Abstract
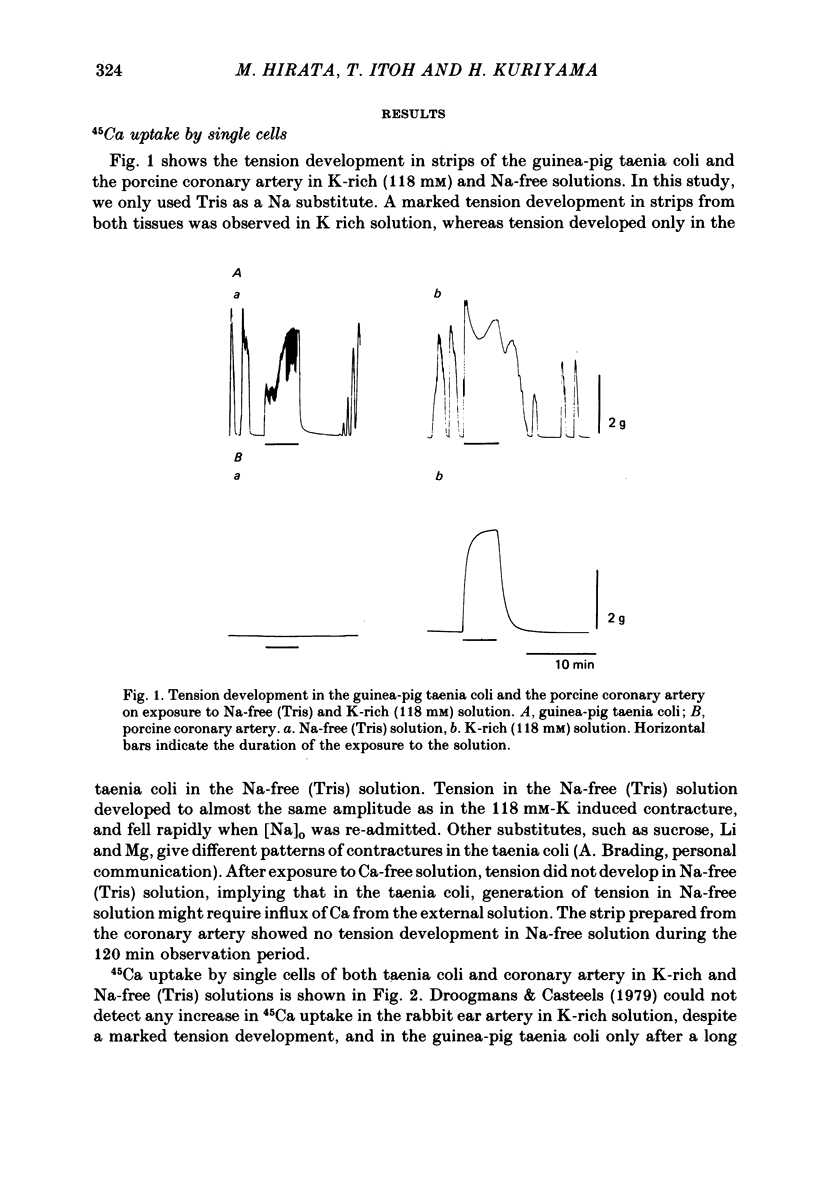
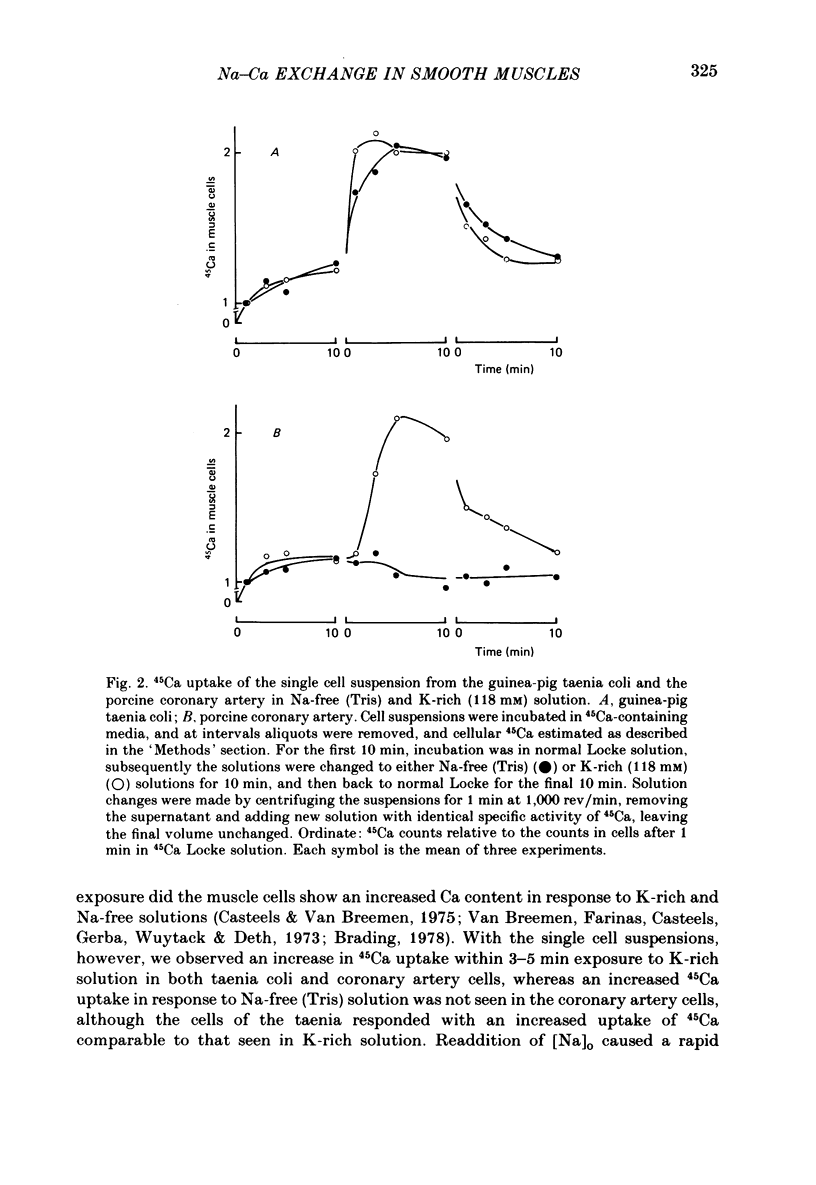
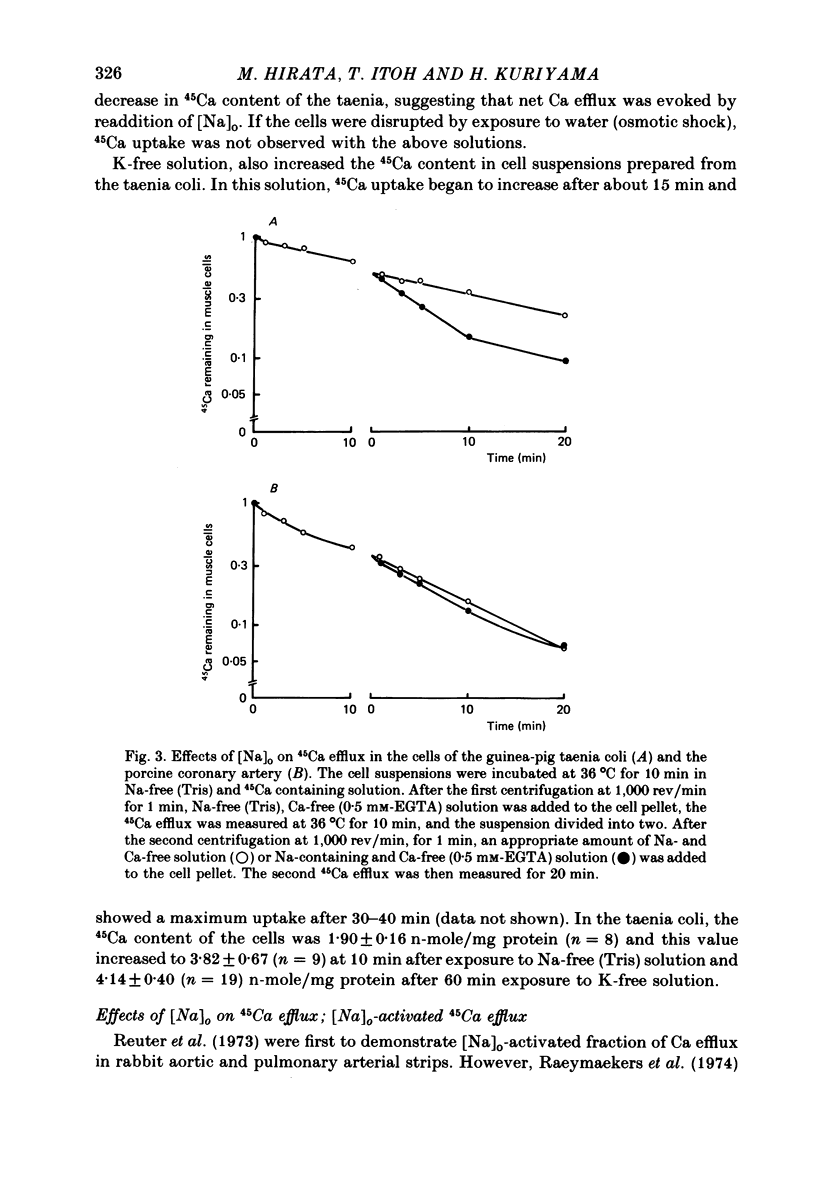
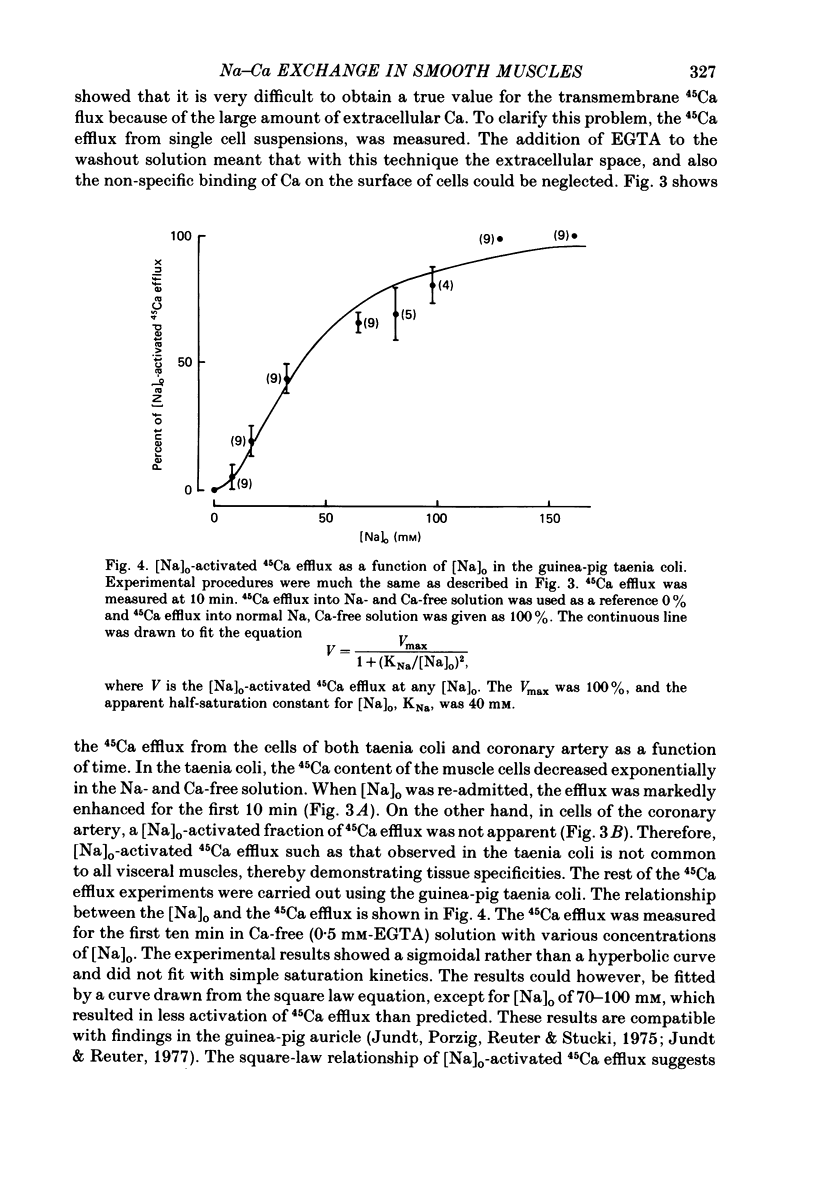
1. Single smooth muscle cells were prepared from the guinea-pig taenia coli and the porcine coronary artery by treatment with collagenase, in order to measure the 45Ca flux with special reference to the effects of external cations. 2. In excess [K]o solution, cell suspensions prepared from both tissues showed an increased 45Ca uptake within 3-5 min. In Na-free solution, the cells prepared from taenia coli, but not from coronary artery showed an increased 45Ca uptake. The Ca uptake of the cells paralleled with the tension increase recorded from tissues of both species. 3. The efflux of 45Ca into Ca-free EGTA containing solution was markedly increased by [Na]o in cells from the taenia coli, but not in cells from the coronary artery. 4. The [Na]o-activated 45Ca efflux from cells of the taenia coli was slightly larger in Ca-free solution than in the Ca-containing (10)-4) M) solution. Depolarization of membranes produced by excess [K]o did not effect the [Na]o-activated 45Ca efflux. 5. Increase in [Na]i by treatment with K-free solution suppressed the [Na]o-activated 45Ca efflux in the taenia coli. Re-addition of [K]o reactivated the [Na]o-activated 45Ca efflux. This re-activation was blocked by ouabain. 6. The efflux of 45Ca was slightly activated by [Ca]o in cells from the taenia coli. This [ca]o-activated 45Ca efflux was larger in Na-free solution than in Na-containing solution, thus suggesting interactions between [Na]o and [Ca]o on the Ca efflux. 7. In cells from the taenia coli, 45Ca efflux could still be observed in nominally Na-and Ca-free solution. This residual 45Ca efflux made a large contribution to the total 45Ca efflux. 8. When 45Ca uptake was measured in Na-free (Tris) solution, the [Na]o-activated, [Ca]o-activated and residual 45Ca effluxes of cells from the taenia coli were accelerated, non-selectively. 9. These results obtained with cells prepared from the guinea-pig taenia coli are comparable to the Ca2+ efflux mechanism seen in the squid axon. However, maintenance of low concentrations of [Ca]i seems to require not only the above three 45Ca efflux mechanisms, but also Ca sequestering mechanisms in the cell.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashley C. C., Ellory J. C., Hainaut K. Calcium movements in single crustacean muscle fibres. J Physiol. 1974 Oct;242(1):255–272. doi: 10.1113/jphysiol.1974.sp010705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., McNaughton P. A. Kinetics and energetics of calcium efflux from intact squid giant axons. J Physiol. 1976 Jul;259(1):103–144. doi: 10.1113/jphysiol.1976.sp011457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., McNaughton P. A. The influence of extracellular calcium binding on the calcium efflux from squid axons. J Physiol. 1978 Mar;276:127–150. doi: 10.1113/jphysiol.1978.sp012223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein M. P., Ector A. C. Carrier-mediated sodium-dependent and calcium-dependent calcium efflux from pinched-off presynaptic nerve terminals (synaptosomes) in vitro. Biochim Biophys Acta. 1976 Jan 21;419(2):295–308. doi: 10.1016/0005-2736(76)90355-2. [DOI] [PubMed] [Google Scholar]
- Blaustein M. P., Russell J. M. Sodium-calcium exchange and calcium-calcium exchange in internally dialyzed squid giant axons. J Membr Biol. 1975 Jul 24;22(3-4):285–312. doi: 10.1007/BF01868176. [DOI] [PubMed] [Google Scholar]
- Blaustein M. P., Santiago E. M. Effects of internal and external cations and of ATP on sodium-calcium and calcium-calcium exchange in squid axons. Biophys J. 1977 Oct;20(1):79–111. doi: 10.1016/S0006-3495(77)85538-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brading A. F. Calcium-induced increase in membrane permeability in the guinea-pig taenia coli: evidence for involvement of a sodium-calcium exchange mechanism. J Physiol. 1978 Feb;275:65–84. doi: 10.1113/jphysiol.1978.sp012178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brading A. F. Maintenance of ionic composition. Br Med Bull. 1979 Sep;35(3):227–234. doi: 10.1093/oxfordjournals.bmb.a071582. [DOI] [PubMed] [Google Scholar]
- Busselen P., van Kerkhove E. The effect of sodium, calcium and metabolic inhibitors on calcium efflux from goldfish heart ventricles. J Physiol. 1978 Sep;282:263–283. doi: 10.1113/jphysiol.1978.sp012462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R., Droogmans G., Hendrickx H. Electrogenic sodium pump in smooth muscle cells of the guinea-pig's taenia coli. J Physiol. 1971 Sep;217(2):297–313. doi: 10.1113/jphysiol.1971.sp009572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R., Van Breemen C. Active and passive Ca2+ fluxes across cell membranes of the guinea-pig taenia coli. Pflugers Arch. 1975 Sep 9;359(3):197–207. doi: 10.1007/BF00587379. [DOI] [PubMed] [Google Scholar]
- Casteels R., van Breemen C., Wuytack F. Effect of metabolic depletion on the membrane permeability of smooth muscle cells and its modification by La 3+ . Nat New Biol. 1972 Oct 25;239(95):249–251. doi: 10.1038/newbio239249a0. [DOI] [PubMed] [Google Scholar]
- Cittadini A., Van Rossum G. D. Properties of the calcium-extruding mechanism of liver cells. J Physiol. 1978 Aug;281:29–43. doi: 10.1113/jphysiol.1978.sp012407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPolo R., Beaugé L. Physiological role of ATP-driven calcium pump in squid axon. Nature. 1979 Mar 15;278(5701):271–273. doi: 10.1038/278271a0. [DOI] [PubMed] [Google Scholar]
- Dipolo R. Effect of ATP on the calcium efflux in dialyzed squid giant axons. J Gen Physiol. 1974 Oct;64(4):503–517. doi: 10.1085/jgp.64.4.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droogmans G., Casteels R. Sodium and calcium interactions in vascular smooth muscle cells of the rabbit ear artery. J Gen Physiol. 1979 Jul;74(1):57–70. doi: 10.1085/jgp.74.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata M., Kuriyama H. Does activation of cyclic AMP dependent phosphorylation induced by beta-adrenergic agent control the tone of vascular muscle? J Physiol. 1980 Oct;307:143–161. doi: 10.1113/jphysiol.1980.sp013428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jundt H., Porzig H., Reuter H., Stucki J. W. The effect of substances releasing intracellular calcium ions on sodium-dependent calcium efflux from guinea-pig auricles. J Physiol. 1975 Mar;246(1):229–253. doi: 10.1113/jphysiol.1975.sp010888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jundt H., Reuter H. Is sodium-activated calcium efflux from mammalian cardiac muscle dependent on metabolic energy [proceedings]? J Physiol. 1977 Mar;266(1):78P–79P. [PubMed] [Google Scholar]
- Katase T., Tomita T. Influences of sodium and calcium on the recovery process from potassium contracture in the guinea-pig taenia coli. J Physiol. 1972 Jul;224(2):489–500. doi: 10.1113/jphysiol.1972.sp009907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb J. F., Lindsay R. Effect of Na, metabolic inhibitors and ATP on Ca movements in L cells. J Physiol. 1971 Nov;218(3):691–708. doi: 10.1113/jphysiol.1971.sp009640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins L. J., Brinley F. J., Jr Sensitivity of calcium efflux from squid axons to changes in membrane potential. J Gen Physiol. 1975 Feb;65(2):135–152. doi: 10.1085/jgp.65.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raeymaekers L., Wuytack F., Casteels R. Na-Ca exchange in Taenia coli of the guinea-pig. Pflugers Arch. 1974 Mar 25;347(4):329–340. doi: 10.1007/BF00587173. [DOI] [PubMed] [Google Scholar]
- Reuter H., Blaustein M. P., Haeusler G. Na-Ca exchange and tension development in arterial smooth muscle. Philos Trans R Soc Lond B Biol Sci. 1973 Mar 15;265(867):87–94. doi: 10.1098/rstb.1973.0011. [DOI] [PubMed] [Google Scholar]
- Reuter H., Seitz N. The dependence of calcium efflux from cardiac muscle on temperature and external ion composition. J Physiol. 1968 Mar;195(2):451–470. doi: 10.1113/jphysiol.1968.sp008467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. M., Blaustein M. P. Calcium efflux from barnacle muscle fibers. Dependence on external cations. J Gen Physiol. 1974 Feb;63(2):144–167. doi: 10.1085/jgp.63.2.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saida K., Nonomura Y. Characteristics of Ca2+- and Mg2+-induced tension development in chemically skinned smooth muscle fibers. J Gen Physiol. 1978 Jul;72(1):1–14. doi: 10.1085/jgp.72.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small J. V. Studies on isolated smooth muscle cells: The contractile apparatus. J Cell Sci. 1977 Apr;24:327–349. doi: 10.1242/jcs.24.1.327. [DOI] [PubMed] [Google Scholar]
- van Breemen C., Farinas B. R., Casteels R., Gerba P., Wuytack F., Deth R. Factors controlling cytoplasmic Ca 2+ concentration. Philos Trans R Soc Lond B Biol Sci. 1973 Mar 15;265(867):57–71. doi: 10.1098/rstb.1973.0009. [DOI] [PubMed] [Google Scholar]


