Abstract
P-glycoprotein (P-gp) overexpression by tumor cells imparts resistance to multiple antineoplastic chemotherapeutic agents (multiple drug resistance). Treatment of tumor cells with chemotherapeutic agents such as anthracyclines, epipodophyllotoxins, and Vinca alkaloids results in induction of P-gp expression. This study was performed to determine if clinically relevant antimicrobial drugs (i.e., drugs that are used to treat bacterial infections in cancer patients) other than antineoplastic agents can induce expression of P-gp in MCF-7 breast carcinoma cells. Expression of P-gp and MDR1 mRNA was determined in samples from MCF-7 cells that were treated in culture with doxorubicin (positive control) and the antimicrobial drugs doxycycline, piperacillin, and cefoperazone. The functional status of P-gp was assessed using laser cytometry to determine intracellular doxorubicin concentrations. The MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) assay was used to determine if the cytotoxicity of experimental drugs was related to their ability to induce P-gp expression. MCF-7 cells treated with doxycycline (MCF-7/doxy) were stimulated to overexpress P-gp, whereas cells treated with piperacillin and cefoperazone did not overexpress P-gp. MCF-7/doxy cells were compared to a positive-control subline, MCF-7/Adr, previously selected for doxorubicin resistance, and to MCF-7 cells treated with doxorubicin (MCF-7/doxo). All three sublines overexpressed P-gp and MDR1 mRNA and accumulated less intracellular doxorubicin than did control MCF-7 cells. P-gp expression was induced only by experimental drugs that were cytotoxic (doxorubicin and doxycycline). Doxycycline, a drug that has been used for treatment of bacterial infections in cancer patients, can induce functional P-gp expression in cancer cells, resulting in multidrug resistance.
In 1970, Biedler and Riehm (3) described an in vitro model of chemotherapeutic multidrug resistance (MDR) in which cultured cells that were selected for growth in actinomycin D developed resistance to a variety of structurally and functionally diverse cytotoxic compounds. Further studies showed that the emergence of MDR was associated with increased levels of a transmembrane glycoprotein (24), P glycoprotein (P-gp). P-gp, the product of the MDR1 gene, is a 170-kDa protein that functions as an energy-dependent drug efflux pump whose substrates include naturally occurring, lipophilic agents with a complex ring structure such as Vinca alkaloids, anthracyclines, epipodophyllotoxins, and certain rhodamine dyes (18, 26, 37). Exposure of tumor cells to any of these substrates can generate overexpression of P-gp, resulting in the MDR phenotype.
Drug exposure is thought to cause overexpression of P-gp by both selection of resistant cells and induction of P-gp expression at the level of the MDR1 promoter (14, 34). The MDR1 promoter contains a heat-shock consensus element (46) and a putative xenobiotic response element that responds directly to treatment with cytotoxic agents (25, 45), supporting the premise that induction of P-gp expression occurs in the presence of chemotherapeutic agents. Additionally, there is a correlation between specific point mutations in the MDR1 promoter and increased inducibility after treatment with chemotherapeutic agents (45). Collectively, these studies provide supportive evidence that induction of P-gp overexpression, as a direct result of exposure to chemotherapeutic agents, does occur in vitro. Such evidence is lacking in the clinical setting, where it is unclear whether apparently increasing levels of P-gp expression are a result of selection of a P-gp-expressing subpopulation of cells or induction of P-gp expression.
However, considerable data document the importance of P-gp-mediated MDR in clinical cancer patients. Expression of P-gp has been documented elsewhere for tumor specimens derived from patients with a variety of histologic types of cancer (8, 20, 31, 38). Results from these clinical investigations were similar to in vitro results described above: P-gp expression was increased in patients with a history of exposure to chemotherapeutic drugs. Clinical studies of different malignant tumors have shown the progressive development of P-gp overexpression during chemotherapy, confirming the hypothesis that exposure to antineoplastic agents results in selection (or induction) of MDR clones of tumor cells (4, 41). Emergence of these resistant clones often leads to relapse of disease and therapeutic failure. For many tumor types, a relationship between P-gp expression and an adverse clinical course has been observed previously (1, 6, 7). Although the role of P-gp in human cancer is not entirely defined, the consensus view is that P-gp overexpression is associated with clinical evidence of drug resistance and treatment failure for a significant number of cancer patients (22, 34).
As a result of these observations, chemotherapeutic treatment protocols are manipulated so as to prevent the development of P-gp expression. Protocols are designed to circumvent the proliferation of resistant tumor cells by judiciously combining multiple drugs and delivering them at optimal doses and intervals (34). Considerable effort is made to avoid drugs that may have only sporadic activity against a specific tumor and yet are likely to select for MDR. In contrast, little attention is granted to the possibility that P-gp expression may be affected by other drugs that are administered to cancer patients. Many cancer patients are immunocompromised, as a direct consequence of their disease or from treatment for their disease, and are predisposed to bacterial infections. Such patients often undergo antimicrobial therapy either prophylactically or for active bacterial infections. Administration of an antimicrobial drug that enhances the development of MDR could promote therapeutic failure.
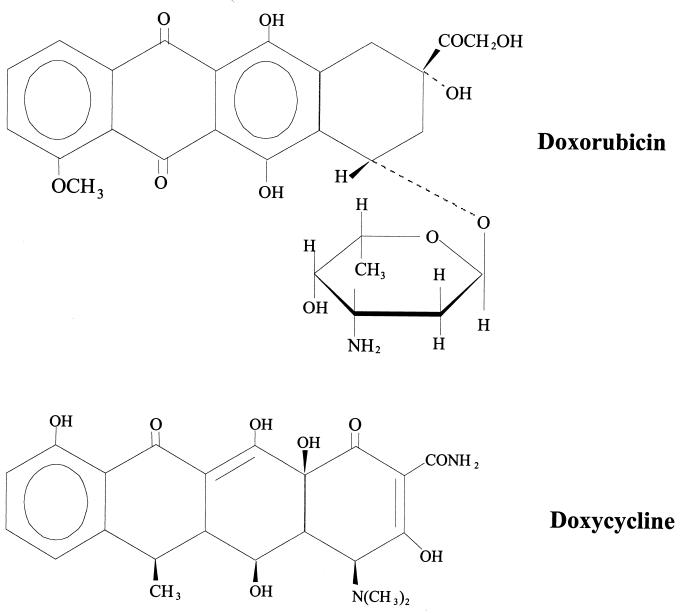
P-gp shares a high degree of homology with bacterial transport proteins (9, 35) and displays characteristics of bacterial multidrug efflux systems (36). Interestingly, one of the same drugs that is used to generate overexpression of P-gp in mammalian cells, rhodamine, is capable of generating expression of a homologous bacterial transport protein. Bacillus subtilis cells selected for rhodamine 6G resistance display amplification of the gene coding for a prokaryotic MDR transporter. This multidrug efflux system transports similar drugs (puromycin, ethidium bromide, and rhodamine) and is sensitive to the same inhibitors (verapamil and reserpine) as is the mammalian multidrug transporter P-gp (36). These observations led to the hypothesis that exposure of cancer cells to antimicrobial drugs would result in the emergence of a P-gp-expressing MDR subline. To test this hypothesis, three agents were selected, each representing a different antimicrobial drug class. Doxycycline, a tetracycline antimicrobial agent, was selected because of its structural similarity to doxorubicin (Fig. 1), and because of its lipophilicity among the tetracyclines (13). Both doxycycline and doxorubicin are elaborated by strains of the genus Streptomyces (5, 42). Cefoperazone, a cephalosporin antimicrobial agent, was selected because of its ability to modulate P-gp function, as it has been reported previously that some P-gp modulators are actually substrates of P-gp (40). Piperacillin, a semisynthetic penicillin, was selected because it contains a piperazine group, a chemical moiety that plays an important role in substrate binding to P-gp (21). The well-defined human breast carcinoma cell line MCF-7 was incubated with increasing concentrations of doxycycline, cefoperazone, piperacillin, and the antineoplastic drug doxorubicin (positive control). Exposure of MCF-7 cells to the antimicrobial agent doxycycline produces a P-gp-overexpressing cell line with properties that are identical to those of well-characterized MDR cell lines generated following incubation of cells with antineoplastic agents. The fact that P-gp overexpression was generated in a previously P-gp-negative cell clone provides supportive evidence that induction of P-gp overexpression, rather than selection of a resistant cell population, is responsible for generating MDR in cell culture systems.
FIG. 1.
Chemical structures of doxorubicin and doxycycline.
MATERIALS AND METHODS
Drugs.
Doxycycline and cefoperazone were generously provided by Pfizer (New York, N.Y.), and piperacillin was provided by Lilly Laboratories (Indianapolis, Ind.). Doxorubicin and verapamil were obtained from Sigma Chemical Company (St. Louis, Mo.).
Cells and culture conditions.
MCF-7 and MCF-7/Adr (27) cell lines were the generous gift of Kenneth Cowan of the National Cancer Institute (Bethesda, Md.) and were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum at 37°C in a humidified atmosphere containing 5% CO2-95% air. MCF-7/Adr cells were periodically incubated in 3 μM doxorubicin to ensure that high levels of P-gp expression were maintained. Prior to experiments, MCF-7/Adr cells were incubated without drug for 48 h. With an enzyme-linked immunosorbent assay-PCR system (Boehringer Mannheim, Indianapolis, Ind.), cells were found to be free of contamination by Mycoplasma species.
In order to ascertain whether development of P-gp expression resulted from selection of a previously resistant clone of cells or from induction of P-gp expression, experimental cells were obtained from a single cell clone. This was accomplished by serially diluting MCF-7 cells to a concentration of 0.5 cells/250 μl of medium and instilling 250 μl per well in a 96-well plate. The plate was examined microscopically to identify wells containing a single cell. One such cell was expanded for utilization in these studies. No P-gp or MDR1 mRNA expression was detected in this clone as determined by immunoblotting or Northern analysis, respectively (data not shown). The expanded clone of MCF-7 cells was grown in 25-cm2 dishes and incubated without drug (negative control) or with doxorubicin (as an internal control), doxycycline, cefoperazone, or piperacillin. Initial drug concentrations were as follows: doxorubicin, 0.01 μg/ml; doxycycline, 3 μg/ml; cefoperazone, 20 μg/ml; and piperacillin, 20 μg/ml. These concentrations represent plasma drug levels achievable by therapeutic dose regimens. Fresh drug was added each time that the medium was changed (approximately every 48 to 72 h). Drug concentrations were increased in a stepwise manner, with subsequent concentrations being approximately 1.5- to 3-fold greater than previous concentrations. Aliquots of cell sublines were cryopreserved at each incremental concentration. After 24 h of incubation at the higher drug concentration, cells were inspected microscopically, and sublines that appeared unhealthy based on visual inspection were subjected to trypan blue exclusion. If cell viability was less than 50 to 60%, drug concentration was reduced to previous levels for approximately 1 week. Drug concentration was then increased to a level approximately 1.25- to 1.3-fold greater than the previous concentration. Final concentrations of drugs were as follows: doxorubicin, 2 μg/ml (3 μM); doxycycline, 100 μg/ml (208 μM); cefoperazone, 2 mg/ml (3 mM); and piperacillin, 8 mg/ml (14 mM). Solubility problems prevented further increases in cefoperazone and piperacillin concentrations. The duration of drug exposure was approximately 12 weeks for doxorubicin and doxycycline and 16 weeks for cefoperazone and piperacillin.
Northern analysis.
Total cellular RNA was extracted from cell pellets by the guanidinium thiocyanate method (11). The RNA concentration was determined spectrophotometrically, and 10 μg was loaded into each well of an agarose gel and electrophoresed. RNA was transferred to a nylon membrane, cross-linked using UV irradiation, and prehybridized (15 min at 65°C) in RapidHyb buffer (Amersham, Cleveland, Ohio). A 32P-labeled cDNA probe (approximately 5 × 106 cpm/ml of hybridization buffer) corresponding to nucleotides 1178 to 2561 of the human MDR1 gene (Michael Gottesman, National Cancer Institute) was allowed to hybridize for 2 h at 65°C. The membrane was then washed twice for 15 min in 0.1× SSC (0.015 M NaCl, 0.0015 M Na3 citrate)-0.1% sodium dodecyl sulfate at 25°C, exposed to autoradiography film, and developed after approximately 24 h. Procedures for the glyceraldehyde-3-phosphate dehydrogenase probe (cDNA probe for human glyceraldehyde-3-phosphate dehydrogenase [ATCC 57091]; American Type Culture Collection, Manassas, Va.) were identical to those used for the MDR1 probe.
Immunoblotting.
Cells were harvested by trypsinization, washed with Dulbecco's phosphate-buffered saline solution (Sigma Chemical Company), and solubilized in tumor solubilization buffer (50 mM Tris HCl [pH 6.8], 50 mM KCl, 5 mM EGTA, 5 mM MgCl2, 2% 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate [CHAPS], 0.1 mM leupeptin, 0.2 mM phenylmethylsulfonyl fluoride, and 10 mM dithiothreitol). Insoluble complexes were cleared by a 1,500-rpm, 5-min spin, and the soluble protein was collected for quantitation by a modified Lowry technique (30). Samples containing 50 μg of protein per well were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and electroblotted onto an Immobilon-P membrane (Millipore, Bedford, Mass.). Membranes were washed with BLOTTO buffer (50 mM Tris HCl, 2 mM CaCl2, 80 mM NaCl, 5% nonfat dry milk, 0.2% Nonidet P-40, 0.03% sodium azide) for 1 h at 25°C and then incubated (25°C for 16 h) with C219 anti-P-gp monoclonal antibody (Signet, Dedham, Mass.). Actin was subsequently detected with a monoclonal antiactin antibody (ICN Immunobiologicals, Costa Mesa, Calif.). Membranes were washed in fresh BLOTTO buffer and incubated with the appropriate alkaline phosphatase-labeled secondary antibody. Membranes were washed with buffer A (50 mM Tris HCl, 2 mM CaCl2, 80 mM NaCl) and developed by using nitroblue tetrazolium and 5-bromo-4-chloro-3-indolylphosphate with an alkaline phosphatase-conjugated substrate kit (Bio-Rad Laboratories, Hercules, Calif.). The color reaction was terminated by washing the reaction mixture in distilled water. Resulting bands were scanned with a Visage 110 camera-based densitometer (BioImage, Ann Arbor, Mich.) and analyzed with whole-band software. Integrated intensity signals for P-gp were normalized to those for actin in the analysis since actin levels were not expected to change under the experimental conditions.
Laser cytometric analysis of doxorubicin accumulation-efflux.
Functional analysis of P-gp took advantage of the inherent fluorescence of doxorubicin, which accumulates in sensitive cells but is actively exported from cells expressing a functional P-gp. The reduced doxorubicin fluorescence in P-gp-expressing cells is blocked by verapamil (16), causing accumulation of cytoplasmic fluorescence. Cells were maintained in drug-free medium for 48 h prior to doxorubicin accumulation studies and then harvested from 25-cm2 tissue culture flasks by trypsinization. Cells were seeded in chambered borosilicate cover glass slides (100,000 cells per well) (Nunc Inc., Naperville, Ill.) and incubated for 36 h (37°C, humidified 5% CO2) to allow cells to adhere to chamber slides. Doxorubicin (5 μM) or doxorubicin plus verapamil (10 μM) were added to the chamber slides. Chamber slides containing cells but no drug were used as negative controls. Cells were incubated with drug for approximately 18 h, washed once with Dulbecco's phosphate-buffered saline, and examined immediately by laser cytometry (Meridian Ultima Workstation; Meridian Instruments, Okemos, Mich.) to quantitate intracellular fluorescence intensity. To relate fluorescence intensities of doxorubicin obtained by laser cytometry from different treatments to intracellular doxorubicin concentration, excitation and detection parameters were kept constant, and a suspension calibration curve was generated with graded concentrations (0 to 2 mM) of doxorubicin in suspension. Correction for differences in optical thickness between suspension analysis and intracellular doxorubicin concentration was accomplished according to previously described methods (2). The laser cytometer was set at an excitation wavelength of 488 nm, and the emitted fluorescence was detected with a barrier filter (band pass 530/30). Ten microscopic fields, each containing aggregates of 10 to 15 cells, were analyzed for each treatment. At least two experiments on different days were performed. For each subline, comparisons between doxorubicin and doxorubicin plus verapamil fluorescence intensity data were performed with the Student t test. Between-treatment group comparisons of sublines were performed with Duncan's new multiple range test of the General Linear Models analysis of variance procedure of SAS-STAT (1985) with significance set at P < 0.05.
Cytotoxicity (MTT) assay.
MCF-7 and MCF-7/Adr cells were plated in 24-well plates at 105 cells per well and allowed to adhere to the plate overnight. Cells underwent a 24-h treatment with experimental drugs alone or in combination with verapamil. Corresponding controls received either no drug treatment or treatment with verapamil only. On the day of assay, treatment medium was replaced with fresh medium containing 0.83 mg of MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) per ml and incubated for 4 h. Medium was then aspirated off, and 200 μl of dimethyl sulfoxide was added to wells to solubilize crystals. The optical density of each sample was read on a microplate reader (model 7520; Cambridge Technology, Inc., Watertown, Mass.) at 570 nm against a blank prepared from cell-free wells. Cell survival was expressed as a fraction of that of untreated controls.
RESULTS
Northern hybridization and immunoblot analysis.
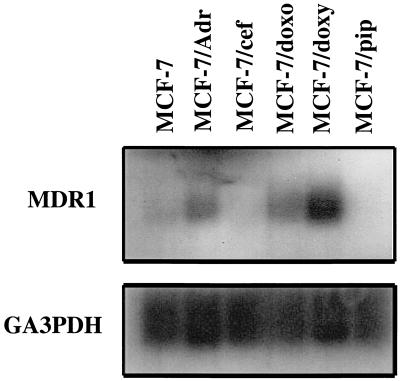
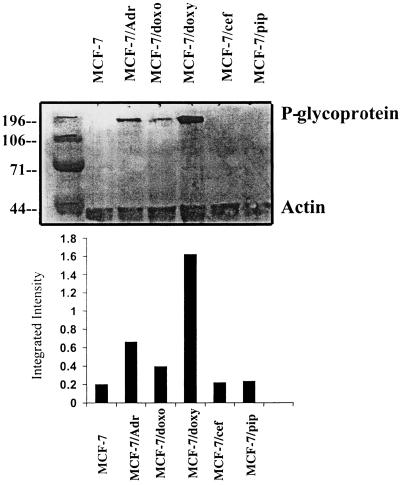
Northern blot analysis showed that P-gp mRNA levels could not be detected in RNA extracts from uninduced parental cells (MCF-7), nor in extracts from cells incubated with either cefoperazone (MCF-7/cef) or piperacillin (MCF-7/pip) (Fig. 2). In contrast, a 4.5-kb band was visible in lanes containing RNA from MCF-7/Adr (positive-control) cells and from cells treated with either doxorubicin (MCF-7/doxo) or doxycycline (MCF-7/doxy), indicating increased MDR1 gene expression. Immunoblotting of cellular extracts with a monoclonal anti-human P-gp antibody (C219) confirmed high levels of P-gp expression (Fig. 3) in protein extracts from MCF-7/Adr, MCF-7/doxo, and MCF-7/doxy cells, whereas immunoreactive P-gp was not detected in extracts of MCF-7, MCF-7/pip, and MCF-7/cef cells.
FIG. 2.
Expression of MDR1 mRNA in MCF-7 cell sublines. Total cellular RNA (10 μg) was electrophoresed, transferred, hybridized with 32P-labeled MDR1 cDNA, and processed for autoradiography. A glyceraldehyde-3-phosphate dehydrogenase (GA3PDH) probe was utilized to normalize RNA load (lower panel). Final concentrations of drugs were as follows: doxorubicin, 2 μg/ml (3 μM); doxycycline, 100 μg/ml (208 μM); cefoperazone, 2 mg/ml (3 mM); and piperacillin, 8 mg/ml (14 mM). Solubility problems prevented further increases in cefoperazone and piperacillin concentrations. The duration of drug exposure was approximately 12 weeks for doxorubicin and doxycycline and 16 weeks for cefoperazone and piperacillin.
FIG. 3.
Immunoblot analysis of P-gp expression in cell lysates from MCF-7 sublines. Cell extracts (50 μg of protein) were electrophoresed, transferred, and immunoblotted with monoclonal (C219) anti-human P-gp antibody. A monoclonal antibody recognizing actin (45 kDa) was used to normalize protein load. The analysis was repeated twice with protein extracts from separate cell lysates. Final concentrations of drugs were as follows: doxorubicin, 2 μg/ml (3 μM); doxycycline, 100 μg/ml (208 μM); cefoperazone, 2 mg/ml (3 mM); and piperacillin, 8 mg/ml (14 mM). Solubility problems prevented further increases in cefoperazone and piperacillin concentrations. The duration of drug exposure was approximately 12 weeks for doxorubicin and doxycycline and 16 weeks for cefoperazone and piperacillin. Numbers to the left of the top panel are molecular masses in kilodaltons.
Laser cytometric analysis of doxorubicin accumulation-efflux.
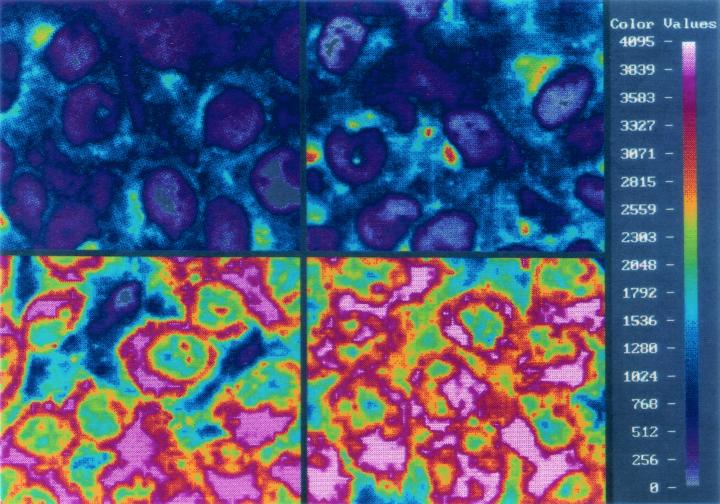
In order to examine the phenotype associated with P-gp expression, intracellular doxorubicin accumulation was assessed by means of laser cytometry. Intracellular doxorubicin accumulation in MCF-7/Adr, MCF-7/doxo, and MCF-7/doxy cells was significantly less than that in MCF-7, MCF-7/cef, and MCF-7/pip cells (data not shown). Intracellular doxorubicin accumulation was also assessed after incubating cells with both doxorubicin and the P-gp-reversing agent verapamil, which prevents P-gp-mediated drug efflux. Figure 4 shows computer-generated images of the fluorescence intensity, representing intracellular doxorubicin accumulation, of MCF-7/Adr and MCF-7/doxy cells in the presence and absence of verapamil. Intracellular concentrations of doxorubicin in MCF-7, MCF-7/Adr, and MCF-7/doxy cells incubated with doxorubicin alone and in combination with verapamil are summarized in Table 1. Both MCF-7/Adr and MCF-7/doxy cells have significantly lower intracellular doxorubicin concentrations than do MCF-7 cells when incubated with doxorubicin, indicating functional expression of P-gp. Addition of verapamil completely reverses the P-gp phenotype, resulting in comparable intracellular doxorubicin concentrations in MCF-7, MCF-7/Adr, and MCF-7/doxy cells. Interestingly, when MCF-7/doxy cells were incubated with doxorubicin alone, they accumulated significantly less doxorubicin than did the positive-control cell line, MCF-7/Adr. These results indicate that P-gp function is independent of the drug used to generate its expression.
FIG. 4.
Digital images of doxorubicin accumulation in MCF-7/Adr (right) and MCF-7/doxy (left) cells. Images at the top represent cells treated with 5 μM doxorubicin for 18 h. Bottom panel represents cells treated with doxorubicin and 10 μM verapamil. Because of the inherent fluorescence of the doxorubicin molecule, fluorescence intensity (scale at far right) corresponds to relative doxorubicin concentrations.
TABLE 1.
Intracellular concentrations of doxorubicin in parental MCF-7 cells and drug-selected sublines as determined by laser cytometry
| Drug | Concn (μM) of doxorubicin in cell linea:
|
||
|---|---|---|---|
| MCF-7 | MCF-7/Adr | MCF-7/doxy | |
| Doxorubicin | 700∗ (7.7) | 329‡ (5.8) | 221§ (5.9) |
| Doxorubicin + verapamil | 770† (5.6) | 770† (5.1) | 756† (7.1) |
Values are expressed as means (standard errors). Values identified with different symbols are significantly different (P < 0.05).
MTT cytotoxicity assay.
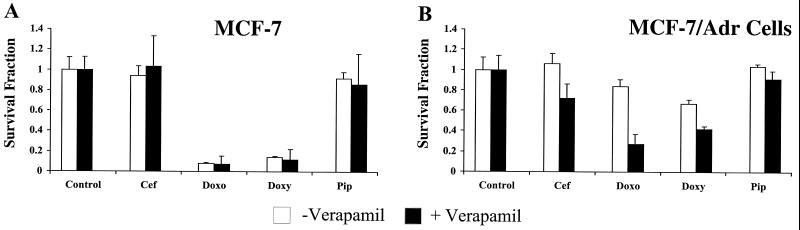
Cytotoxicity assays were performed to determine whether the experimental drug's ability to generate P-gp overexpression was related to its degree of cytotoxicity. Such a relationship would lend support to the theory that P-gp-overexpressing cell lines are generated by selection of a small population of MDR cells. From a teleological perspective, the role of P-gp is to protect cells from potentially toxic xenobiotics (43). Therefore, cells that express P-gp have the greatest chance of survival in the presence of cytotoxic agents, provided that the cytotoxic drug is a substrate for P-gp. Based on this rationale, one would predict that an experimental drug must be both cytotoxic and a substrate for P-gp in order to generate a subline of P-gp-overexpressing, MDR cells. As shown in Fig. 5A, doxorubicin and doxycycline were cytotoxic to P-gp-negative MCF-7 cells whereas no cytotoxicity was observed after a 24-h treatment with cefoperazone or piperacillin, drugs that failed to generate P-gp overexpression. Experiments were then performed to determine if MCF-7/Adr cells were protected against doxycycline-induced cytotoxicity (Fig. 5B). After incubation with doxycycline for 24 h, MCF-7/Adr cells were more resistant to doxycycline-induced cytotoxicity than were P-gp-negative MCF-7 cells. When P-gp function was inhibited by addition of verapamil, MCF-7/Adr cells became susceptible to drug-induced cytotoxicity. Interestingly, it appears that addition of verapamil to MCF-7/Adr cells increased the cytotoxicity of all drugs, not just doxorubicin and doxycycline (Fig. 5B). Since verapamil had no effect on doxycycline-induced cytotoxicity in P-gp-negative MCF-7 cells, it is reasonable to assume that P-gp-mediated drug efflux is at least partially responsible for protection of MCF-7/Adr cells. However, it is likely that induction of P-gp expression requires a combination of several factors, one of which may be exposure to a cytotoxic drug that is a P-gp substrate.
FIG. 5.
Effect of experimental drugs, with or without verapamil, on the sensitivity of parental (MCF-7) and P-gp-expressing (MCF-7/Adr) cells. Cells were incubated in the presence of drug for 24 h, and cell survival was determined by MTT metabolism. Cell survival (mean ± standard deviation) is expressed as a fraction of either untreated or verapamil-treated controls.
DISCUSSION
In the present study, the effects of antimicrobial agents on P-gp expression in MCF-7 breast carcinoma cells were investigated. Results indicate that doxycycline, but not cefoperazone or piperacillin, increases P-gp expression and confers the MDR phenotype, namely, reduced intracellular accumulation of doxorubicin and reversal of this response by the chemosensitizing agent verapamil. In all aspects studied, MCF-7/doxy cells appear identical to the well-characterized P-gp-overexpressing positive-control cell line, MCF-7/Adr. This is the first report of a clinically relevant, nonanticancer drug generating P-gp expression in a cancer cell line. Doxycycline is used for treating a variety of infectious diseases in cancer patients (29, 33, 44). It has also been used previously for chemical pleurodesis in patients with malignant pleural effusions (15, 39) and is currently being investigated as an antiangiogenic agent for the treatment of cancer (19, 23). Administration of doxycycline to cancer patients could result in clinical chemotherapeutic failure as a consequence of generating P-gp-expressing, MDR tumor cell clones.
It is interesting that the most common form of tetracycline resistance among bacteria is via a plasmid-derived, inducible membrane protein that promotes energy-dependent efflux of the tetracyclines (28). Human MDR1 cDNA was recently cloned into a prokaryotic expression vector, resulting in expression of a full-length, immunoreactive, apparently functional P-gp molecule in the membrane fraction of transfected Escherichia coli (17). These cells displayed increased resistance to the P-gp substrates puromycin, tetraphenylphosphonium ion, and tetraphenylarsonium ion. Moreover, cells expressing P-gp demonstrated reduced intracellular accumulation of tetracycline compared to that of cells that did not express P-gp. These results are in agreement with our findings that suggest that doxycycline (a semisynthetic derivative of tetracycline) is a substrate for P-gp.
The conditions under which MDR is acquired during chemotherapy remain poorly understood. Both induction of P-gp expression in previously negative cells and selection of chemoresistant, P-gp-positive cells may be involved in the development of an MDR phenotype in a clinical setting. In cell culture systems, overexpression of P-gp may be a result of transcriptional upregulation (10, 32), gene amplification (12), or RNA stabilization (18). For resistant cell lines generated in this study, it is doubtful that selection alone was responsible for increased MDR1 mRNA and P-gp expression. Cells utilized in this study were expanded from a single cell clone that did not express MDR1 mRNA or P-gp; therefore, selection of a previously drug-resistant population did not occur.
Acknowledgments
This work was supported by National Institutes of Health grant CA 68001 (to K. Mealey; S. Safe, sponsor; D. Kochevar, cosponsor).
REFERENCES
- 1.Baldini, N., K. Scotlandi, G. Barbanti-Brodano, M. C. Manara, D. Maurici, G. Bacci, F. Bertoni, P. Picci, S. Sottili, M. Campanacci, and M. Serra. 1995. P-glycoprotein expression and outcome in high-grade osteosarcoma. N. Engl. J. Med. 333:1380-1385. [DOI] [PubMed] [Google Scholar]
- 2.Barhoumi, R., R. H. Bailey, and R. C. Burghardt. 1995. Kinetic analysis of glutathione in anchored cells with monochlorobimane. Cytometry 19:226-234. [DOI] [PubMed] [Google Scholar]
- 3.Biedler, J. L., and H. Riehm. 1970. Cellular resistance to actinomycin D in Chinese hamster ovary cells in vitro: cross-resistance, autoradiographic, and cytogenetic studies. Cancer Res. 30:1174-1184. [PubMed] [Google Scholar]
- 4.Bradley, G., and V. Ling. 1994. P-glycoprotein, multidrug resistance and tumor progression. Cancer Metastasis Rev. 13:222-233. [DOI] [PubMed] [Google Scholar]
- 5.Chabner, B. A., C. J. Allegra, G. A. Curt, and P. Calabresi. 1996. Antineoplastic agents, p. 1233-1287. In J. G. Hardman, L. E. Limbird, P. B. Molinoff, R. W. Ruddon, and A. G. Gilman (ed.), The pharmacological basis of therapeutics. McGraw-Hill, New York, N.Y.
- 6.Chan, H. S., P. S. Thorner, G. Haddad, and V. Ling. 1990. Immunohistochemical detection of P-glycoprotein: prognostic correlation in soft tissue sarcoma of childhood. J. Clin. Oncol. 8:689-704. [DOI] [PubMed] [Google Scholar]
- 7.Chan, H. S., G. Haddad, P. S. Thorner, G. DeBoer, Y. P. Lin, N. Ondrusek, H. Yeger, and V. Ling. 1991. P-glycoprotein expression as a predictor of the outcome of therapy for neuroblastoma. N. Engl. J. Med. 325:1608-1614. [DOI] [PubMed] [Google Scholar]
- 8.Chan, H. S., T. M. Grogan, G. Haddad, G. DeBoer, and V. Ling. 1997. P-glycoprotein expression: critical determinant in the response to osteosarcoma chemotherapy. J. Natl. Cancer Inst. 89:1706-1715. [DOI] [PubMed] [Google Scholar]
- 9.Chen, C. J., J. E. Chin, D. P. Clark, K. Ueda, I. Pastan, M. M. Gottesman, and I. B. Roninson. 1986. Internal duplication and homology with bacterial transport proteins in the mdr1 (P-glycoprotein) gene from multidrug-resistant human cells. Cell 47:381-389. [DOI] [PubMed] [Google Scholar]
- 10.Chin, K. V., S. S. Chauhan, I. Pastan, and M. Gottesman. 1990. Regulation of mdr RNA levels in response to cytotoxic drugs in rodent cells. Cell Growth Differ. 1:361-365. [PubMed] [Google Scholar]
- 11.Chomczynski, P., and N. Sacchi. 1987. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162:156-159. [DOI] [PubMed] [Google Scholar]
- 12.Davies, R., J. Budworth, J. Riley, R. Snowden, A. Gescher, and T. W. Gant. 1996. Regulation of P-glycoprotein 1 and 2 gene expression and protein activity in two MCF-7/Dox cell line subclones. Br. J. Cancer 73:307-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edlind, T. D. 1989. Tetracyclines as antiparasitic agents: lipophilic derivatives are highly active against Giardia lamblia in vitro. Antimicrob. Agents Chemother. 33:2144-2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Endicott, J. A., and V. Ling. 1989. The biochemistry of P-glycoprotein-mediated multidrug resistance. Annu. Rev. Biochem. 58:137-171. [DOI] [PubMed] [Google Scholar]
- 15.Fenton, K. N., and J. D. Richardson. 1995. Diagnosis and management of malignant pleural effusions. Am. J. Surg. 170:69-74. [DOI] [PubMed] [Google Scholar]
- 16.Fort, J. M., and W. N. Hait. 1990. Pharmacology of drugs that alter multidrug resistance in cancer. Pharmacol. Rev. 42:155-199. [PubMed] [Google Scholar]
- 17.George, A. M., M. W. Dvey, and A. A. Mir. 1996. Functional expression of the human MDR1 gene in Escherichia coli. Arch. Biochem. Biophys. 333:66-74. [DOI] [PubMed] [Google Scholar]
- 18.Germann, U. A. 1996. P-glycoprotein—a mediator of multidrug resistance in tumor cells. Eur. J. Cancer 32A:927-944. [DOI] [PubMed]
- 19.Gilbertson-Beadling, S., E. A. Powers, M. Stamp-Cole, P. S. Scott, T. L. Wallace, J. Copeland, G. Petzold, M. Mitchell, S. Ledbetter, and R. Poorman. 1995. The tetracycline analogs minocycline and doxycycline inhibit angiogenesis in vitro by a non-metalloproteinase-dependent mechanism. Cancer Chemother. Pharmacol. 36:418-424. [DOI] [PubMed] [Google Scholar]
- 20.Goldstein, L. J., H. Glaski, and A. Fojo. 1989. Expression of a human multidrug resistance gene in human cancers. J. Natl. Cancer Inst. 81:116-124. [DOI] [PubMed] [Google Scholar]
- 21.Gosland, M. P., B. L. Lum, and B. I. Sikic. 1989. Reversal by cephalosporins of resistance to etoposide, doxorubicin, and vinblastine in multidrug resistant human sarcoma cells. Cancer Res. 49:6901-6905. [PubMed] [Google Scholar]
- 22.Gottesman, M. M., I. Pastan, and S. V. Ambudkar. 1996. P-glycoprotein and multidrug resistance. Curr. Opin. Genet. Dev. 6:610-617. [DOI] [PubMed] [Google Scholar]
- 23.Guerin, C., J. Laterra, T. Masnyk, L. M. Golub, and H. Brem. 1992. Selective endothelial growth inhibition by tetracyclines that inhibit collagenase. Biochem. Biophys. Res. Commun. 188:740-745. [DOI] [PubMed] [Google Scholar]
- 24.Juliano, R. L., and V. Ling. 1976. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochim. Biophys. Acta 455:152-162. [DOI] [PubMed] [Google Scholar]
- 25.Kohno, K., S. Sato, H. Takano, K. Matsuo, and M. Kuwano. 1989. The direct activation of human multidrug resistance gene (MDR1) by anticancer agents. Biochem. Biophys. Res. Commun. 165:1415-1421. [DOI] [PubMed] [Google Scholar]
- 26.Lampidis, T. J., C. Castello, A. del Giglio, B. C. Pressman, P. Viallet, K. W. Trevorrow, G. K. Valet, H. Tapiero, and N. Savaraj. 1989. Relevance of the chemical charge of rhodamine dyes to multiple drug resistance. Biochem. Pharmacol. 38:4267-4271. [DOI] [PubMed] [Google Scholar]
- 27.Leonessa, F., M. Jacobson, B. Boyle, J. Lippman, M. McGarvey, and R. Clarke. 1994. Effect of tamoxifen on the multidrug-resistant phenotype in human breast cancer cells: isobologram, drug accumulation, and M(r) 170,000 glycoprotein (gp170) binding studies. Cancer Res. 54:441-447. [PubMed] [Google Scholar]
- 28.Levy, S. B. 1992. Active efflux mechanisms for antimicrobial resistance. Antimicrob. Agents Chemother. 36:695-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liston, T. E., and J. E. Koehler. 1996. Granulomatous hepatitis and necrotizing splenitis due to Bartonella henselae in a patient with cancer: case report and review of hepatosplenic manifestations of bartonella infection. Clin. Infect. Dis. 22:951-957. [DOI] [PubMed] [Google Scholar]
- 30.Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265-275. [PubMed] [Google Scholar]
- 31.Marie, J. P., D. C. Zhou, S. Gurbuxani, O. Legrand, and R. Zittoun. 1996. MDR1/P-glycoprotein in haematological neoplasms. Eur. J. Cancer 32A:1034-1038. [DOI] [PubMed]
- 32.Miyazaki, M., K. Kohno, T. Uchiumi, H. Tanimura, K. Matsuo, M. Nasu, and M. Kuwano. 1992. Activation of human multidrug resistance gene-1 promoter in response to heat shock stress. Biochem. Biophys. Res. Commun. 187:677-684. [DOI] [PubMed] [Google Scholar]
- 33.Moreno, F., J. H. Jorgensen, and M. H. Weine. 1994. An old antibiotic for a new multiple-resistant Enterococcus faecium? Diagn. Microbiol. Infect. Dis. 20:41-43. [DOI] [PubMed] [Google Scholar]
- 34.Morrow, C. S., and K. H. Cowan. 1997. Drug resistance and its clinical circumvention, p. 799-815. In J. F. Holland, R. C. Bast, D. L. Morton, E. Frei III, D. W. Kufe, and R. R. Weichselbaum (ed.), Cancer medicine, 4th ed. The Williams & Wilkins Co., Baltimore, Md.
- 35.Neyfakh, A. A. 1997. Natural functions of bacterial multidrug transporters. Trends Microbiol. 5:309-313. [DOI] [PubMed] [Google Scholar]
- 36.Neyfakh, A. A., V. E. Bidnenko, and L. B. Chen. 1991. Efflux-mediated multidrug resistance in Bacillus subtilis: similarities and dissimilarities with the mammalian system. Proc. Natl. Acad. Sci. USA 88:4781-4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nielsen, D., C. Maare, and T. Skovsgaard. 1996. Cellular resistance to anthracyclines. Gen. Pharmacol. 27:251-255. [DOI] [PubMed] [Google Scholar]
- 38.Nussler, V., R. Pelka-Fleischer, H. Zwierzina, C. Nerl, B. Beckert, F. Gieseler, H. Diem, G. Ledderose, E. Gullis, H. Sauer, and W. Wilmanns. 1996. P-glycoprotein expression in patients with acute leukemia—clinical relevance. Leukemia 10(Suppl. 3):S23-S31. [PubMed] [Google Scholar]
- 39.Pulsiripunya, C., P. Youngchaiyud, R. Pushpakom, N. Maranetra, A. Nana, and S. Charoenratanakul. 1996. The efficacy of doxycycline as a pleural sclerosing agent in malignant pleural effusion: a prospective study. Respirology 1:69-72. [DOI] [PubMed] [Google Scholar]
- 40.Saeki, T., K. Ueda, Y. Tanigawara, R. Hori, and T. Komano. 1993. P-glycoprotein-mediated transcellular transport of MDR-reversing agents. FEBS Lett. 324:99-102. [DOI] [PubMed] [Google Scholar]
- 41.Salmon, S. E., T. M. Grogan, T. P. Miller, and W. S. Dalton. 1990. Multidrug resistance. Relevance to adjuvant therapy? Adjuv. Ther. Cancer 6:26-36. [Google Scholar]
- 42.Sande, M. A., and G. L. Mandell. 1996. Tetracyclines, chloramphenicol, erythromycin and miscellaneous antibacterial agents, p. 1123-1153. In J. G. Hardman, L. E. Limbird, P. B. Molinoff, R. W. Ruddon, and A. G. Gilman (ed.), The pharmacological basis of therapeutics. McGraw-Hill, New York, N.Y.
- 43.Sarkadi, B., M. Muller, and Z. Hollo. 1996. The multidrug transporters—proteins of an ancient immune system. Immunol. Lett. 54:215-219. [DOI] [PubMed] [Google Scholar]
- 44.Skiest, D. J., and M. E. Levi. 1998. Catheter-related bacteremia due to Mycobacterium smegmatis. South. Med. J. 91:36-37. [DOI] [PubMed] [Google Scholar]
- 45.Stein, U., W. Walther, and R. H. Shoemaker. 1996. Vincristine induction of mutant and wild-type human multidrug resistance promoters is cell-type-specific and dose-dependent. J. Cancer Res. Clin. Oncol. 122:275-282. [DOI] [PubMed] [Google Scholar]
- 46.van Groenigen, M., L. J. Valentijn, and F. Baas. 1993. Identification of a functional initiator sequence in the human MDR1 promoter. Biochim. Biophys. Acta 1172:138-146. [DOI] [PubMed] [Google Scholar]