Abstract
Amoxicillin at doses of 0.2 to 5 mg/kg of body weight was administered for the treatment of pneumococcal otitis media in a gerbil model. Doses greater than or equal to 2.5 mg/kg, which resulted in concentrations in middle ear fluid of ≥1.4 μg/ml and concentrations in serum higher than the MIC (1 μg/ml) for ≥14% of the dosing interval, were both clinically and bacteriologically effective.
The emergence of penicillin-insensitive Streptococcus pneumoniae strains is now a worldwide problem and causes great concern (2, 3, 12). To overcome this problem in cases of acute otitis media (AOM), a proposal has been made to increase the amoxicillin dose (5) in order to achieve concentrations in serum above the MIC for the pathogen during at least 40% of the dosing interval (4).
The aim of this work was to determine, by means of an experimental model of AOM caused by a penicillin-resistant pneumococcus (penicillin MIC, 2 μg/ml), the minimum dose of amoxicillin able to achieve both clinical and bacteriological success.
One S. pneumoniae serotype 23F strain was used. Amoxicillin trihydrate (SmithKline Beecham Pharmaceuticals, Worthing, England) was used for in vitro studies. For in vivo studies, vials of commercially produced amoxicillin (Clamoxyl; SmithKline Beecham Pharmaceuticals, Toledo, Spain) were employed.
MICs and minimum bactericidal concentrations were determined five times by a microdilution method (10, 11), and median values were considered. Eight- to 9-week-old adult female Mongolian gerbils were inoculated bilaterally with ∼5 × 106 S. pneumoniae CFU per 20 μl, as previously reported (13). AOM and otitis media with effusion (OME) were defined as previously described (13).
The antibiotic was tested at doses of 0.2, 0.4, 0.8, 1.25, 2.5, and 5 mg/kg of body weight and administered subcutaneously (s.c.) in 500-μl volumes at 2, 10, and 18 h postinoculation (p.i.). Animals in the control group received apyrogen sterile distilled water. Treated and control animals were studied daily for otorrhea, weight, and behavior. On day 2 p.i., ears were examined with an otoscope and middle ear (ME) samples were obtained by washing the ME fossa with 20 μl of saline solution (ME with wash fluid [MEWF] samples) for determining the volume and presence of bacteria and cells. Amoxicillin levels in serum at 15, 30, 60, and 120 min after drug administration were determined in groups of six healthy animals after single s.c. injections of the doses that had demonstrated the highest efficacies.
Concentrations of the antibiotic in ME fluid without washing (MEF samples) were also determined for groups of 10 bilaterally inoculated animals. Single s.c. doses of the antibiotic or control were administered 46 h after bacterial inoculation. MEF samples were obtained 90 min later, and aliquots were pooled for determination of the antibiotic concentrations.
Antibiotic concentrations were determined by using Micrococcus luteus ATCC 9341. Assay variability for individual samples was <10%.
Differences in eradication (and presence of otorrhea at day 1) were analyzed by chi-square or Fisher's exact test. Reductions of log10 CFU (assuming values at the detection limit for negative samples) and weights were compared by analysis of covariance, and MEWF volumes were compared by analysis of variance. When P was <0.05, contrasts between groups were determined by the Tukey-Kramer test.
The study was performed in accordance with prevailing regulations regarding the care and use of laboratory animals in the European Community.
The MIC and minimum bactericidal concentration of amoxicillin for the pneumococcal strain used were 1 μg/ml.
After S. pneumoniae inoculation, bilateral AOM was obtained in all untreated animals at day 2, with 100% of the MEWF samples being culture positive. Most MEWF specimens contained moderate amounts of polymorphonuclear cells with intra- and extracellular organisms. Animals were hypoactive, with otorrhea and significant weight loss.
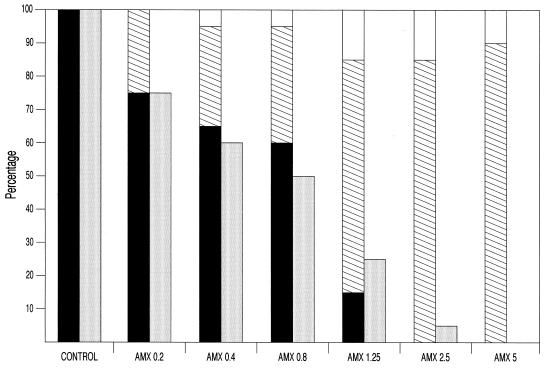
Figure 1 shows the otoscopic results at day 2 in relation to the percentages of samples positive for the pneumococcal strain. All animals receiving amoxicillin at any dose had lower frequencies of AOM as well as fewer culture-positive MEWF samples (with significant reductions in bacterial counts compared to those of the control group, P < 0.05). The lowest dose (0.2 mg/kg) reduced the number of MEWF samples that were culture positive compared to those of the control group, the differences being statistically significant for doses of ≥0.4 mg/kg (P = 0.001). Doses of ≥1.25 mg/kg were more efficacious, with the frequencies of AOM being 15% after the administration of 1.25-mg/kg doses and 0% for 2.5- and 5-mg/kg doses. However, in these groups, only 10 to 15% of the animals showed no signs of otitis, with the majority of them (70 to 90%) showing OME. The pathogen was isolated only in a few MEWF samples (25 and 5% of MEWF samples after doses of 1.25 and 2.5 mg/kg, respectively). No MEWF samples from animals receiving 5 mg/kg were culture positive. Doses of ≥2.5 mg/kg significantly reduced the number of MEWF samples that were culture positive compared to those of the control group and the samples treated with doses of ≤0.8 mg/kg (P < 0.05).
FIG. 1.
Otoscopic results at day 2. Solid bars, acute otitis media; hatched bars, otitis media with effusion; open bars, no otitis; shaded bars, percentages of middle ear samples positive for S. pneumoniae. AMX, amoxicillin. Doses are given in milligrams per kilogram.
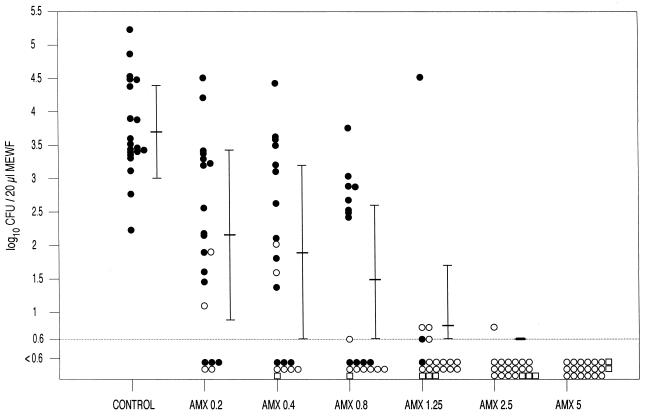
Figure 2 shows the results of bacterial counts and type of otitis (AOM or OME) determined for each group of animals. The number of organisms decreased in a dose-related manner. Regardless of the treatment group, 87.7% of the MEWF specimens with OME were culture negative.
FIG. 2.
Bacterial counts in the MEWF samples from control and treated animals. Solid circles, acute otitis media; open circles, otitis media with effusion; open squares, no otitis. AMX, amoxicillin. Doses are given in milligrams per kilogram.
Table 1 presents comparisons of the therapeutic results for the control and treated animal groups. There was a direct relationship between culture-positive samples and the presence of otorrhea, with otorrhea occurring less frequently in animals with lower percentages of culture-positive specimens. Doses of ≥1.25 mg/kg significantly reduced the incidence of otorrhea in the treated animal group compared to that in the control group (P ≤ 0.05), and doses of ≥2.5 mg/kg significantly reduced the incidence of otorrhea compared to that in any other treated or untreated group (P < 0.05). Analysis of the fluid volumes recovered showed that after the administration of 5-mg/kg doses, significantly greater volumes were obtained compared to those obtained from animals in the control group. All treated animals lost less weight than the animals in the untreated, control group, although significant differences were found only for those animals receiving doses of ≥1.25 mg/kg (P < 0.05). Significant differences were also found in weight loss between the animals in the groups treated with low doses (≤0.8 mg/kg) and those in the groups treated with high doses (≥2.5 mg/kg).
TABLE 1.
Relationships between animals with culture-positive ME samples and frequencies of otorrhea, volumes of MEWF recovered, and variations in body weight in an experimental model of otitis media caused by S. pneumoniaea
| Group and antibiotic dose (mg/kg) | No. (%) of animals with culture-positive ME samples
|
Frequency of otorrhea (%) | MEWF vol (μl) (mean ± SD) | Body wt loss (%) (mean ± SD) | ||
|---|---|---|---|---|---|---|
| Bilateral | Unilateral | Total | ||||
| Untreated controls | 10 (100) | 0 (0) | 10 (100) | 100 | 20.3 ± 7.7 | 10.4 ± 1.7 |
| Amoxicillin-treated animals | ||||||
| 0.2 | 6 (60) | 3 (30) | 9 (90) | 85 | 24.4 ± 8.6 | 9.3 ± 2.7 |
| 0.4 | 5 (50) | 2 (20) | 7 (70) | 80 | 25.5 ± 7.2 | 7.6 ± 3.8 |
| 0.8 | 4 (40) | 2 (20) | 6 (60) | 80 | 26.0 ± 9.1 | 7.4 ± 3.8 |
| 1.25 | 2 (20) | 1 (10) | 3 (30) | 40b | 22.7 ± 9.5 | 3.9 ± 2.7b |
| 2.5 | 0 (0) | 1 (10) | 1 (10) | 10b | 24.9 ± 8.9 | 1.8 ± 2.6b |
| 5.0 | 0 (0) | 0 (0) | 0 (0) | 10b | 30.9 ± 11.1b | 2.1 ± 2.7b |
Data at day 2, except those for frequency of otorrhea (day 1).
Significantly different (P ≤ 0.05) compared with value for control group.
After the administration of either 2.5 or 5 mg of amoxicillin/kg, the samples taken 15 min after drug administration showed the highest concentrations of the antibiotic (C15min) and were used for calculating the serum inhibitory quotient (C15min/MIC).
Table 2 presents the serum and MEF pharmacodynamic data in relation to bacterial eradication. After the administration of amoxicillin at doses of either 2.5- or 5-mg/kg, which correlates with a serum inhibitory quotient of >4 and a time during which concentrations in serum are above the MIC (T > MIC) of ≥69 min (equivalent to ≥14.3% of the dosing interval), bacteriological eradication was obtained in ≥95% of the ME samples. The MEF antibiotic concentrations were ≥1.4 μg/ml, which corresponded to MEF inhibitory quotients (MEF concentration/MIC) of >1. The concentrations obtained in serum and ME in the gerbils after the administration of 2.5-mg/kg treatment doses were similar to those usually achieved in children after the administration of standard doses of this antibiotic (6), the latter concentration being in the range of 1 to 6 μg/ml (1, 6, 8), which might be expected to eradicate many penicillin-insensitive pneumococci. Our results show that amoxicillin, even at a very low dose (0.2 mg/kg), improved clinical cure rates and enhanced bacteriological eradication, which agrees with the observation that such antibiotic concentrations may act synergistically with polymorphonuclear neutrophils (7, 9). Higher doses that achieve ME antibiotic concentrations close to the MIC allowed more efficient clearing of the organism. The antibiotic concentration in the ME seems to be more reliable than the serum antibiotic concentration for predicting efficacy, because a short T > MIC (less than 20% of the dosing interval) in serum may be associated with cure if a sufficient antibiotic concentration in the ME is achieved. Due to this fact, we believe that amoxicillin remains the antimicrobial drug of first choice for the treatment of uncomplicated pneumococcal AOM (5), although higher doses might be necessary if highly penicillin-resistant pneumococci are involved.
TABLE 2.
Serum and MEF pharmacodynamic data of amoxicillin in relation to the eradication of S. pneumoniae
| Amoxicillin treatment group dose (mg/kg) | Serum
|
MEF antibiotic concn/ MIC | % Eradication | ||
|---|---|---|---|---|---|
| C15min/MIC | T > MIC (min) (% dose interval) | AUC/MICb | |||
| 2.5 | 4.7 | 69 (14.3) | 4.3 | 1.4 | 95 |
| 5.0 | 12.2 | 93 (19.4) | 10.2 | 2.4 | 100 |
Antibiotic concentration in MEF 90 min after drug administration.
AUC, area under the curve.
Acknowledgments
This work was supported by a grant from SmithKline Beecham Pharmaceuticals, Madrid, Spain. A.P. and G.G.-C. were aided by scholarships from the Fundación Conchita Rábago, Madrid, Spain.
We thank A. Carcas (Clinical Pharmacology, Universidad Autónoma, Madrid, Spain) for the pharmacokinetic analysis.
REFERENCES
- 1.Brook, I., and P. Yocum. 1995. Bacteriology and beta-lactamase activity in ear aspirates of acute otitis media that failed amoxicillin therapy. Pediatr. Infect. Dis. J. 14:805-808. [PubMed] [Google Scholar]
- 2.Butler, J. C., J. Hofmann, M. S. Cetron, J. A. Elliott, R. R. Facklam, R. F. Breiman, and the Pneumococcal Sentinel Surveillance Working Group. 1996. The continued emergence of drug-resistant Streptococcus pneumoniae in the United States: an update from the Centers for Disease Control and Prevention's Pneumococcal Sentinel Surveillance System. J. Infect. Dis. 174: 986-993. [DOI] [PubMed] [Google Scholar]
- 3.Castillo, F., F. Baquero-Artigao, and A. García-Perea. 1998. Influence of recent antibiotic therapy on antimicrobial resistance of Streptococcus pneumoniae in children with acute otitis media in Spain. Pediatr. Infect. Dis. J. 17:94-97. [DOI] [PubMed] [Google Scholar]
- 4.Craig, W. A., and D. Andes. 1996. Pharmacokinetics and pharmacodynamics of antibiotics in otitis media. Pediatr. Infect. Dis. J. 15:255-259. [DOI] [PubMed] [Google Scholar]
- 5.Dowell, S. F., J. C. Butler, J. S. Giebink, M. R. Jacobs, D. Jernigan, D. M. Musher, A. Rakowsky, B. Schwartz, and the Drug-resistant Streptococcus pneumoniae Therapeutic Working Group. 1999. Acute otitis media: management and surveillance in an era of pneumococcal resistance—a report from the Drug-Resistant Streptococcus pneumoniae Therapeutic Working Group. Pediatr. Infect. Dis. J. 18:1-9. [PubMed] [Google Scholar]
- 6.Ginsburg, C. M., G. H. McCracken, Jr., and J. D. Nelson. 1981. Pharmacology of oral antibiotics used for treatment of otitis media and tonsillopharyngitis in infants and children. Ann. Otol. Rhinol. Laryngol. 90(Suppl.):37-43. [DOI] [PubMed] [Google Scholar]
- 7.Gómez-Lus, M. L., M. J. Giménez, J. Prieto, M. Martín, J. Frías, and L. Aguilar. 1998. Effect of polymorphonuclear neutrophils on serum bactericidal activity against Streptococcus pneumoniae after amoxicillin administration. Eur. J. Clin. Microbiol. Infect. Dis. 17:40-43. [DOI] [PubMed] [Google Scholar]
- 8.Krause, P. J., N. J. Owens, C. H. Nightingale, J. J. Klimek, W. B. Lehmann, and R. Quintiliani. 1982. Penetration of amoxicillin, cefaclor, erythromycin-sulfisoxazole, and trimethoprim-sulfamethoxazole into the middle ear fluid of patients with chronic serous otitis media. J. Infect. Dis. 145: 815-821. [DOI] [PubMed] [Google Scholar]
- 9.Martín, M., M. L. Gómez-Lus, L. Aguilar, P. Martínez, M. J. Giménez, and J. Prieto. 1997. Effect of clavulanic acid and/or polymorphonuclear neutrophils on amoxicillin bactericidal activity against Streptococcus pneumoniae. Eur. J. Clin. Microbiol. Infect. Dis. 16:512-516. [DOI] [PubMed] [Google Scholar]
- 10.National Committee for Clinical Laboratory Standards. 1997. Methods for dilution antimicrobial susceptibility test for bacteria that grow aerobically. Document M7-A4. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 11.National Committee for Clinical Laboratory Standards. 1992. Methods for determining bactericidal activity of antimicrobial agents. Document M26-T. National Committee for Clinical Laboratory Standards, Villanova, Pa.
- 12.Sahm, D. F., M. E. Jones, M. L. Hickey, D. R. Diakun, S. V. Mani, and C. Thornsberry. 2000. Resistance surveillance of Streptococcus pneumoniae, Haemophilus influenzae and Moraxella catarrhalis isolated in Asia and Europe, 1997-1998. J. Antimicrob. Chemother. 45:457-466. [DOI] [PubMed] [Google Scholar]
- 13.Soriano, F., A. Parra, C. Cenjor, E. Nieto, G. García-Calvo, M. J. Giménez, L. Aguilar, and C. Ponte. 2000. Role of Streptococcus pneumoniae and Haemophilus influenzae in the development of acute otitis media and otitis media with effusion in a gerbil model. J. Infect. Dis. 181: 646-652. [DOI] [PubMed] [Google Scholar]