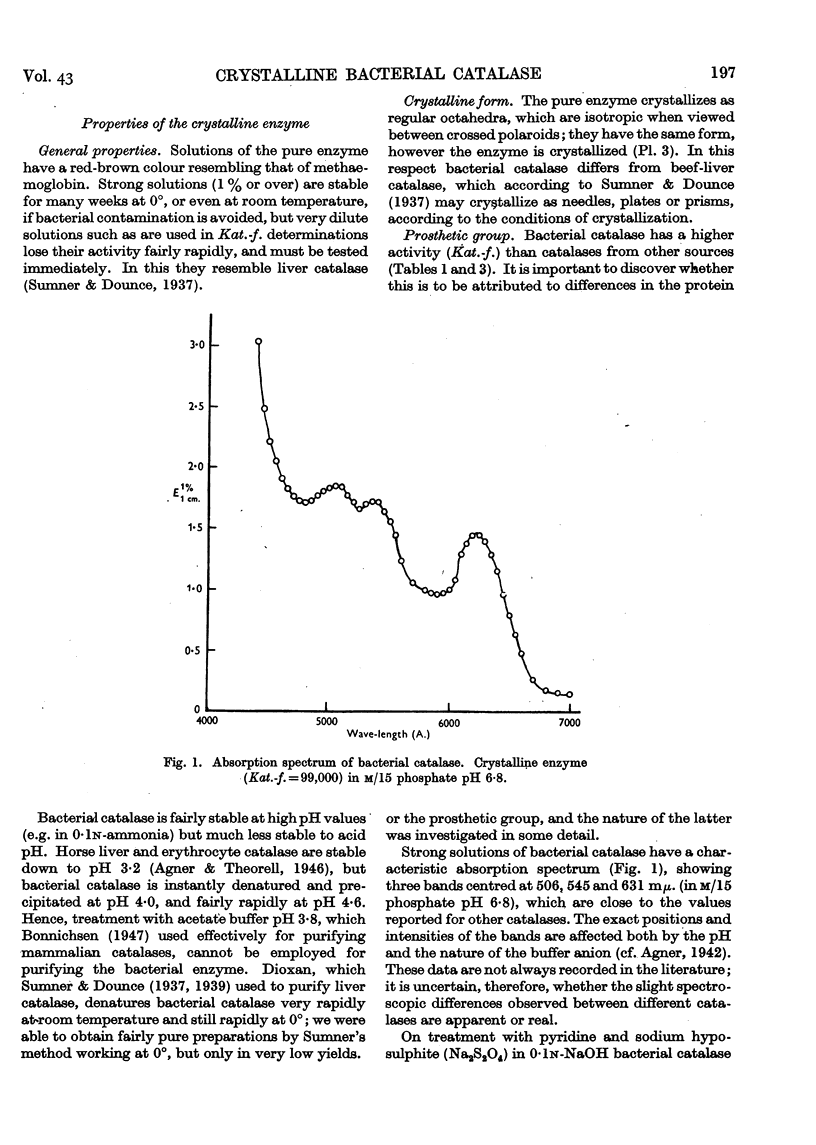
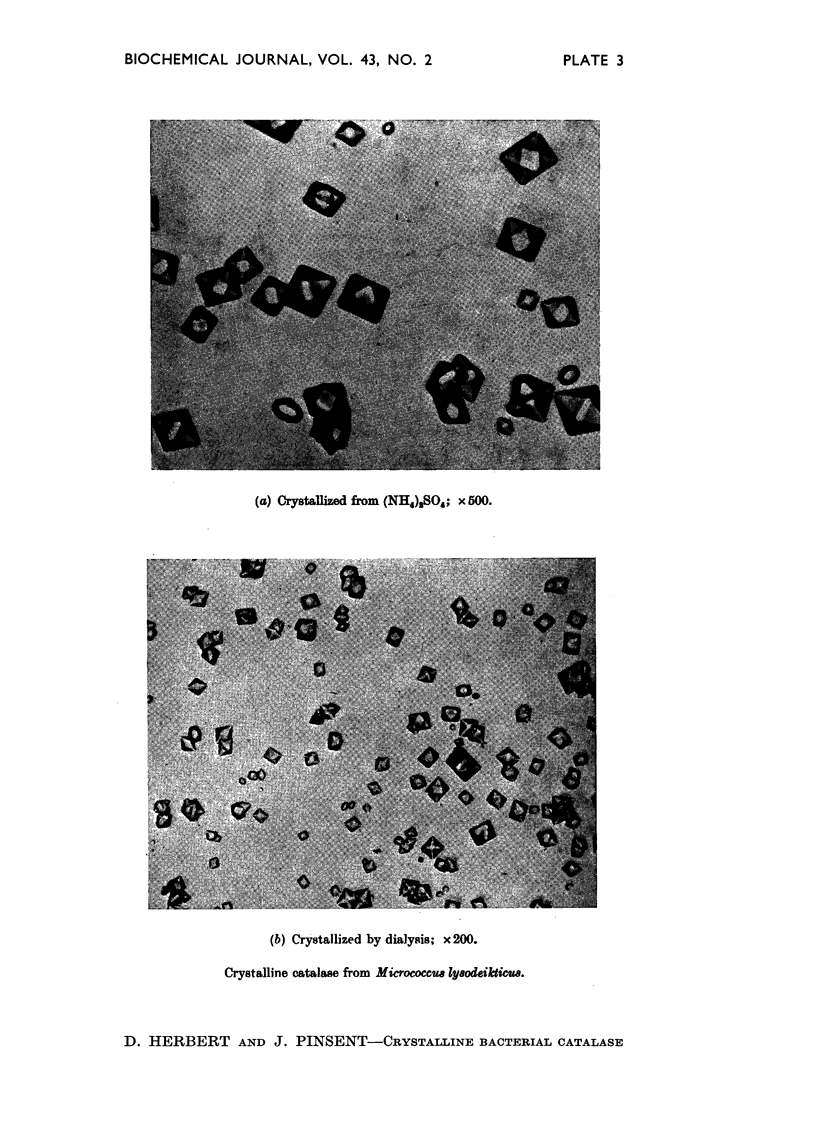
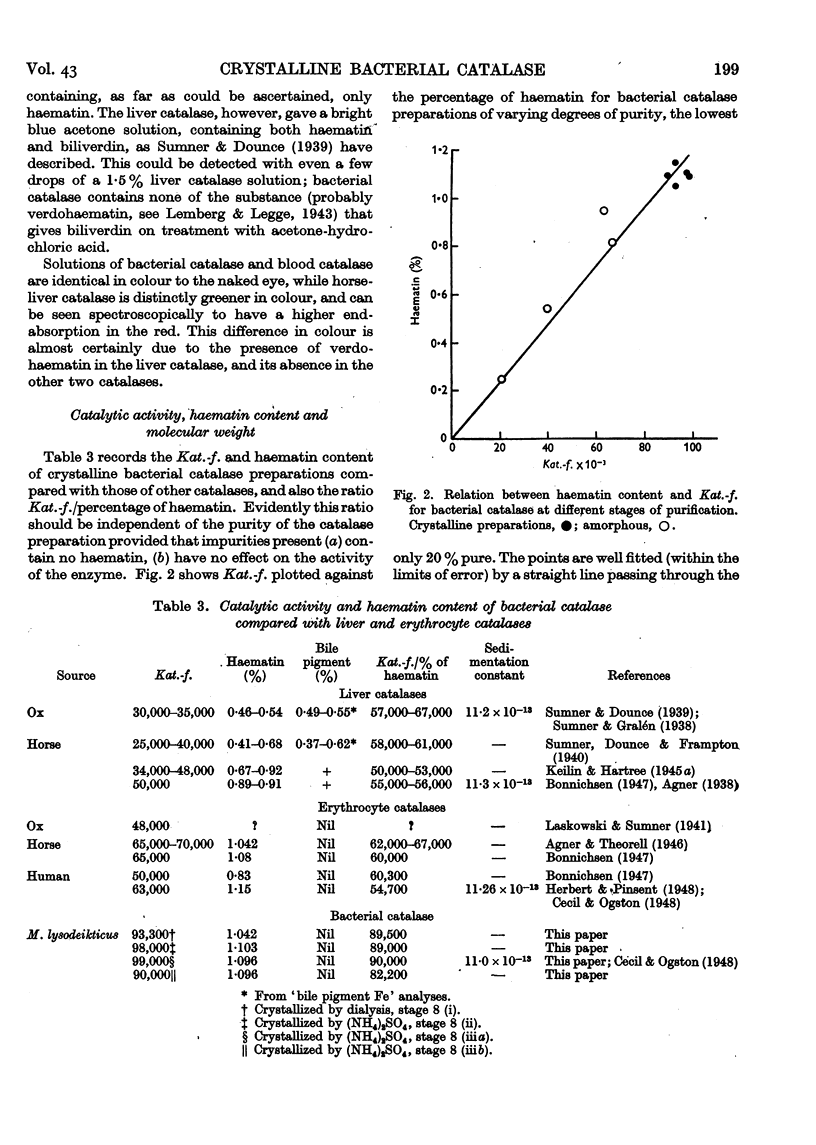
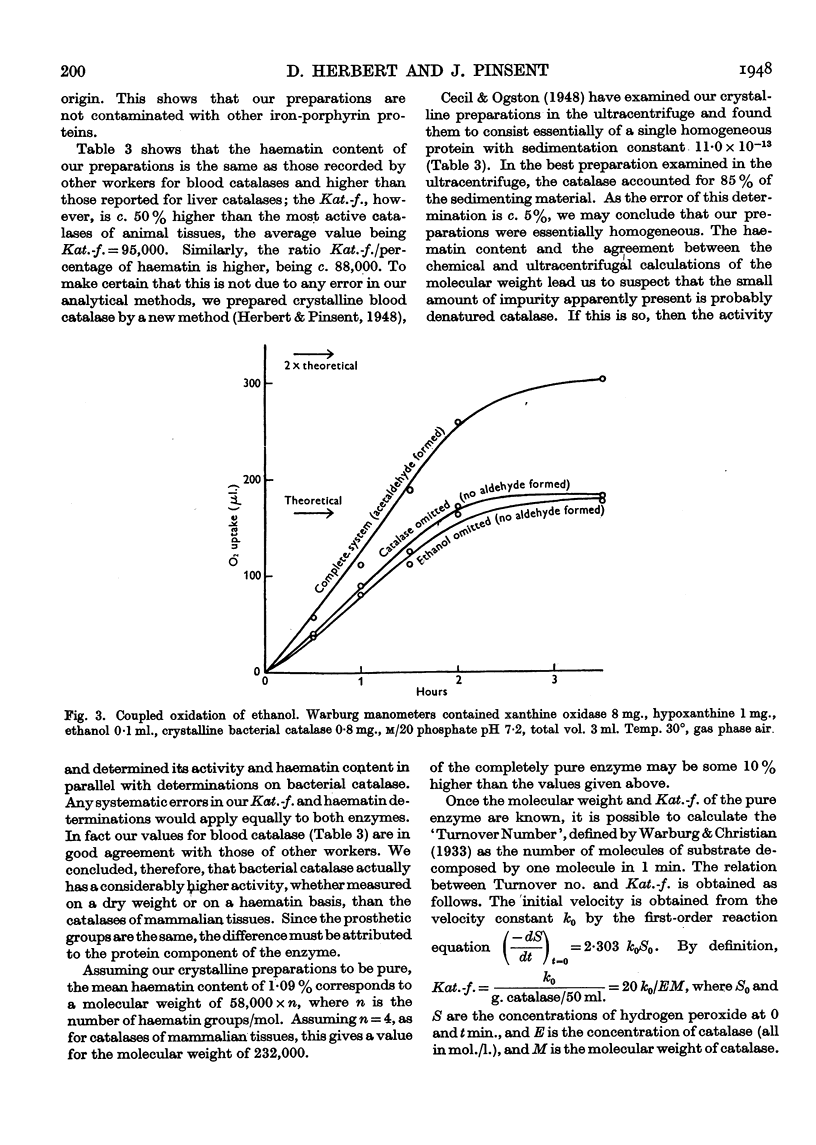
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agner K. The preparation and properties of a highly active catalase from horse liver. Biochem J. 1938 Oct;32(10):1702–1706. doi: 10.1042/bj0321702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth V. H., Green D. E. A wet-crushing mill for micro-organisms. Biochem J. 1938 May;32(5):855–861. doi: 10.1042/bj0320855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecil R., Ogston A. G. Examination of crystalline catalases in the ultracentrifuge. Biochem J. 1948;43(2):205–206. [PMC free article] [PubMed] [Google Scholar]
- Curran H. R., Evans F. R. The Killing of Bacterial Spores in Fluids by Agitation with Small Inert Particles. J Bacteriol. 1942 Feb;43(2):125–139. doi: 10.1128/jb.43.2.125-139.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epps H. M., Gale E. F. The influence of the presence of glucose during growth on the enzymic activities of Escherichia coli: comparison of the effect with that produced by fermentation acids. Biochem J. 1942 Sep;36(7-9):619–623. doi: 10.1042/bj0360619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert D., Pinsent J. Crystalline human erythrocyte catalase. Biochem J. 1948;43(2):203–205. doi: 10.1042/bj0430203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalnitsky G., Utter M. F., Werkman C. H. Active Enzyme Preparations from Bacteria. J Bacteriol. 1945 Jun;49(6):595–602. doi: 10.1128/jb.49.6.595-602.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keilin D., Hartree E. F. Properties of azide-catalase. Biochem J. 1945;39(2):148–157. doi: 10.1042/bj0390148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keilin D., Hartree E. F. Properties of catalase. Catalysis of coupled oxidation of alcohols. Biochem J. 1945;39(4):293–301. [PMC free article] [PubMed] [Google Scholar]
- Krampitz L. O., Werkman C. H. The enzymic decarboxylation of oxaloacetate. Biochem J. 1941 Jun;35(5-6):595–602. doi: 10.1042/bj0350595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskowski M., Sumner J. B. CRYSTALLINE CATALASE FROM BEEF ERYTHROCYTES. Science. 1941 Dec 26;94(2452):615–615. doi: 10.1126/science.94.2452.615. [DOI] [PubMed] [Google Scholar]
- Lemberg R., Legge J. W. Liver catalase. Biochem J. 1943 Apr;37(1):117–127. doi: 10.1042/bj0370117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpf P. K., Green D. E., Smith F. W. Ultrasonic Disintegration as Method of Extracting Bacterial Enzymes. J Bacteriol. 1946 Apr;51(4):487–493. [PMC free article] [PubMed] [Google Scholar]



