin B (percentage of dose) in the urine (left panel) and feces (right panel) of subjects receiving 2 mg of liposomal amphotericin B/kg (closed circles) or 0.6 mg of amphotericin B deoxycholate/kg (open circles). Values are means ± standard deviations.
The total recovery of unchanged drug in collected excreta was nearly two-thirds of the dose for amphotericin B deoxycholate but less than 10% of the dose for liposomal amphotericin B (Table 3). From the concentration of amphotericin B deoxycholate in plasma (estimated by extrapolation to be 0.08 μg/ml at 168 h) and its volume of distribution (V, 2,340 ml/kg), it was estimated that 180 μg/kg or 30.2% of the dose remained in the body 1 week after infusion. Thus, 93.4% of the amphotericin B deoxycholate was accounted for in this study (Table 3). A similar calculation for liposomal amphotericin B indicated that only 15.5% of the dose remained in the body 1 week after infusion, such that only 24% of the administered liposomal amphotericin B was accounted for in this study.
TABLE 3.
Amphotericin B mass balance 1 week after administration of 2 mg of liposomal amphotericin B/kg or 0.6 mg of amphotericin B deoxycholate/kg
| Source | Mean mass balance (% of dose) ± SD after administration of:
|
P | |
|---|---|---|---|
| Liposomal amphotericin B | Amphotericin B deoxycholatea | ||
| Urine | 4.5 ± 0.6 | 20.6 ± 3.6 | <0.01 |
| Feces | 4.0 ± 3.8 | 42.5 ± 11.2 | <0.01 |
| Bodyb | 15.5 ± 6.2 | 30.2 ± 8.8 | <0.05 |
| Total | 24.0 ± 4.6 | 93.4 ± 20.3 | |
n = 4 (fecal recovery was incomplete in one subject).
Estimated from concentration in plasma at 168 h and volume of distribution.
Metabolism.
HPLC chromatograms of plasma obtained after the administration of liposomal amphotericin B and amphotericin B deoxycholate were compared to chromatograms of each subject's plasma prior to dosing and to blank plasma spiked with amphotericin B. No peaks were observed to indicate the presence of metabolites that could be separated and detected in this chromatographic system. In addition, the 2-, 6-, and 24-h plasma samples from three male liposomal amphotericin B-treated subjects and three male amphotericin B deoxycholate-treated subjects and the 8-to-12-h urine sample from a male liposomal amphotericin B-treated subject were subjected to a metabolite screen by single quadrupole electrospray mass spectrometry by using both positive- and negative-ion modes. Amphotericin B was clearly evident (peak m/z, 924.4 to 924.8) in all plasma samples from liposomal amphotericin B-treated subjects (amphotericin B concentrations, ≥10 μg/ml) but was not positively identified in plasma samples from amphotericin B deoxycholate-treated subjects or in the liposomal amphotericin B-treated subject's urine sample (amphotericin B concentration, <2 μg/ml). No other peaks were observed in the mass spectra to indicate the presence of detectable amphotericin B metabolites in these samples.
DISCUSSION
This study demonstrated, for the first time, that amphotericin B deoxycholate is mostly excreted as unchanged drug in the urine and feces, suggesting that humans do not metabolize amphotericin B extensively. The study further characterized the multiphasic plasma concentration profiles of liposomal amphotericin B and amphotericin B deoxycholate in healthy volunteers, showing that both formulations have terminal half-lives of longer than 5 days. Liposomal amphotericin B is known to alter the pharmacokinetics of amphotericin B (5, 12, 32). The observation that urinary and fecal clearances of liposomal amphotericin B were 10-fold lower than those of amphotericin B deoxycholate further supports the hypothesis that liposomal amphotericin B significantly alters the disposition of amphotericin B as a result of encapsulation in liposomes.
The pharmacokinetics of 2 mg of liposomal amphotericin B/kg in healthy subjects were similar to those in neutropenic patients receiving 2.5 mg of liposomal amphotericin B/kg daily (32). The higher values for half-life, AUC, V, and Vss in our study reflect the contribution of the slow terminal elimination phase not observed in the earlier study which analyzed 0- to 24-h data. However, the possibility that population means of some pharmacokinetic parameters differ between healthy subjects and patients cannot be excluded. Previous studies suggested that liposomal amphotericin B pharmacokinetics are characterized by significant interpatient variability (20) and are nonlinear due to saturable uptake of liposomes by the mononuclear phagocyte system (MPS) (32). The intersubject variability of liposomal amphotericin B pharmacokinetic parameters in our study was generally 40 to 75% (relative standard deviation), apparently higher than the variability in amphotericin B deoxycholate-treated subjects (15 to 25% relative standard deviation). This variability, observed with other liposomal drugs (17), may result from differences in MPS function between individuals. The effects of MPS function on liposome disposition have been extensively studied (29).
In this study, liposomal amphotericin B had a long terminal half-life (t1/2γ, 152 h). Earlier estimates of liposomal amphotericin B's half-life (6 to 10 h) (32) probably reflect the second of the three phases we observed (t1/2β, 6.0 h). The terminal half-lives of liposomal amphotericin B and amphotericin B deoxycholate were similar in our study, suggesting that the rate of amphotericin B elimination is similar for the two formulations. However, the terminal phase contained over 80% of the total AUC for amphotericin B deoxycholate but less than half of the total AUC for liposomal amphotericin B. Thus, the earlier phases of liposomal amphotericin B disposition make a significant contribution to its overall pharmacokinetic profile, which may explain the relatively minor accumulation observed in plasma after repeated daily administration of liposomal amphotericin B in humans (32) and animals (9). A dosing interval of 24 h is therefore appropriate for liposomal amphotericin B, despite its long terminal half-life.
The low initial volume of distribution (V1) for liposomal amphotericin B suggests that circulating liposomes sequester drug in the plasma compartment, contributing to the high Cmax observed for liposomal amphotericin B. The values for V and Vss suggest that a substantial fraction of the amphotericin B in liposomal amphotericin B eventually entered the tissue compartment, consistent with the interpretation that the β phase, contributing nearly half of the total AUC for liposomal amphotericin B, represents the slow release of drug from long-circulating liposomes (8) or the slow uptake of liposomes into tissues.
This study extends previous observations in healthy subjects (22), neutropenic patients (4), and patients with disseminated fungal infections (3) that amphotericin B deoxycholate pharmacokinetics are multiphasic and that the drug is distributed widely to tissues and has a long elimination half-life and a total clearance substantially lower than the plasma flow to any major eliminating organ. However, earlier studies either employed bioassays (3) that may substantially underestimate amphotericin B concentrations (13) or collected plasma over periods (24 to 48 h) too short to adequately characterize the drug's long elimination phase (4, 22). We measured amphotericin B phospholipase C for 168 h after dosing, allowing a more detailed characterization of its pharmacokinetics and excretion. Due to the long residence time of amphotericin B, even this period was insufficient to completely characterize the drug's excretion. The currently tabulated half-life for amphotericin B deoxycholate (15 days) was estimated by bioassay in two male diabetic patients after chronic amphotericin B deoxycholate therapy (3). The plasma half-life we observed (127 ± 30 h) may be a closer approximation to the population mean for this parameter.
In this study, two-thirds of the amphotericin B deoxycholate was recovered in the excreta with over 90% of the dose accounted for in mass balance calculations at 1 week. The urinary recovery of unchanged amphotericin B deoxycholate (20.6%) was markedly higher than the urinary recovery reported in two human subjects (3%) by using a bioassay (3) but similar to the urinary recovery reported in animals (25%) by using an HPLC assay (23). The urinary and fecal clearances of unchanged amphotericin B deoxycholate amounted to 75% of the total clearance observed in our study, suggesting that at least 75% of the drug was excreted unchanged. The observation that urinary and fecal excretion of amphotericin B in humans is larger than previously assumed explains why previous mass balance calculations did not account for most of the amphotericin B administered.
Amphotericin B is reported to be highly bound in the plasma (10; Bekersky et al., 40th ICAAC). The volumes of distribution we observed for amphotericin B deoxycholate indicate that the drug is even more strongly bound in tissues with a free fraction severalfold lower than that in the plasma (30). This is consistent with the low solubility of amphotericin B in water and its affinity for sterol-containing membranes (19). Amphotericin B plasma clearance was at least 50-fold lower than plasma flow to either the kidneys or the hepato-portal system (14), suggesting that none of these tissues extensively extracted or eliminated the drug, an observation consistent with the drug's low unbound fraction (Bekersky et al., 40th ICAAC).
The low urinary clearance observed for amphotericin B deoxycholate (approximately 4% of the glomerular filtration rate in humans) is consistent with renal filtration of a highly protein-bound drug (10; Bekersky et al., 40th ICAAC). The extensive fecal recovery of amphotericin B deoxycholate is consistent with the fact that it has many of the properties typical of other drugs that are excreted into the bile: high molecular weight, strong protein binding, and both charged and hydrophobic regions (24). In addition, the composition of bile is likely to increase the solubility of amphotericin B. Although amphotericin B deoxycholate is reported to alter hepatic P-450 activity (21), its extensive excretion as unchanged drug suggests that the pharmacokinetics of amphotericin B are unlikely to be influenced by hepatic enzyme levels but could be affected in renal or hepatic disease.
In contrast to the high recoveries of amphotericin B deoxycholate, less than 10% of the drug was recovered in the urine and feces after liposomal amphotericin B administration. The reduced excretion of unchanged drug could result from the sequestration of amphotericin B within circulating liposomes, which prevents its renal filtration or biliary secretion, or from alterations in tissue distribution, which prolong the drug's residence time (28), alter its cellular location in tissues (15), or lead to increased metabolism. Since MPS tissues play a key role in determining the disposition of liposomes (29), they are expected to be involved in the tissue uptake, retention, and possible metabolism of liposomal amphotericin B. Liposomal formulations reduce nephrotoxicity by reducing concentrations of amphotericin B in the kidneys (15) and can increase efficacy by targeting sites of infection (28). Our observation that liposomal amphotericin B reduces excretion of unchanged drug supports the hypothesis that MPS uptake of liposomal drugs alters their distribution in tissue, affecting not only safety and efficacy but the excretion of encapsulated drugs as well.
The reduced urinary and fecal excretion of liposomal amphotericin B suggests that its distribution within the tissue compartment differs from that of amphotericin B deoxycholate. As suggested for other liposomes, drugs sequestered within MPS tissues may not be in diffusional equilibrium with the plasma compartment (18), so that some of the amphotericin B in liposomal amphotericin B may be eliminated from the tissue compartment rather than from the central compartment. It has recently been demonstrated that conventional volumes of distribution can substantially underestimate the amount of liposomal drug in the body (18) and that liposomes achieve more extensive and prolonged tissue distribution than their plasma profiles suggest (16). If this is the case for liposomal amphotericin B, drug residing in deep tissue compartments, unmeasured by pharmacokinetic analysis, may contribute to the mass balance deficit observed for this liposomal formulation. In animals, liposomal amphotericin B (9, 31) and other liposomes (16) significantly increase and prolong drug levels in tissues, and the long tissue half-lives of liposomal drugs may result in accumulation in tissue after repeated dosing. This accumulation does not result in increased toxicity, suggesting that liposomal drugs are sequestered in deep or protected tissue compartments, such as tissue macrophages (9, 15, 17). Thus, some of the amphotericin B unrecovered after liposomal amphotericin B administration may remain in unmeasured compartments within tissues, from which it is slowly eliminated without returning to the central compartment.
Previous studies of amphotericin B in humans failed to account for a majority of the dose, leaving open the possibility that the drug is extensively metabolized (19). Although no explicit evidence for amphotericin B metabolism was reported, amphotericin B is degraded by rat liver homogenates (34). In our study, over 90% of the amphotericin B deoxycholate was accounted for in mass balance calculations, with renal and fecal clearances of unchanged drug amounting to 74% of the total clearance, suggesting that metabolism plays at most a minor role in amphotericin B elimination in humans. Although the sensitivities of the metabolite screens we employed were limited, they also suggested the absence of major metabolites after liposomal amphotericin B and amphotericin B deoxycholate administration. The possibility that minor metabolites could accumulate to measurable levels after repeated dosing with either formulation cannot be excluded.
In summary, a comparative study of liposomal amphotericin B and amphotericin B deoxycholate showed that amphotericin B has a terminal half-life of 127 h in healthy subjects and is extensively excreted as unchanged drug in the urine and feces. Liposomal amphotericin B produced higher plasma concentrations and lower apparent distribution volumes and greatly reduced renal and fecal clearances, suggesting that it constitutes a stable intravenous drug delivery system that remains in the circulation for a period of time and significantly alters the pharmacokinetics, distribution, and elimination of its drug payload in the body.
REFERENCES
- 1.Adler-Moore, J., and R. T. Proffitt. 1998. AmBisome: long circulating liposomal formulation of amphotericin B, p. 185-206. In M. C. Woodle and G. Storm (ed.), Long-circulating liposomes: old drugs, new therapeutics. Landes Bioscience, Georgetown, Tex.
- 2.Alak, A., S. Moy, and I. Bekersky. 1996. A high-performance liquid chromatographic assay for the determination of amphotericin B serum concentrations after the administration of AmBisome, a liposomal amphotericin B formulation. Ther. Drug Monit. 18:604-609. [DOI] [PubMed] [Google Scholar]
- 3.Atkinson, A. J., and J. E. Bennett. 1978. Amphotericin B pharmacokinetics in humans. Antimicrob. Agents Chemother. 13:271-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ayestarán, A., R. M. López, J. B. Montoro, A. Estíbalez, L. Pou, A. Julià, A. López, and B. Pascual. 1996. Pharmacokinetics of conventional formulation versus fat emulsion formulation of amphotericin B in a group of patients with neutropenia. Antimicrob. Agents Chemother. 40:609-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bekersky, I., R. M. Fielding, D. Buell, and I. Lawrence. 1999. Lipid-based amphotericin B formulations: from animals to man. Pharm. Sci. Technol. Today 2:230-236. [DOI] [PubMed] [Google Scholar]
- 6.Bekersky, I., D. Buell, M. Tomishima, K. Maki, I. Lawrence, and R. M. Fielding. 1999. New approaches to systemic antifungal therapy: case studies of AmBisome and FK463. Recent Res. Dev. Antimicrob. Agents Chemother. 3:407-413. [Google Scholar]
- 7.Bekersky, I., G. W. Boswell, R. Hiles, R. M. Fielding, D. Buell, and T. J. Walsh. 1999. Safety and toxicokinetics of intravenous liposomal amphotericin B (AmBisome) in beagle dogs. Pharm. Res. 16:1694-1701. [DOI] [PubMed] [Google Scholar]
- 8.Bekersky, I., R. M. Fielding, D. E. Dressler, S. Kline, D. N. Buell, and T. J. Walsh. 2001. Pharmacokinetics, excretion and mass balance of 14C after administration of 14C-cholesterol labeled AmBisome to healthy volunteers. J. Clin. Pharmacol. 41:963-971. [DOI] [PubMed] [Google Scholar]
- 9.Bekersky, I., G. W. Boswell, R. Hiles, R. M. Fielding, D. N. Buell, and T. J. Walsh. 2000. Safety, toxicokinetics, and tissue distribution of long-term intravenous liposomal amphotericin B (AmBisome): a 91-day study in rats. Pharm. Res. 17:1494-1502. [DOI] [PubMed] [Google Scholar]
- 10.Block, E. R., J. E. Bennett, L. G. Livoti, W. J. Klein, R. R. MacGregor, and L. Henderson. 1974. Flucytosine and amphotericin B: hemodialysis effects on the plasma concentration and clearance. Ann. Intern. Med. 80:613-617. [DOI] [PubMed] [Google Scholar]
- 11.Boswell, G. W., I. Bekersky, D. Buell, R. Hiles, and T. J. Walsh. 1998. Toxicological profile and pharmacokinetics of a unilamellar liposomal vesicle formulation of amphotericin B in rats. Antimicrob. Agents Chemother. 42:263-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boswell, G. W., D. Buell, and I. Bekersky. 1998. AmBisome (liposomal amphotericin B): a comparative review. J. Clin. Pharmacol. 38:583-592. [DOI] [PubMed] [Google Scholar]
- 13.Collette, N., P. Van Der Auwera, A. P. Lopez, C. Heymans, and F. Meunier. 1989. Tissue concentrations and bioactivity of amphotericin B in cancer patients treated with amphotericin B-deoxycholate. Antimicrob. Agents Chemother. 33:362-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davies, B., and T. Morris. 1993. Physiological parameters in laboratory animals and humans. Pharm. Res. 10:1093-1095. [DOI] [PubMed] [Google Scholar]
- 15.Fielding, R. M., A. W. Singer, L. H. Wang, S. Babbar, and L. S. S. Guo. 1992. Relationship of pharmacokinetics and tissue distribution to reduced toxicity of colloidal amphotericin B in dogs. Antimicrob. Agents Chemother. 36:299-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fielding, R. M., R. O. Lewis, and L. Moon-McDermott. 1998. Altered tissue distribution and elimination of amikacin encapsulated in unilamellar, low-clearance liposomes (MiKasome). Pharm. Res. 15:1775-1781. [DOI] [PubMed] [Google Scholar]
- 17.Fielding, R. M., G. Mukwaya, and R. A. Sandhaus. 1998. Clinical and preclinical studies with low-clearance liposomal amikacin (MiKasome), p. 213-225. In M. C. Woodle and G. Storm (ed.), Long-circulating liposomes: old drugs, new therapeutics. Landes Bioscience, Georgetown, Tex.
- 18.Fielding, R. M. 2001. Relationship of pharmacokinetically calculated volumes of distribution to the physiologic distribution of liposomal drugs in tissues: implications for the characterization of liposomal formulations. Pharm. Res. 18:238-242. [DOI] [PubMed] [Google Scholar]
- 19.Groll, A., S. Piscitell, and T. J. Walsh. 1998. Clinical pharmacology of systemic antifungal agents: a comprehensive review of agents in clinical use, current investigational compounds, and putative targets for antifungal drug development. Adv. Pharmacol. 44:343-500. [DOI] [PubMed] [Google Scholar]
- 20.Heinemann, V., D. Bosse, U. Jehn, B. Kähny, K. Wachholz, A. Debus, P. Scholz, H.-J. Kolb, and W. Wilmanns. 1997. Pharmacokinetics of liposomal amphotericin B (AmBisome) in critically ill patients. Antimicrob. Agents Chemother. 41:1275-1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inselmann, G., A. Volkmann, and H. T. Heidemann. 2000. Comparison of the effects of liposomal amphotericin B and conventional amphotericin B on propafenone metabolism and hepatic cytochrome P-450 in rats. Antimicrob. Agents Chemother. 44:131-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kan, V. L., J. E. Bennett, M. A. Amantea, M. C. Smolskis, E. McManus, D. M. Grasela, and J. W. Sherman. 1991. Comparative safety, tolerance, and pharmacokinetics of amphotericin B lipid complex and amphotericin B desoxycholate in healthy male volunteers. J. Infect. Dis. 164:418-421. [DOI] [PubMed] [Google Scholar]
- 23.Kim, H., D. Loebenberg, A. Marco, S. Symchowicz, and C. Lin. 1984. Comparative pharmacokinetics of Sch 28191 and amphotericin B in mice, rats, dogs, and cynomolgus monkeys. Antimicrob. Agents Chemother. 26:446-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klaassen, C. D., and J. B. Watkins. 1984. Mechanisms of bile formation, hepatic uptake, and biliary excretion. Pharmacol. Rev. 36:1-67. [PubMed] [Google Scholar]
- 25.Lee, J. W., M. Amantea, E. Navarro, P. Francis, E. McManus, R. Schaufele, J. Bacher, P. A. Pizzo, and T. J. Walsh. 1994. Pharmacokinetics and safety of a unilamellar liposomal formulation of amphotericin B (AmBisome) in rabbits. Antimicrob. Agents Chemother. 38:713-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee, J. W., M. E. Petersen, P. Lin, D. Dressler, and I. Bekersky. 2001. Quantitation of free and total amphotericin B in human biological matrices by a liquid chromatography tandem mass spectrometric method. Ther. Drug Monit. 23:268-276. [DOI] [PubMed] [Google Scholar]
- 27.Loo, J. C. K., and S. Riegelman. 1970. Assessment of pharmacokinetic constants from postinfusion blood curves obtained after I.V. infusion. J. Pharm. Sci. 59:53-55. [DOI] [PubMed] [Google Scholar]
- 28.Lopez-Berestein, G., M. G. Rosenblum, and R. Mehta. 1984. Altered tissue distribution of amphotericin B by liposomal encapsulation: comparison of normal mice to mice infected with Candida albicans. Cancer Drug Rev. 1:199-205. [DOI] [PubMed] [Google Scholar]
- 29.Papahadjopoulos, D. 1996. Fate of liposomes in vivo: a brief introductory review. J. Liposome Res. 6:3-17. [Google Scholar]
- 30.Rowland, M., and T. N. Tozer. 1994. Distribution, p. 137-155. In Clinical pharmacokinetics: concepts and applications, 3rd ed. Lea and Febiger, Philadelphia, Pa.
- 31.Townsend, R. W., A. Zutshi, and I. Bekersky. 2001. Biodistribution of 4-[14C] cholesterol-AmBisome following a single intravenous administration to rats. Drug Metab. Dispos. 29:681-685. [PubMed] [Google Scholar]
- 32.Walsh, T. J., V. Yeldandi, M. McEvoy, C. Gonzalez, S. Chanock, A. Freifeld, N. I. Seibel, P. O. Whitcomb, P. Jarosinski, G. Boswell, I. Bekersky, A. Alak, D. Buell, J. Barret, and W. Wilson. 1998. Safety, tolerance and pharmacokinetics of a small unilamellar liposomal formulation of amphotericin B (AmBisome) in neutropenic patients. Antimicrob. Agents Chemother. 42:2391-2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walsh, T. J., R. W. Finberg, C. Arndt, J. Hiemenz, C. Schwartz, D. Bodensteiner, P. Pappas, N. Seibel, R. N. Greenberg, S. Dummer, M. Schuster, and J. S. Holcenberg. 1999. Liposomal amphotericin B for empirical therapy in patients with persistent fever and neutropenia. N. Engl. J. Med. 340:764-771. [DOI] [PubMed] [Google Scholar]
- 34.Wang, L. H., P. C. Smith, K. L. Anderson, and R. M. Fielding. 1992. High-performance liquid chromatographic analysis of amphotericin B in plasma, blood, urine and tissues for pharmacokinetic and tissue distribution studies. J. Chromatogr. 579:259-268. [DOI] [PubMed] [Google Scholar]
- 35.Wasan, K. M., R. E. Morton, M. G. Rosenblum, and G. Lopez-Berestein. 1994. Decreased toxicity of liposomal amphotericin B due to association of amphotericin B with high-density lipoproteins: role of lipid transfer protein. J. Pharm. Sci. 83:1006-1010. [DOI] [PubMed] [Google Scholar]