Abstract
New drugs and molecular targets are needed against Trypanosoma brucei, the protozoan that causes African sleeping sickness. Tryptanthrin (indolo[2,1-b]quinazoline-6,12-dione), a traditional antifungal agent, and 11 analogs were tested against T. brucei in vitro. The greatest activity was conferred by electron-withdrawing groups in the 8 position of the tryptanthrin ring system; the most potent compound had a 50% effective concentration of 0.40 μM.
Members of the Trypanosoma brucei species are flagellated protozoa that are transmitted by tsetse flies. They cause sleeping sickness in humans and a related disease in cattle. The characteristic meningoencephalitis of African sleeping sickness is fatal if it is not treated, and currently available drugs are limited by toxicity and growing parasite resistance (7, 9, 12). Unfortunately, in recent years there has been a dramatic and devastating resurgence of sleeping sickness (14). The need for safe and effective new antitrypanosomal agents is pressing.
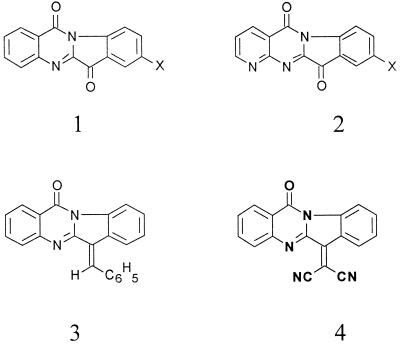
Tryptanthrin (indolo[2,1-b]quinazoline-6,12-dione; in Fig. 1, X is H in tryptanthrin) is a weakly basic alkaloid found in a number of plant species (11). This alkaloid is unusual in that its synthesis was described a half century before it was discovered as a natural product (6). Tryptanthrin is the active principal of a traditional Japanese herbal remedy for fungal infections (8). Subsequent studies extended the spectrum of antimicrobial activity to include bacteria (10, 11), particularly Mycobacterium tuberculosis (1). The activity of tryptanthrin against this intracellular organism prompted us to evaluate tryptanthrin and a series of derivatives of tryptanthrin against intracellular parasites, including Plasmodium falciparum, which causes malaria (13). In this report we describe the activities of a series of substituted tryptanthrins and 4-azatryptanthrins (Fig. 1) against Trypanosoma brucei, an extracellular protozoan parasite.
FIG. 1.
Compounds synthesized and analyzed for antitrypanosomal activities include tryptanthrins (ring system 1), 4-azatryptanthrins (ring system 2), a 6-benzylidene analog (ring system 3), and a dicyanomethylidene derivative (ring system 4).
Tryptanthrins.
Tryptanthrin compounds were synthesized as described by Baker and Mitscher (1) and were characterized by their infrared and mass spectra. Stock solutions were prepared in dimethyl sulfoxide (99.8%; 27,685-5; Aldrich) and were then serially diluted into culture medium.
Assay.
Bloodstream-form T. brucei (MiTat 1.2, strain 427) organisms were grown axenically (5) at 37°C in HEPES-buffered Iscove's modified Dulbecco's medium that did not contain phenol red but that was supplemented with glutamate, hypoxanthine, cysteine, thymidine, sodium pyruvate, mercaptoethanol, bathocuproinedisulfonate, 10% Serum Plus, and 10% heat-inactivated fetal bovine serum, as described previously (2). Ten concentrations of each tryptanthrin were assayed in quadruplicate. Exponentially growing cells were incubated in 96-well plates (Falcon no. 3072 plates; Becton Dickinson) with or without test compound for 20 h and were then lysed and incubated for 3 to 6 h with p-nitrophenol phosphate. The acid phosphatase activity was determined, and 50% effective concentrations (EC50s) were obtained from curves fit to the data (2, 4). Each compound was assayed twice in this fashion. As an experimental control, the EC50 of berenil, a recognized antitrypanosomal drug, was determined concurrently.
Antitrypanosomal activity.
We tested tryptanthrin and 11 derivatives for their activities against axenically cultured bloodstream-form T. brucei (Table 1). These compounds exhibited significant antitrypanosomal activities. The least soluble of the analogs exhibited the lowest activity. Thus, tryptanthrin (compound 1a) and 8-trifluoromethoxytryptanthrin (compound 1f) had EC50s of 23 and 38 μM, respectively. At its maximal solubility of 40 μM, 4-azatryptanthrin was less than 20% effective. The activity of 6-dicyanomethylidenetryptanthrin (compound 4) at its solubility limit of 8.4 μM was also limited to 20%. The relatively high antitrypanosomal activity of 6-benzylidenetryptanthrin (compound 3) is rather surprising in view of its increased molecular weight and the loss of the aqueous solubility-conferring 6-keto group. Antitrypanosomal activity was markedly improved by the presence of an electron-withdrawing group (halogen or nitro) at position 8 of the tryptanthrin or azatryptanthrin ring system: these analogs were up to 100-fold more active than the unsubstituted parent. In this regard, it is interesting that the most active analog, 4-aza-8-bromotryptanthrin (compound 2d; EC50, 0.40 μM) possessed both 4-aza- and 8-bromo substituents. Submicromolar activity was also exhibited by compound 1e (8-nitrotryptanthrin; EC50, 0.82 μM). In our hands, the antitrypanosomal activities of the tryptanthrins (low micromolar EC50s) are comparable to those that we obtained for clinically useful antitrypanosomal agents, such as pentamidine and difluoromethylornithine (0.02 and 22 μM, respectively) (3). The tryptanthrins represent a new class of agents with significant in vitro activity against T. brucei. Further investigation of this lead may reveal a novel molecular target amenable to exploitation in the search for much-needed new chemotherapeutic agents against sleeping sickness.
TABLE 1.
Activities of tryptanthrins against T. brucei in vitro
| Compound | Structurea
|
Antitrypanosomal activity (EC50)b
|
||
|---|---|---|---|---|
| Ring system | X | μg/ml | μM | |
| 1a (tryptanthrin) | 1 | H | 5.6 | 23.0 |
| 1b | 1 | F | 0.59 | 2.2 |
| 1c | 1 | Cl | 0.52 | 1.8 |
| 1d | 1 | Br | 1.6 | 4.9 |
| 1e | 1 | NO2 | 0.24 | 0.82 |
| 1f | 1 | OCF3 | 12.5c | 38.0c |
| 2a | 2 | H | 10.0d | 40.0d |
| 2b | 2 | F | 0.37 | 1.4 |
| 2c | 2 | Cl | 1.3 | 4.6 |
| 2d | 2 | Br | 0.13 | 0.4 |
| 3 | 3 | 0.81 | 2.5 | |
| 4 | 4 | 2.5e | 8.4e | |
See Fig. 1 for the structures of ring systems 1 to 4 and the positions of the x substituents.
The results are the averages of two independent experiments. Differences between the average value and the individual EC50s do not exceed 30%. The r2 (goodness of fit) value obtained for each concentration-cell killing curve was ≥0.95 (0.98 ± 0.02 [mean ± standard deviation]). The EC50 for concurrent berenil controls was 0.31 ± 0.08 μM.
Fifty percent inhibition at the indicated limit of solubility.
Less than 20% inhibition at the indicated limit of solubility.
About 20% inhibition at the indicated limit of solubility.
Acknowledgments
This work was supported by Public Health Service grant AI28855 from the National Institutes of Health and by the Burroughs Wellcome Fund.
REFERENCES
- 1.Baker, W. R., and L. A. Mitscher. August 1995. Indolo[2,1-bi]quinazoline-6,12-dione antibacterial compounds and methods of use thereof. U.S. patent 5,441,955.
- 2.Bodley, A. L., M. W. McGarry, and T. A. Shapiro. 1995. Drug cytotoxicity assay for African trypanosomes and Leishmania species. J. Infect. Dis. 172:1157-1159. [DOI] [PubMed] [Google Scholar]
- 3.Bodley, A. L., and T. A. Shapiro. 1995. Molecular and cytotoxic effects of camptothecin, a topoisomerase I inhibitor, on trypanosomes and Leishmania. Proc. Natl. Acad. Sci. USA 92:3726-3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bodley, A. L., M. C. Wani, M. E. Wall, and T. A. Shapiro. 1995. Antitrypanosomal activity of camptothecin analogs: structure-activity correlations. Biochem. Pharmacol. 50:937-942. [DOI] [PubMed] [Google Scholar]
- 5.Carruthers, V. B., and G. A. M. Cross. 1992. High efficiency clonal growth of bloodstream- and insect-form Trypanosoma brucei on agarose plates. Proc. Natl. Acad. Sci. USA 89:8818-8821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friedlander, P., and R. Roschdestwensky. 1915. An oxidation product of indigo blue. Ber. Dtsh. Chem. Ges. 48:1841-1847. [Google Scholar]
- 7.Hajduk, S. L., P. T. Englund, and D. H. Smith. 1990. African trypanosomiasis, p. 268-281. In K. S. Warren and A. A. F. Mahmoud (ed.), Tropical and geographical medicine. McGraw-Hill Book Co., New York, N.Y.
- 8.Honda, G., and M. Tabata. 1979. Isolation of antifungal principle tryptanthrin, from Strobilanthes cusia. Planta Med. 36:85-90. [DOI] [PubMed] [Google Scholar]
- 9.Mäser, P., and R. Kaminsky. 1997. The mechanisms of drug resistance in Trypanosoma brucei spp. Recent Res. Dev. Antimicrob. Agents Chemother. 2:113-125. [Google Scholar]
- 10.Mitscher, L. A., and W. Baker. 1998. A search for novel chemotherapy against tuberculosis amongst natural products. Pure Appl. Chem. 70:365-371. [Google Scholar]
- 11.Mitscher, L. A., W.-C. Wong, T. DeMeulenaere, J. Sulko, and S. Drake. 1981. Antimicrobial agents from higher plants. New synthesis and bioactivity of tryptanthrin (indolo[2,1b]quinazolin-6,12-dione) and its analogs. Heterocycles 15:1017-1018. [Google Scholar]
- 12.Pépin, J., F. Milord, A. Khonde, T. Niyonsenga, L. Loko, and B. Mpia. 1994. Gambiense trypanosomiasis: frequency of, and risk factors for, failure of melarsoprol therapy. Trans. R. Soc. Trop. Med. Hyg. 88:447-452. [DOI] [PubMed] [Google Scholar]
- 13.Pitzer, K. K., D. E. Kyle, and L. Gerena. September 2001. Indolo[2,1-b]quinazole-6,12-dione antimalarial compounds and methods of treating malaria therewith. U.S. patent 6,284,772.
- 14.Smith, D. H., J. Pepin, and A. H. R. Stich. 1998. Human African trypanosomiasis: an emerging public health crisis. Br. Med. Bull. 54:341-355. [DOI] [PubMed] [Google Scholar]