Abstract
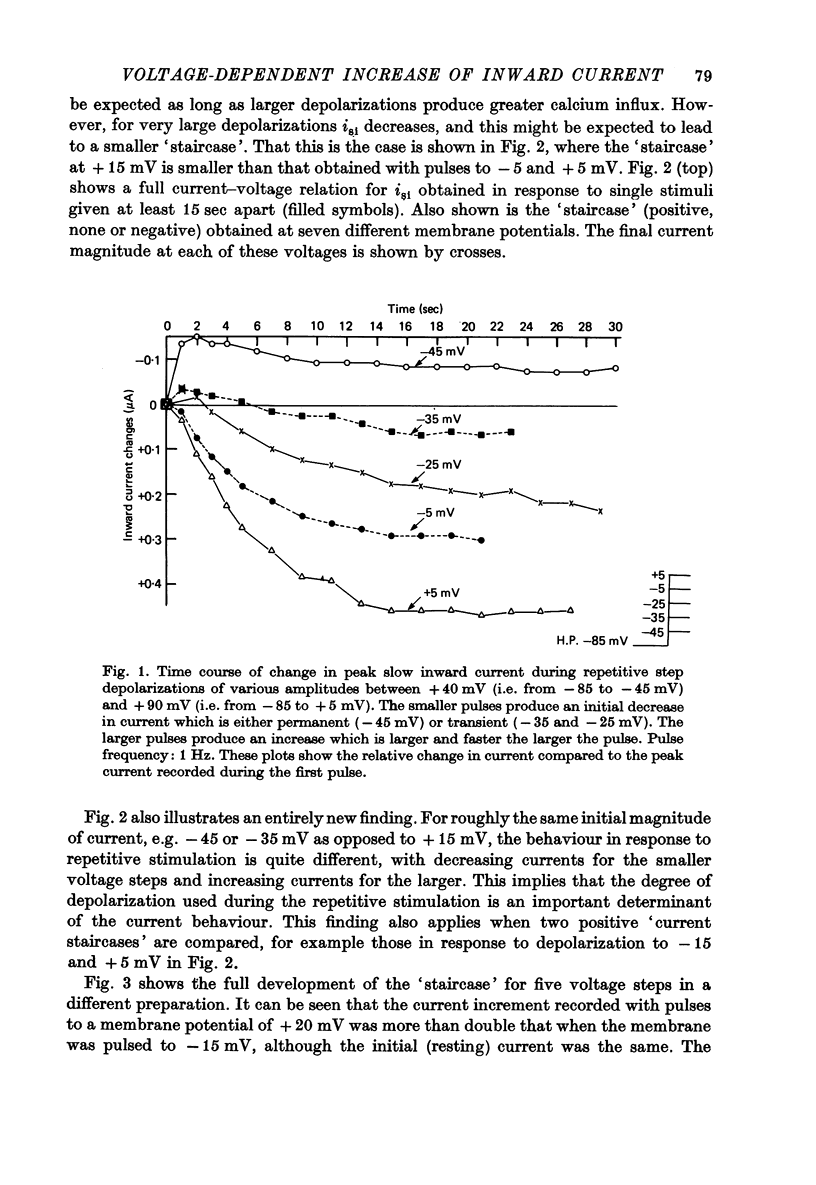
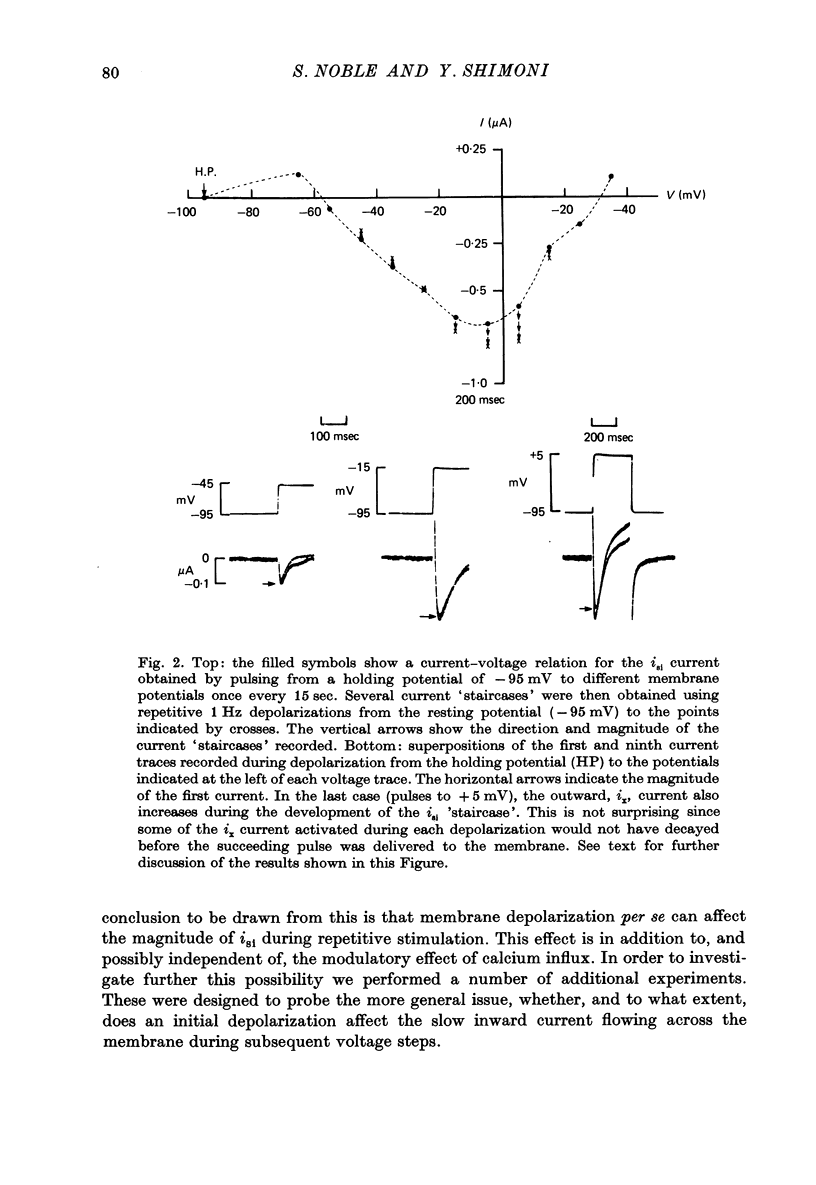
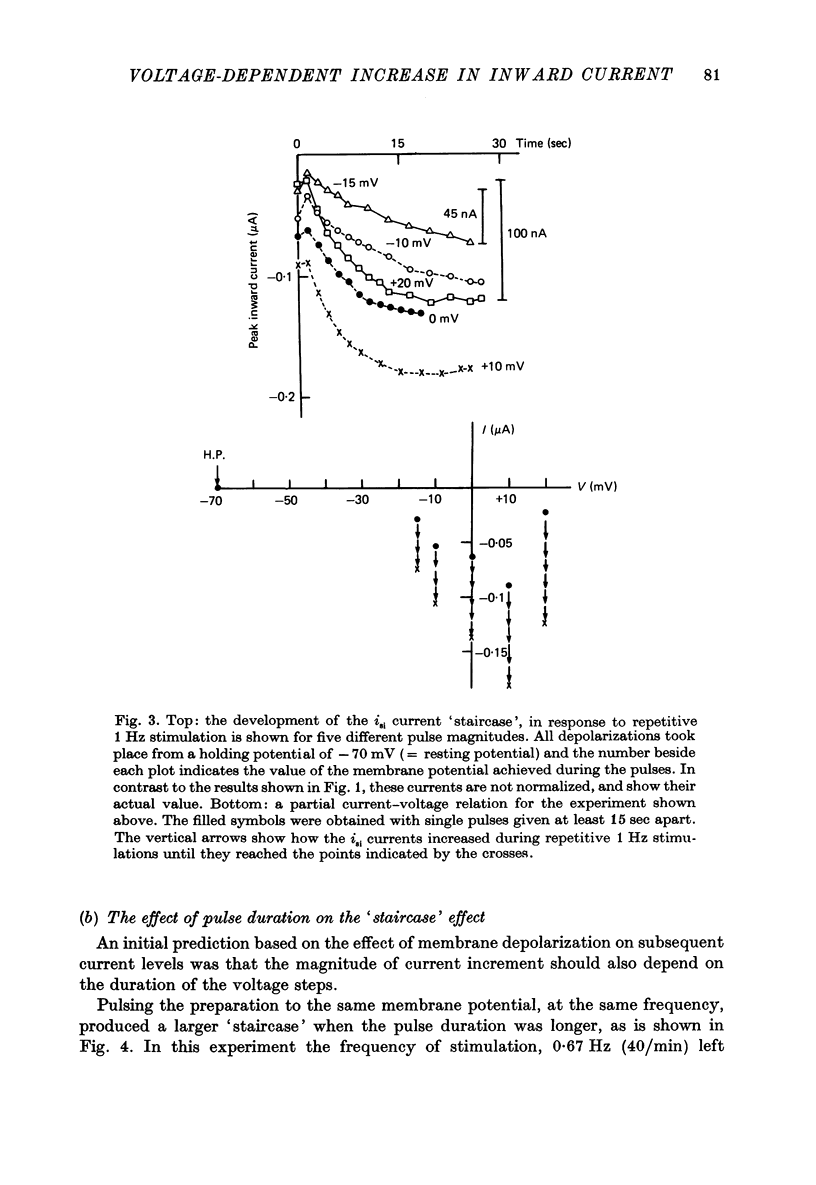
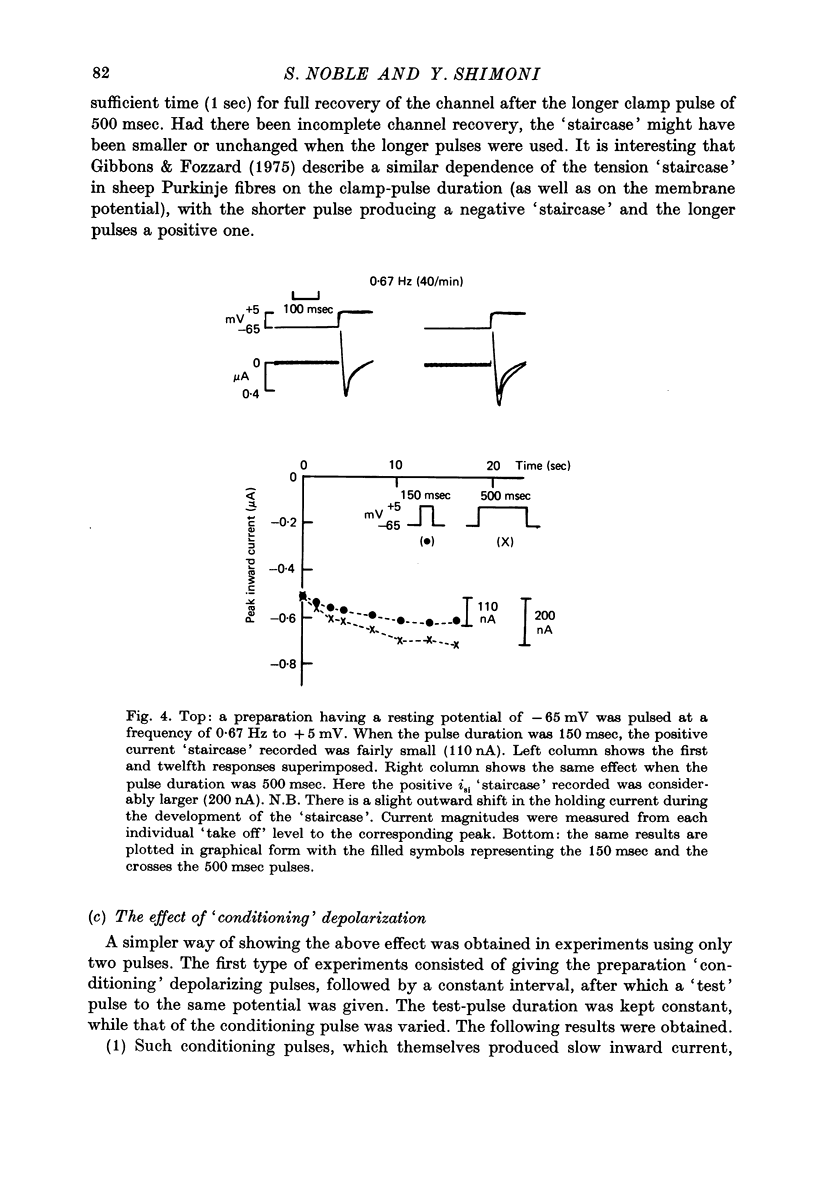
1. Slow inward currents, isi, were measured using a double sucrose-gap voltage-clamp technique during experiments involving different depolarization protocols. 2. Following short rest periods, repetitive stimulation gave rise to slow inward currents which changed progressively in a voltage-dependent manner. Small voltage depolarizations gave rise to initial small decreases in current (negative 'staircase'). The current usually then showed a secondary increase but still remained, in most cases, below the control amplitude. Larger depolarizations produced increasingly larger and more rapidly rising positive current 'staircases'. 3. The amount of increase in current magnitude during repetitive depolarization was more strongly dependent on the size of the voltage step than on the amplitude of the initial current. 4. Twin-pulse experiments having a fixed interval between pulses (usually 1.0 sec), showed that 'test' pulses produced more slow inward current than preceding 'conditioning' depolarizations. The augmentation was larger following conditioning pulses of longer duration. Current augmentation was larger during 'staircase' obtained with longer pulses. 5. Following constant duration 'conditioning' depolarizations, varying the interpulse interval showed that the slow inward current flowing during the 'test' pulse could be augmented following intervals of as long as 6-8 sec. 6. Small pre-pulses also augmented the slow inward currents obtained in response to 'tet' pulses. 7. Experiments in calcium-free solutions (with EGTA) showed qualitatively similar 'staircase' effects and current augmentation following preceding depolarizations. 8. Experiments in which sodium was replaced by lithium gave rise to larger slow inward currents. The 'staircase' for such currents developed in a similar manner to that seen under control conditions. 9. It is concluded that slow inward current augmentation is produced by membrane depolarization in a fashion which is at least partially independent of calcium or sodium ion influx.
Full text
PDF

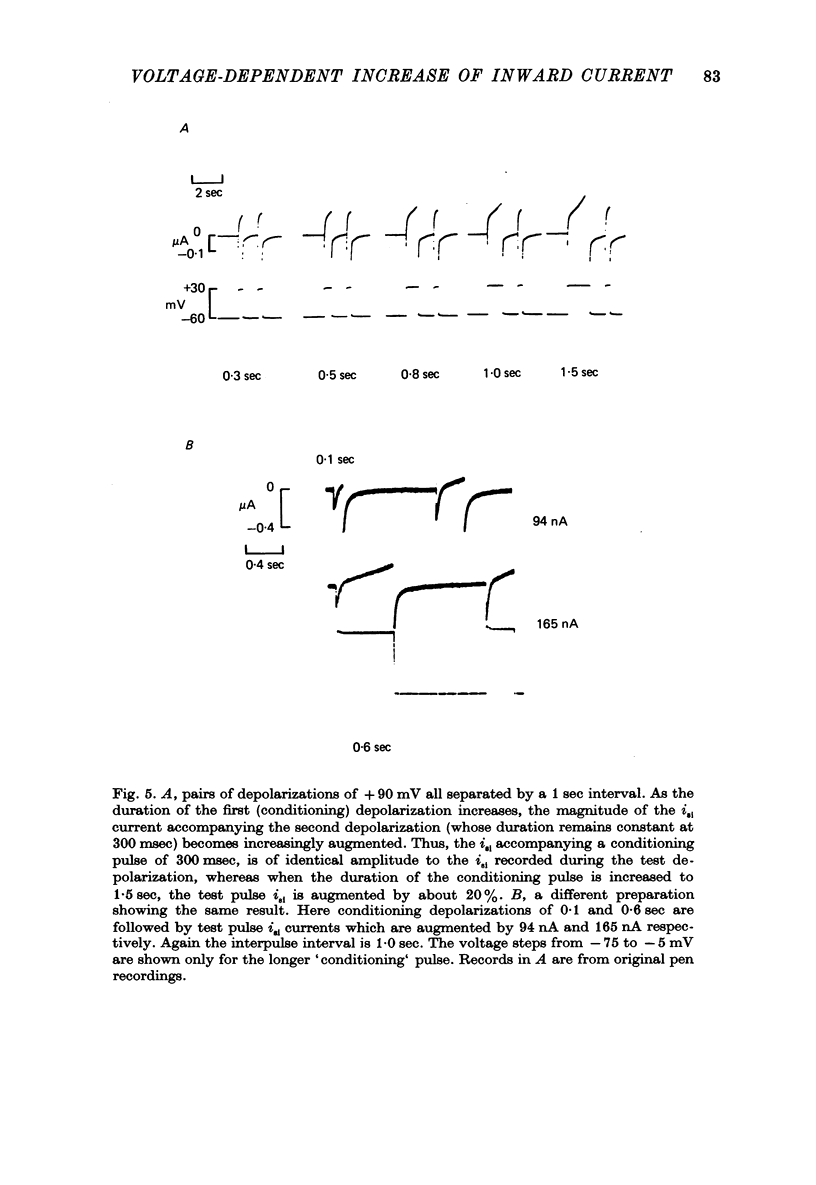
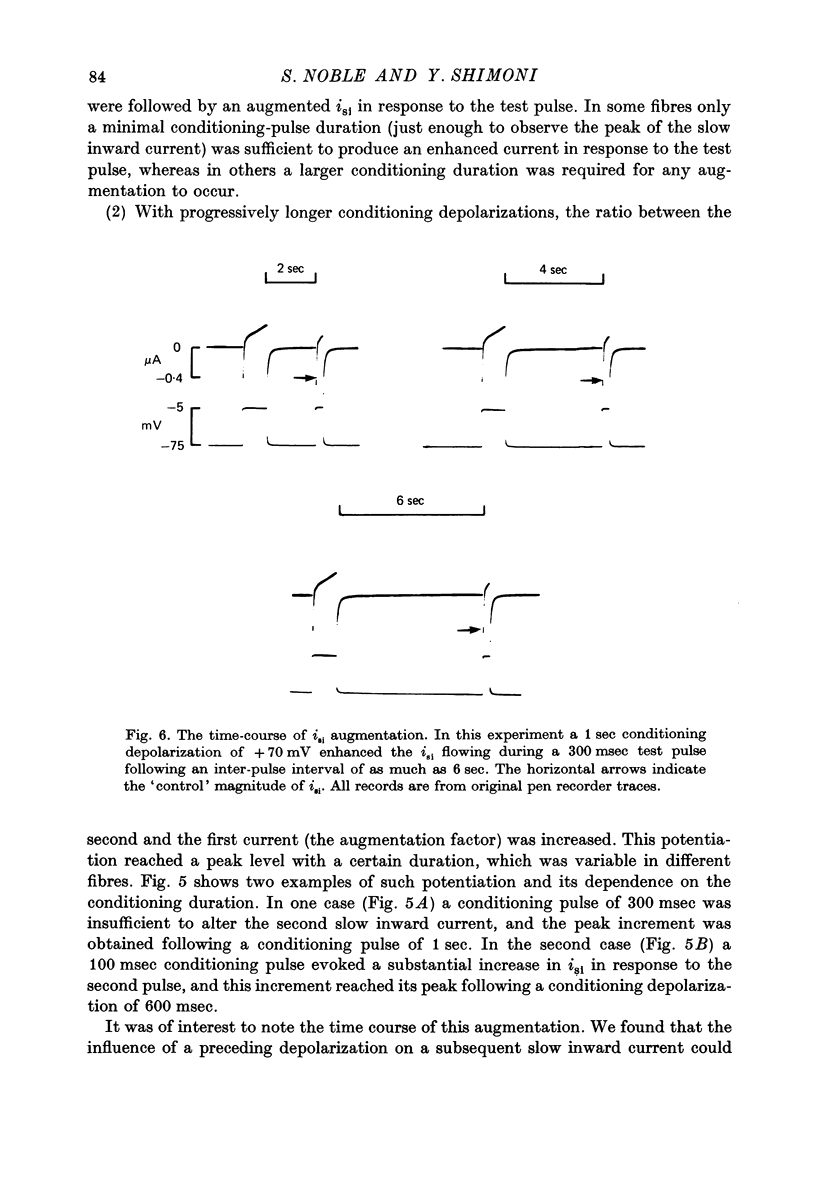
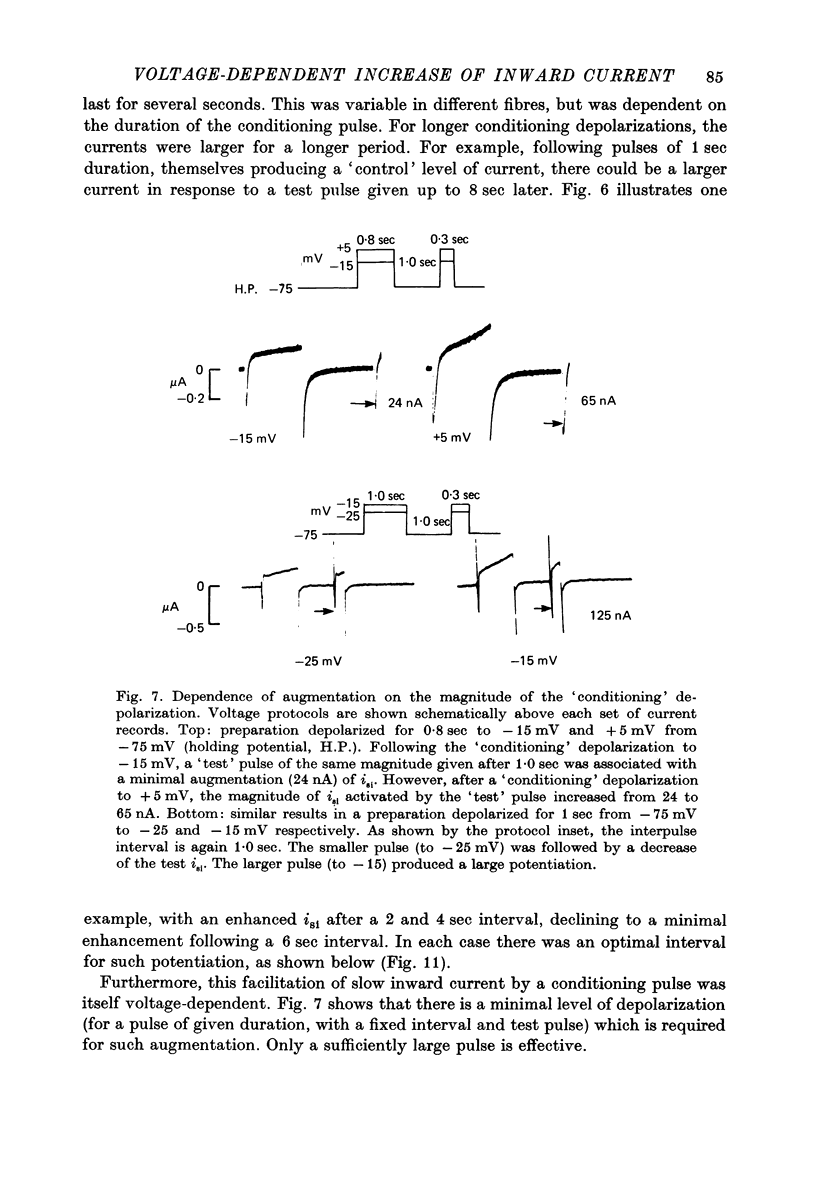

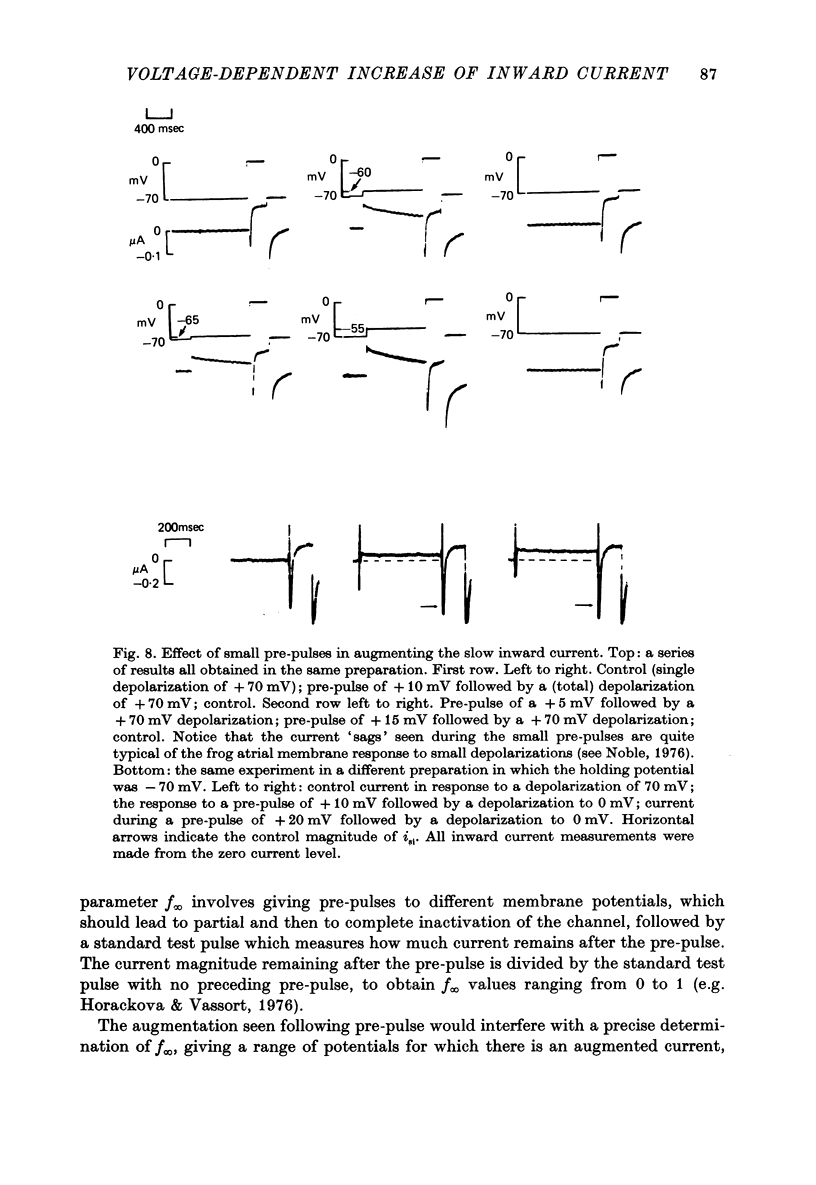



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Besseau A. Analyse, selon le modèle de Hodgkin-Huxley, des conductances membranaires du myocarde de grenouille (Rana esculenta. J Physiol (Paris) 1972;64(6):647–670. [PubMed] [Google Scholar]
- Chandler W. K., Rakowski R. F., Schneider M. F. A non-linear voltage dependent charge movement in frog skeletal muscle. J Physiol. 1976 Jan;254(2):245–283. doi: 10.1113/jphysiol.1976.sp011232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesnais J. M., Coraboeuf E., Sauviat M. P., Vassas J. M. Sensitivity to H, Li and Mg ions of the slow inward sodium current in frog atrial fibres. J Mol Cell Cardiol. 1975 Sep;7(9):627–642. doi: 10.1016/0022-2828(75)90140-6. [DOI] [PubMed] [Google Scholar]
- Gibbons W. R., Fozzard H. A. Relationships between voltage and tension in sheep cardiac Purkinje fibers. J Gen Physiol. 1975 Mar;65(3):345–365. doi: 10.1085/jgp.65.3.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horackova M., Vassort G. Calcium conductance in relation to contractility in frog myocardium. J Physiol. 1976 Aug;259(3):597–616. doi: 10.1113/jphysiol.1976.sp011485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass R. S., Siegelbaum S., Tsien R. W. Incomplete inactivation of the slow inward current in cardiac Purkinje fibres [proceedings]. J Physiol. 1976 Dec;263(1):127P–128P. [PubMed] [Google Scholar]
- Miller D. J., Mörchen A. On the effects of divalent cations and ethylene glycol-bis-(beta-aminoethyl ether) N,N,N',N'-tetraacetate on action potential duration in frog heart. J Gen Physiol. 1978 Jan;71(1):47–67. doi: 10.1085/jgp.71.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIEDERGERKE R. MOVEMENTS OF CA IN FROG HEART VENTRICLES AT REST AND DURING CONTRACTURES. J Physiol. 1963 Jul;167:515–550. doi: 10.1113/jphysiol.1963.sp007166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima Y., Endo M. Release of calcium induced by 'depolarisation' of the sarcoplasmic reticulum membrane. Nat New Biol. 1973 Dec 19;246(155):216–218. doi: 10.1038/newbio246216a0. [DOI] [PubMed] [Google Scholar]
- Noble S. J. Potassium accumulation and depletion in frog atrial muscle. J Physiol. 1976 Jul;258(3):579–613. doi: 10.1113/jphysiol.1976.sp011436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble S., Shimoni Y. The calcium and frequency dependence of the slow inward current 'staircase' in frog atrium. J Physiol. 1981 Jan;310:57–75. doi: 10.1113/jphysiol.1981.sp013537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochi R., Trautwein W. The dependence of cardiac contraction on depolarization and slow inward current. Pflugers Arch. 1971;323(3):187–203. doi: 10.1007/BF00586383. [DOI] [PubMed] [Google Scholar]
- Reuter H. Divalent cations as charge carriers in excitable membranes. Prog Biophys Mol Biol. 1973;26:1–43. doi: 10.1016/0079-6107(73)90016-3. [DOI] [PubMed] [Google Scholar]
- Reuter H. Properties of two inward membrane currents in the heart. Annu Rev Physiol. 1979;41:413–424. doi: 10.1146/annurev.ph.41.030179.002213. [DOI] [PubMed] [Google Scholar]
- Reuter H., Seitz N. The dependence of calcium efflux from cardiac muscle on temperature and external ion composition. J Physiol. 1968 Mar;195(2):451–470. doi: 10.1113/jphysiol.1968.sp008467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rougier O., Vassort G., Garnier D., Gargouil Y. M., Coraboeuf E. Existence and role of a slow inward current during the frog atrial action potential. Pflugers Arch. 1969;308(2):91–110. doi: 10.1007/BF00587018. [DOI] [PubMed] [Google Scholar]
- Schneider M. F., Chandler W. K. Voltage dependent charge movement of skeletal muscle: a possible step in excitation-contraction coupling. Nature. 1973 Mar 23;242(5395):244–246. doi: 10.1038/242244a0. [DOI] [PubMed] [Google Scholar]
- Vassort G., Rougier O. Membrane potential and slow inward current dependence of frog cardiac mechanical activity. Pflugers Arch. 1972;331(3):191–203. doi: 10.1007/BF00589126. [DOI] [PubMed] [Google Scholar]


