Abstract
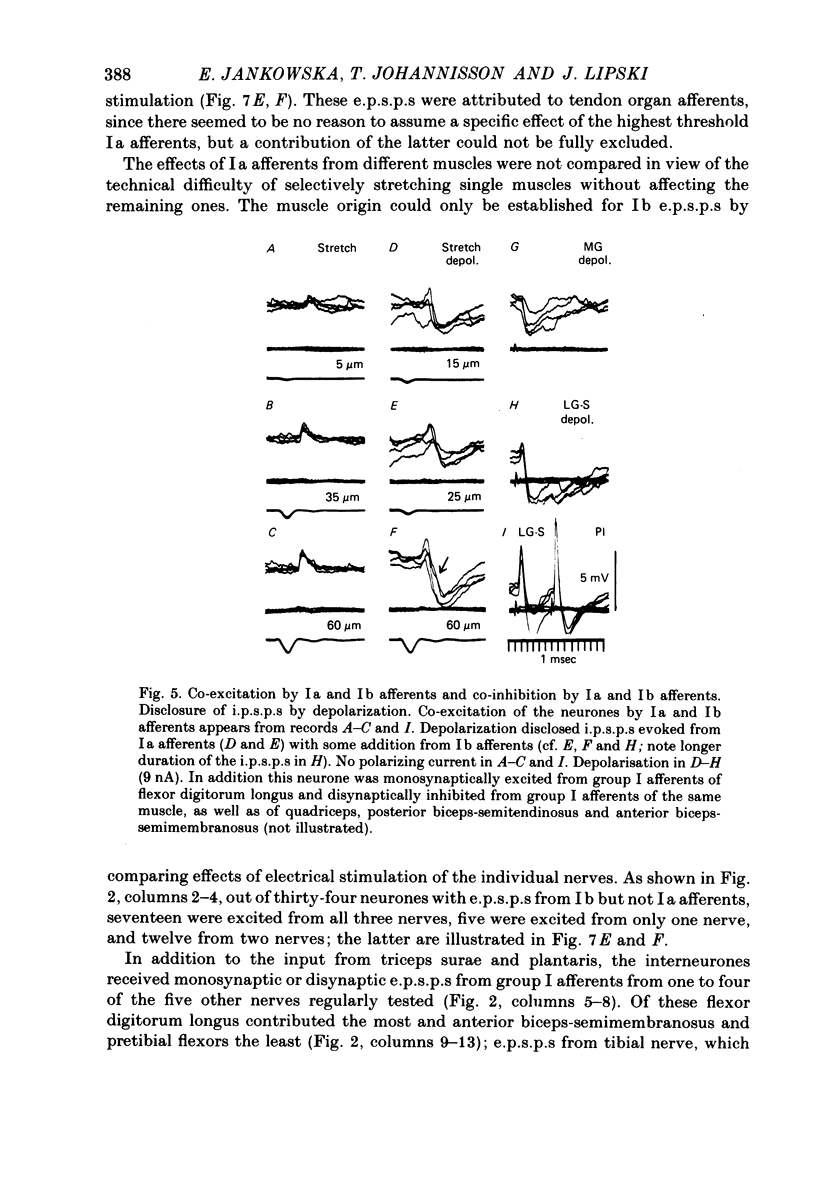
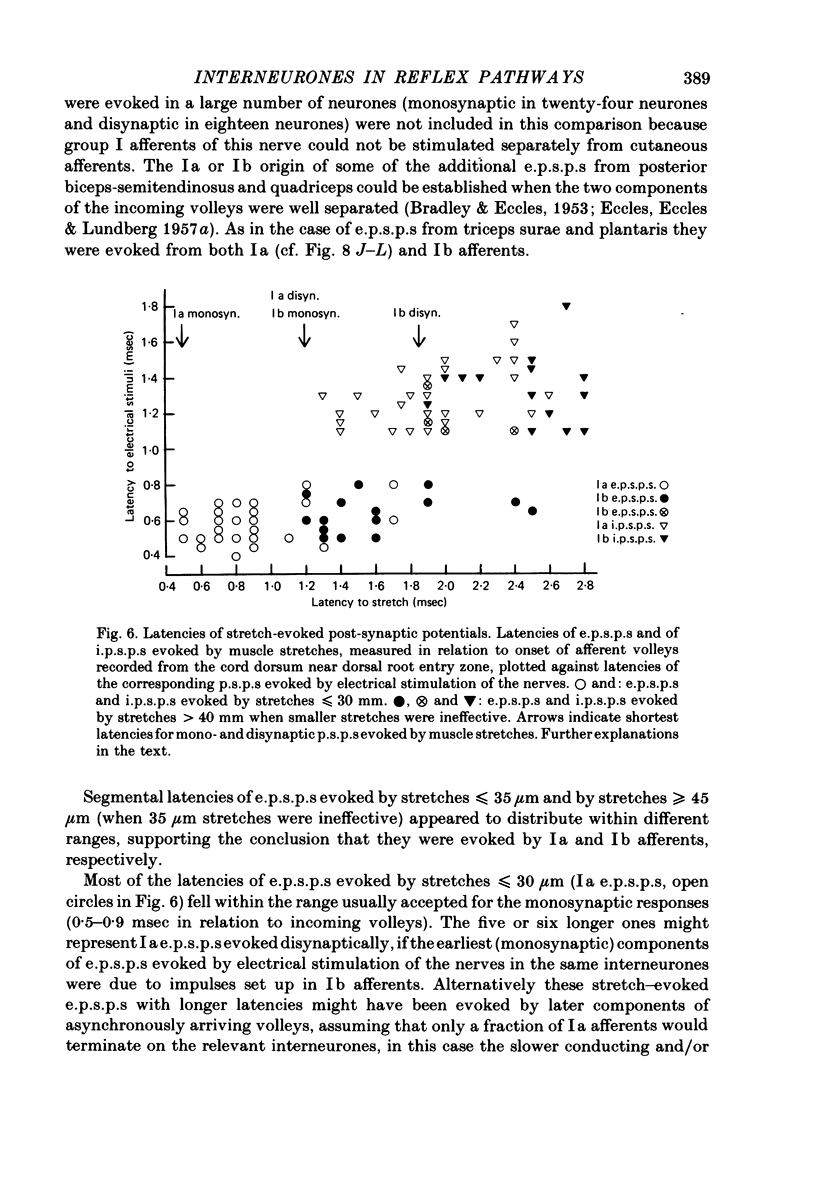
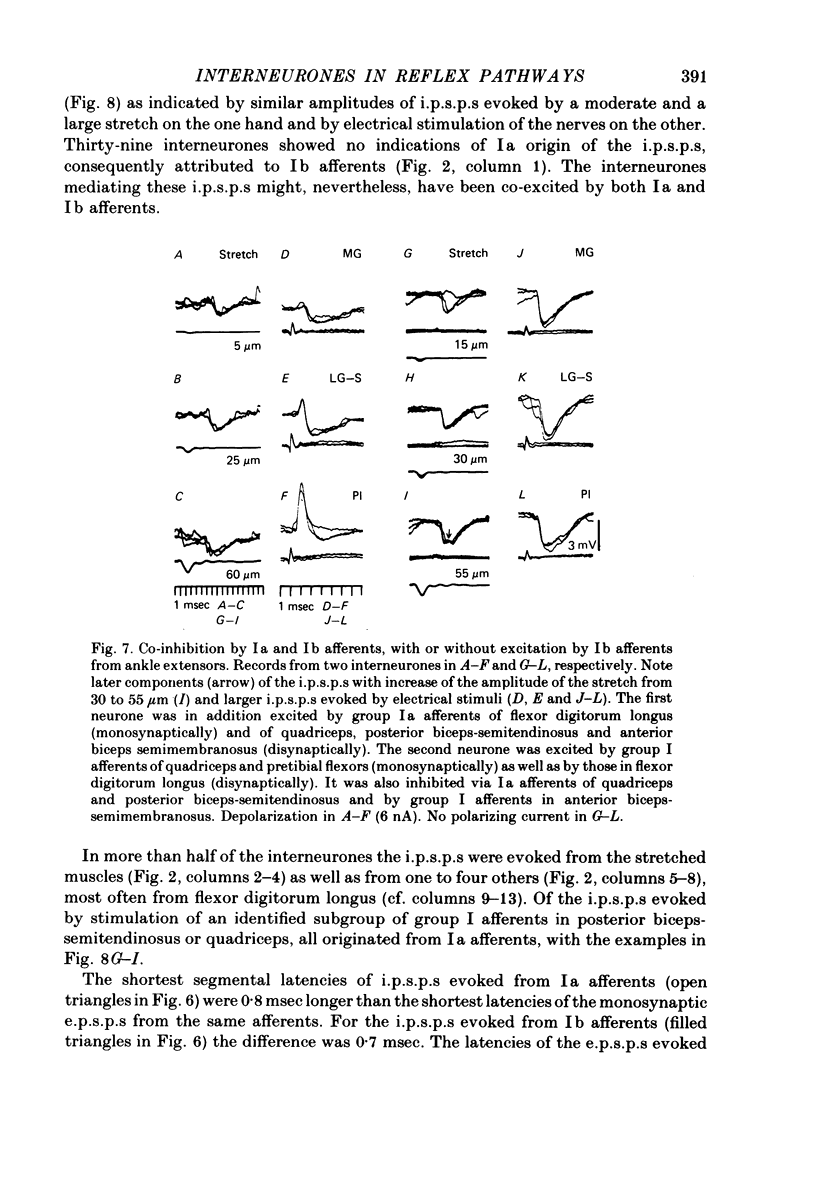
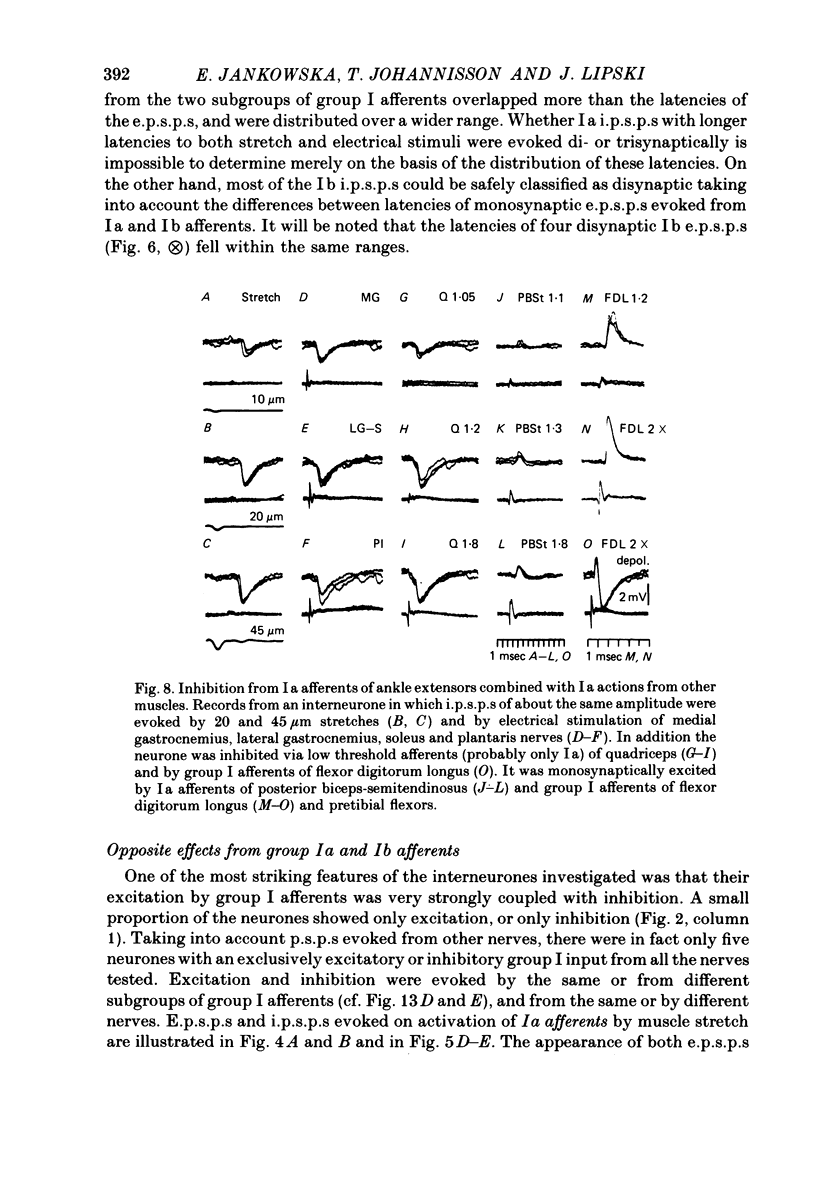
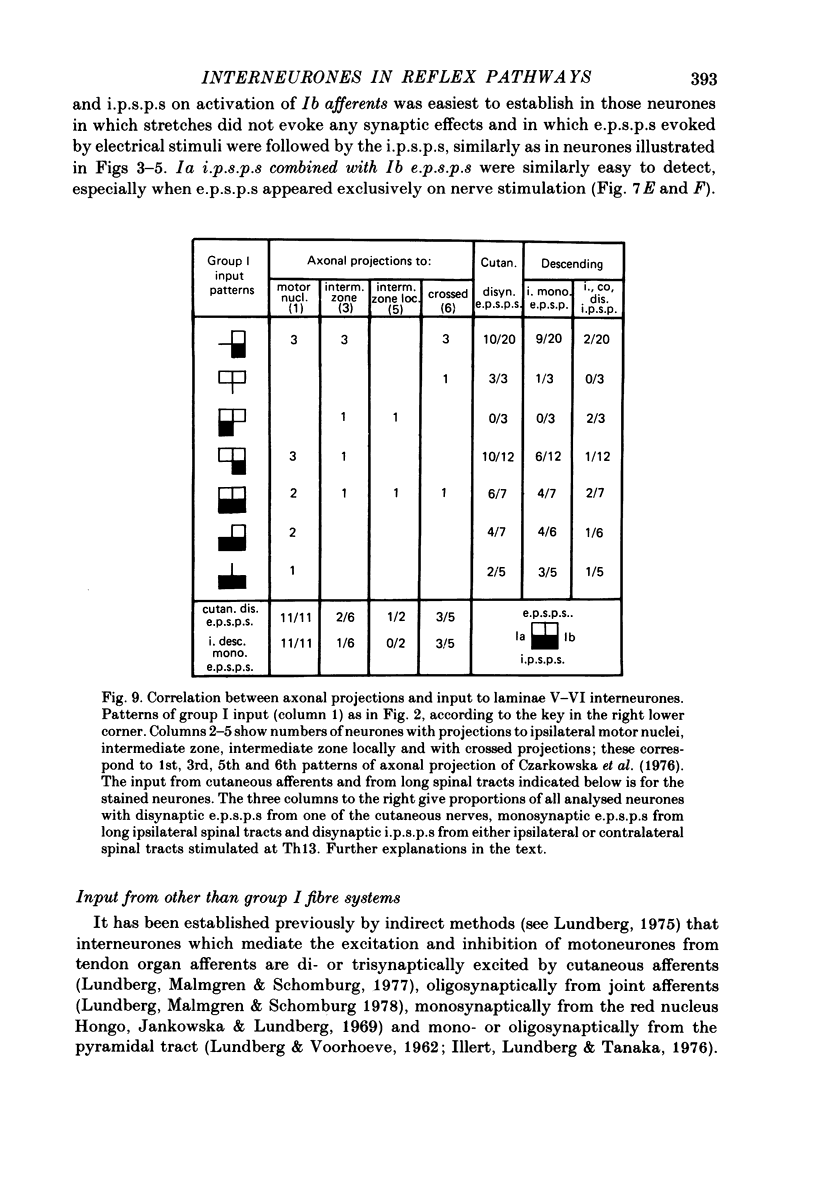
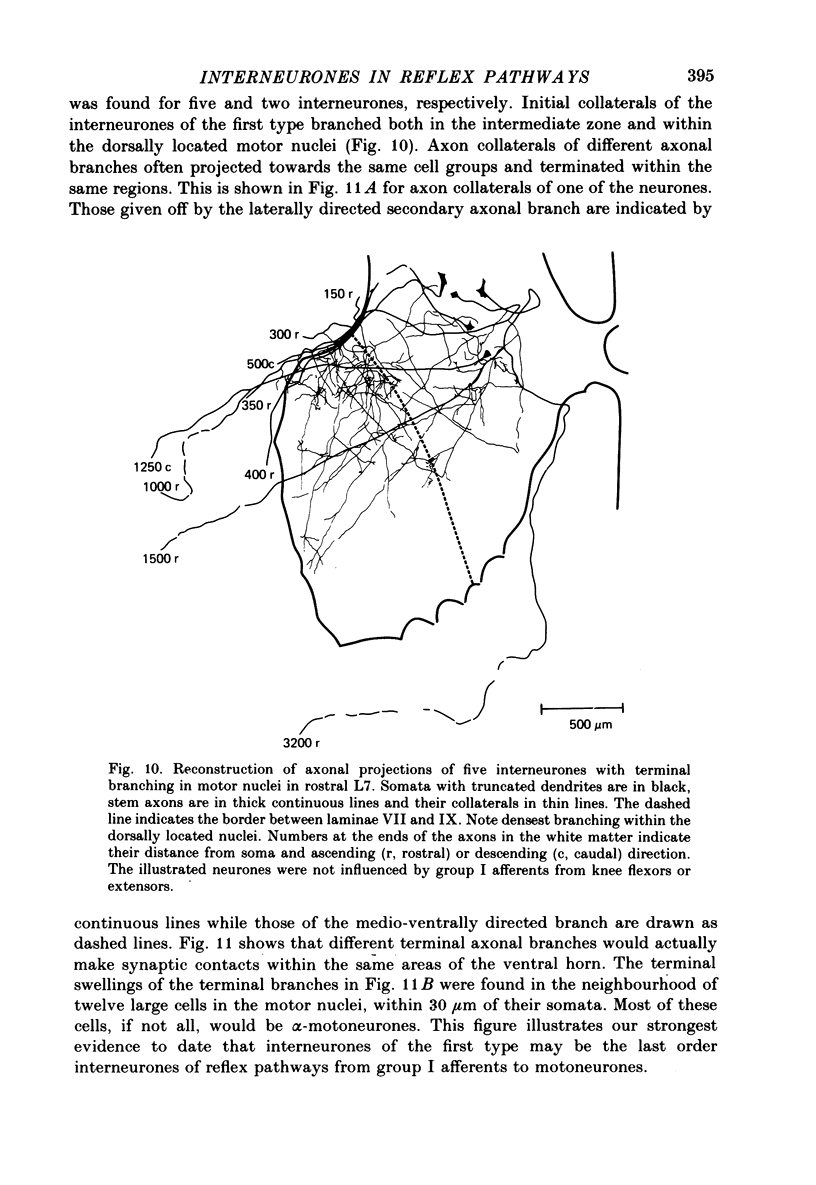
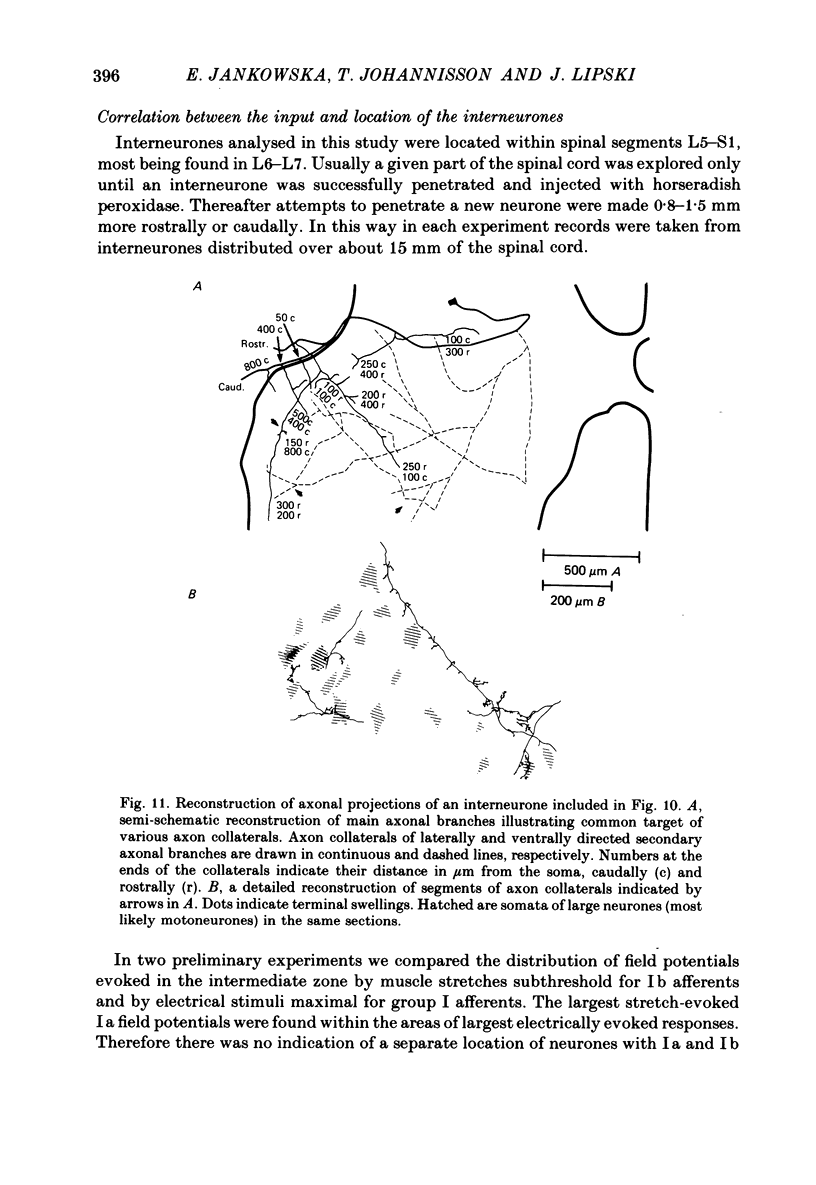
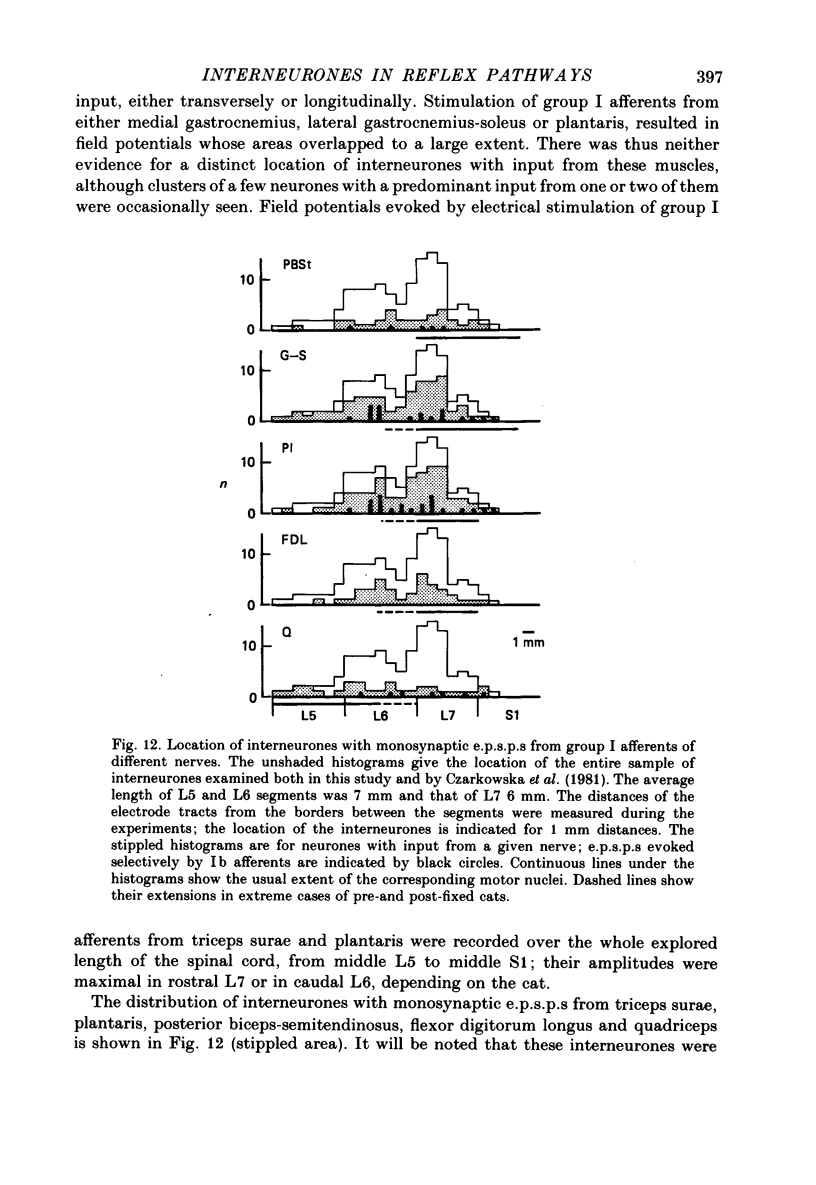
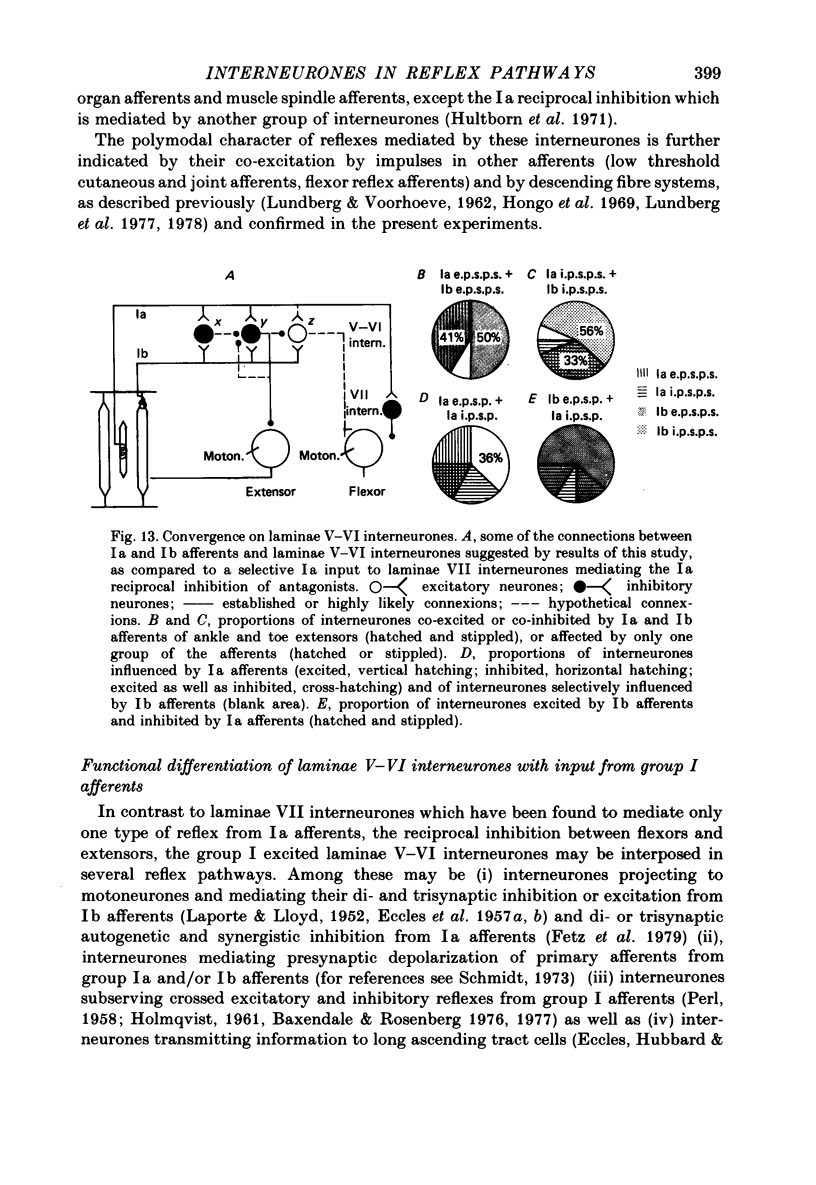
1. Input from group I afferents of ankle and toe extensors, other muscles, skin nerves and descending tracts to interneurones of Rexed's laminae V-VI in the cat spinal cord was analysed using intracellular recording from these interneurones. Adequate stimuli (muscle stretches) were used to activate selectively group Ia muscle spindle afferents of triceps surae and plantaris while other fibre systems were excited electrically. 2. Ia and Ib afferents of ankle and toe extensors were found to co-excite, co-inhibit or exert opposite synaptic actions in 41, 33, and 50% of the analysed interneurones, respectively. Taking into account both excitatory and inhibitory input from these two groups of afferents, 64% of the interneurones appeared to be used in common in reflex pathways from muscle spindles and tendon organs of ankle and toe extensors. 3. Selective input from Ib afferents of triceps surae and plantaris (excitation and/or inhibition) was found in 36% of the interneurones; there was evidence for a similarly selective input from Ia afferents. 4. A great majority (over 90%) of the interneurones excited by group I afferents were also inhibited by group I afferents, from either the same or other muscles. 5. Both monosynaptic and disynaptic e.p.s.p.s from Ia and/or Ib afferents from other muscles and from fibres in the ipsilateral funiculi were found in a great proportion of the same interneurones, together with disynaptic e.p.s.p.s from low threshold cutaneous afferents. 6. Intracellular staining with horseradish peroxidase revealed four different patterns of axonal projections of the analysed interneurones: (i) projections to motor nuclei and the intermediate region, (ii and III) projections only to the intermediate region, locally or combined with projections to different rostro-caudal levels, and (iv) projections to the opposite side of the spinal cord. 7. A large proportion of interneurones projecting to motor nuclei displayed input from both Ia and Ib afferents although such an input was a feature of interneurones with other projections as well. No systematic differences in the input from group I afferents were found for interneurones with different axonal projections. In contrast disynaptic e.p.s.p.s of cutaneous origin and monosynaptic e.p.s.p.s upon stimulation of ipsilateral spinal tracts appeared predominantly in interneurones projecting to motor nuclei.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. C. Technical considerations on the use of horseradish peroxidase as a neuronal marker. Neuroscience. 1977;2(1):141–145. doi: 10.1016/0306-4522(77)90074-4. [DOI] [PubMed] [Google Scholar]
- BRADLEY K., ECCLES J. C. Analysis of the fast afferent impulses from thigh muscles. J Physiol. 1953 Dec 29;122(3):462–473. doi: 10.1113/jphysiol.1953.sp005014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxendale R. H., Rosenberg J. R. Crossed reflexes evoked by selective activation of muscle spindle primary endings in the decerebrate cat. Brain Res. 1976 Oct 15;115(2):324–327. doi: 10.1016/0006-8993(76)90517-5. [DOI] [PubMed] [Google Scholar]
- Baxendale R. H., Rosenberg J. R. Crossed reflexes evoked by selective activation of tendon organ afferent axons in the decerebrate cat. Brain Res. 1977 May 27;127(2):323–326. doi: 10.1016/0006-8993(77)90549-2. [DOI] [PubMed] [Google Scholar]
- Burke R. E. Composite nature of the monosynaptic excitatory postsynaptic potential. J Neurophysiol. 1967 Sep;30(5):1114–1137. doi: 10.1152/jn.1967.30.5.1114. [DOI] [PubMed] [Google Scholar]
- Czarkowska J., Jankowska E., Sybirska E. Axonal projections of spinal interneurones excited by group I afferents in the cat, revealed by intracellular staining with horseradish peroxidase. Brain Res. 1976 Dec 10;118(1):115–118. doi: 10.1016/0006-8993(76)90844-1. [DOI] [PubMed] [Google Scholar]
- Czarkowska J., Jankowska E., Sybirska E. Common interneurones in reflex pathways from group 1a and 1b afferents of knee flexors and extensors in the cat. J Physiol. 1981 Jan;310:367–380. doi: 10.1113/jphysiol.1981.sp013555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., LUNDBERG A. Synaptic actions on motoneurones caused by impulses in Golgi tendon organ afferents. J Physiol. 1957 Sep 30;138(2):227–252. doi: 10.1113/jphysiol.1957.sp005849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., LUNDBERG A. Synaptic actions on motoneurones in relation to the two components of the group I muscle afferent volley. J Physiol. 1957 May 23;136(3):527–546. doi: 10.1113/jphysiol.1957.sp005778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., LUNDBERG A. Types of neurone in and around the intermediate nucleus of the lumbosacral cord. J Physiol. 1960 Nov;154:89–114. doi: 10.1113/jphysiol.1960.sp006566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., HUBBARD J. I., OSCARSSON O. Intracellular recording from cells of the ventral spinocerebellar tract. J Physiol. 1961 Oct;158:486–516. doi: 10.1113/jphysiol.1961.sp006782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellaway P. H., Trott J. R. Autogenetic reflex action on to gamma motoneurones by stretch of triceps surae in the decerebrated cat. J Physiol. 1978 Mar;276:49–66. doi: 10.1113/jphysiol.1978.sp012219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetz E. E., Jankowska E., Johannisson T., Lipski J. Autogenetic inhibition of motoneurones by impulses in group Ia muscle spindle afferents. J Physiol. 1979 Aug;293:173–195. doi: 10.1113/jphysiol.1979.sp012884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Hanker J. S., Yates P. E., Metz C. B., Rustioni A. A new specific, sensitive and non-carcinogenic reagent for the demonstration of horseradish peroxidase. Histochem J. 1977 Nov;9(6):789–792. doi: 10.1007/BF01003075. [DOI] [PubMed] [Google Scholar]
- Hongo T., Jankowska E., Lundberg A. Convergence of excitatory and inhibitory action on interneurones in the lumbosacral cord. Exp Brain Res. 1966;1(4):338–358. doi: 10.1007/BF00237706. [DOI] [PubMed] [Google Scholar]
- Hongo T., Jankowska E., Lundberg A. The rubrospinal tract. II. Facilitation of interneuronal transmission in reflex paths to motoneurones. Exp Brain Res. 1969;7(4):365–391. doi: 10.1007/BF00237321. [DOI] [PubMed] [Google Scholar]
- Hongo T., Jankowska E., Lundberg A. The rubrospinal tract. IV. Effects on interneurones. Exp Brain Res. 1972;15(1):54–78. doi: 10.1007/BF00234958. [DOI] [PubMed] [Google Scholar]
- Hultborn H., Illert M., Santini M. Convergence on interneurones mediating the reciprocal Ia inhibition of motoneurones. I. Disynaptic Ia inhibition of Ia inhibitory interneurones. Acta Physiol Scand. 1976 Feb;96(2):193–201. doi: 10.1111/j.1748-1716.1976.tb10188.x. [DOI] [PubMed] [Google Scholar]
- Hultborn H., Jankowska E., Lindström S. Recurrent inhibition of interneurones monosynaptically activated from group Ia afferents. J Physiol. 1971 Jul;215(3):613–636. doi: 10.1113/jphysiol.1971.sp009488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illert M., Lundberg A., Tanaka R. Integration in descending motor pathways controlling the forelimb in the cat. 2. Convergence on neurones mediating disynaptic cortico-motoneuronal excitation. Exp Brain Res. 1976 Dec 22;26(5):521–540. doi: 10.1007/BF00238825. [DOI] [PubMed] [Google Scholar]
- Jankowska E., Rastad J., Zarzecki P. Segmental and supraspinal input to cells of origin of non-primary fibres in the feline dorsal columns. J Physiol. 1979 May;290(2):185–200. doi: 10.1113/jphysiol.1979.sp012767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensson K., Olsson Y. Retrograde axonal transport of protein. Brain Res. 1971 Jun 18;29(2):363–365. doi: 10.1016/0006-8993(71)90044-8. [DOI] [PubMed] [Google Scholar]
- LAPORTE Y., LLOYD D. P. C. Nature and significance of the reflex connections established by large afferent fibers of muscular origin. Am J Physiol. 1952 Jun;169(3):609–621. doi: 10.1152/ajplegacy.1952.169.3.609. [DOI] [PubMed] [Google Scholar]
- LUNDBERG A., VOORHOEVE P. Effects from the pyramidal tract on spinal reflex arcs. Acta Physiol Scand. 1962 Nov-Dec;56:201–219. doi: 10.1111/j.1748-1716.1962.tb02498.x. [DOI] [PubMed] [Google Scholar]
- LUNDBERG A., WINSBURY G. Selective adequate activation of large afferents from muscle spindles and Golgi tendon organs. Acta Physiol Scand. 1960 Jul 15;49:155–164. doi: 10.1111/j.1748-1716.1960.tb01939.x. [DOI] [PubMed] [Google Scholar]
- Lucas M. E., Willis W. D. Identification of muscle afferents which activate interneurons in the intermediate nucleus. J Neurophysiol. 1974 Mar;37(2):282–293. doi: 10.1152/jn.1974.37.2.282. [DOI] [PubMed] [Google Scholar]
- Lundberg A., Malmgren K., Schomburg E. D. Cutaneous facilitation of transmission in reflex pathways from Ib afferents to motoneurones. J Physiol. 1977 Mar;265(3):763–780. doi: 10.1113/jphysiol.1977.sp011742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg A., Malmgren K., Schomburg E. D. Role of joint afferents in motor control exemplified by effects on reflex pathways from Ib afferents. J Physiol. 1978 Nov;284:327–343. doi: 10.1113/jphysiol.1978.sp012543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg A. Multisensory control of spinal reflex pathways. Prog Brain Res. 1979;50:11–28. doi: 10.1016/S0079-6123(08)60803-1. [DOI] [PubMed] [Google Scholar]
- Lundberg A., Weight F. Functional organization of connexions to the ventral spinocerebellar tract. Exp Brain Res. 1971;12(3):295–316. doi: 10.1007/BF00237922. [DOI] [PubMed] [Google Scholar]
- PERL E. R. Crossed reflex effects evoked by activity in myelinated afferent fibers of muscle. J Neurophysiol. 1958 Mar;21(2):101–112. doi: 10.1152/jn.1958.21.2.101. [DOI] [PubMed] [Google Scholar]
- ROMANES G. J. The motor cell columns of the lumbo-sacral spinal cord of the cat. J Comp Neurol. 1951 Apr;94(2):313–363. doi: 10.1002/cne.900940209. [DOI] [PubMed] [Google Scholar]
- Ryall R. W. Renshaw cell mediated inhibition of Renshaw cells: patterns of excitation and inhibition from impulses in motor axon collaterals. J Neurophysiol. 1970 Mar;33(2):257–270. doi: 10.1152/jn.1970.33.2.257. [DOI] [PubMed] [Google Scholar]
- Stuart D. G., Mosher C. G., Gerlach R. L., Reinking R. M. Selective activation of Ia afferents by transient muscle stretch. Exp Brain Res. 1970 Jun 25;10(5):477–487. doi: 10.1007/BF00234264. [DOI] [PubMed] [Google Scholar]


