Abstract
1. The electrical activities of longitudinal and circular smooth muscle of the anal sphincteric area have been studied in the cat. 2. Electromyographic recordings were achieved with extracellular electrodes, in vivo on acute and chronic animals, and in vitro on the isolated organ. In addition, electrical and mechanical activities were recorded from muscle strips with the sucrose gap technique. 3. Circular muscle coat electrical activity consisted exclusively of slow variations of the membrane potential of the smooth muscle cells. Each slow potential variation was followed by a contraction. 4. The electrical activity and the concomitant contractions were tetrodotoxin resistant (10(-6) g/ml.). Both disappeared in Ca-free solution or in the presence of Mn ions (10(-3) M). 5. On circular muscle, noradrenaline (10(-8)-10(-7) g/ml. in vitro, or 0.1-0.15 mg/kg in vivo) had an excitatory effect consisting in an increase of slow potential frequency. The action of noradrenaline was antagonized by phentolamine (10(-6)-10(-5) g/ml. in vitro, or 0.2 mg/kg in vivo). 6. On circular muscle, acetylcholine (10(-8)-10(-6) g/ml.), used exclusively on muscle strips, did never produce any clear cut effect. 7. Longitudinal muscle coat electrical activity consisted of spike potentials superimposed on slow time course depolarizations which were never observed alone. Each spike was followed by a contraction. This electrical activity was tetrodotoxin resistant (10(-6) g/ml.). 8. Longitudinal muscle activity was abolished by noradrenaline (10(-6) g/ml.) and enhanced by acetylcholine (10(-8)-10(-6) g/ml.). The action of noradrenaline was antagonized by propranolol (0.2 mg/kg I.V.; 10(-6) g/ml.) and that of acetylcholine by atropine (10(-7) g/ml.). 9. Electrophysiological and pharmacological data indicate that electromechanical coupling is achieved (1) in circular muscle, through Ca dependent slow variations in membrane potential of the muscle cells and (2) in longitudinal muscle, through spike potentials. Noradrenaline has opposite effects on the two muscle coats: circular muscle is excited through alpha-receptors located on muscle cells membrane; longitudinal muscle is inhibited through beta-receptors. Acetylcholine excites longitudinal muscle through muscarinic receptors, but it has no effect on circular muscle.
Full text
PDF

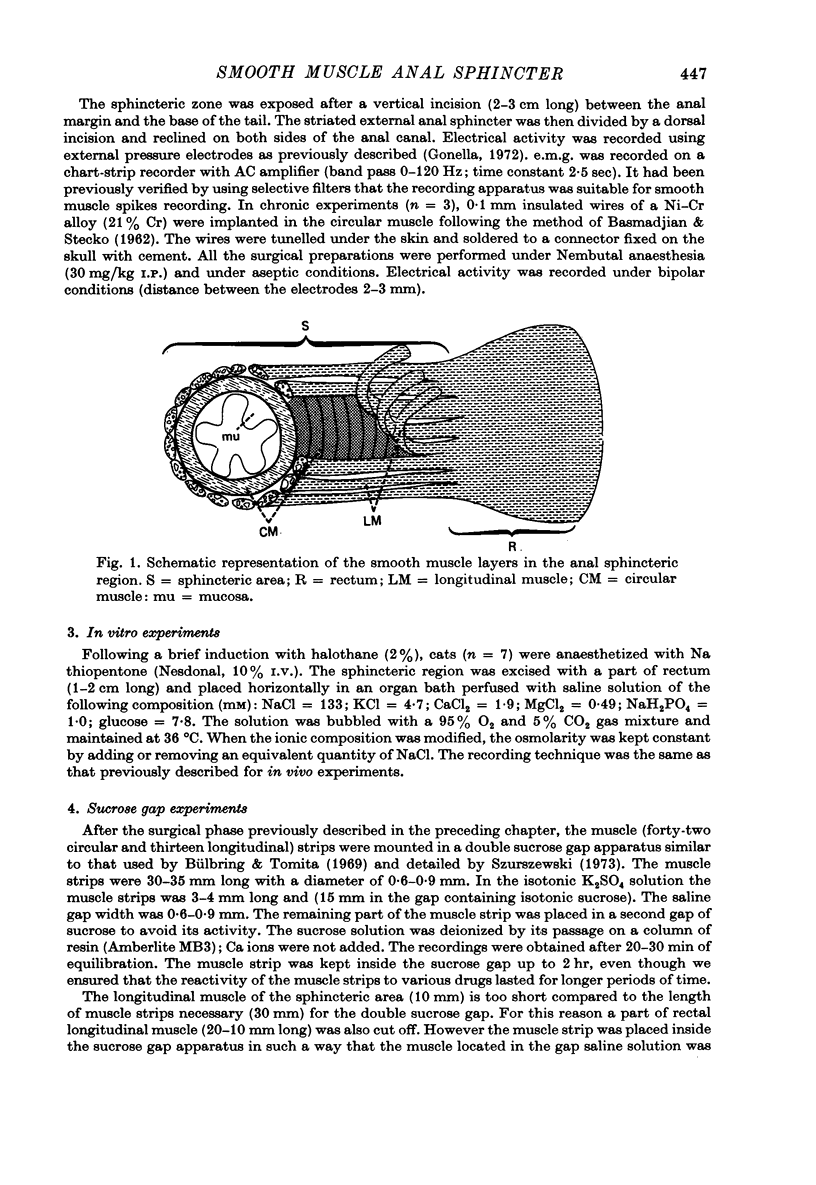


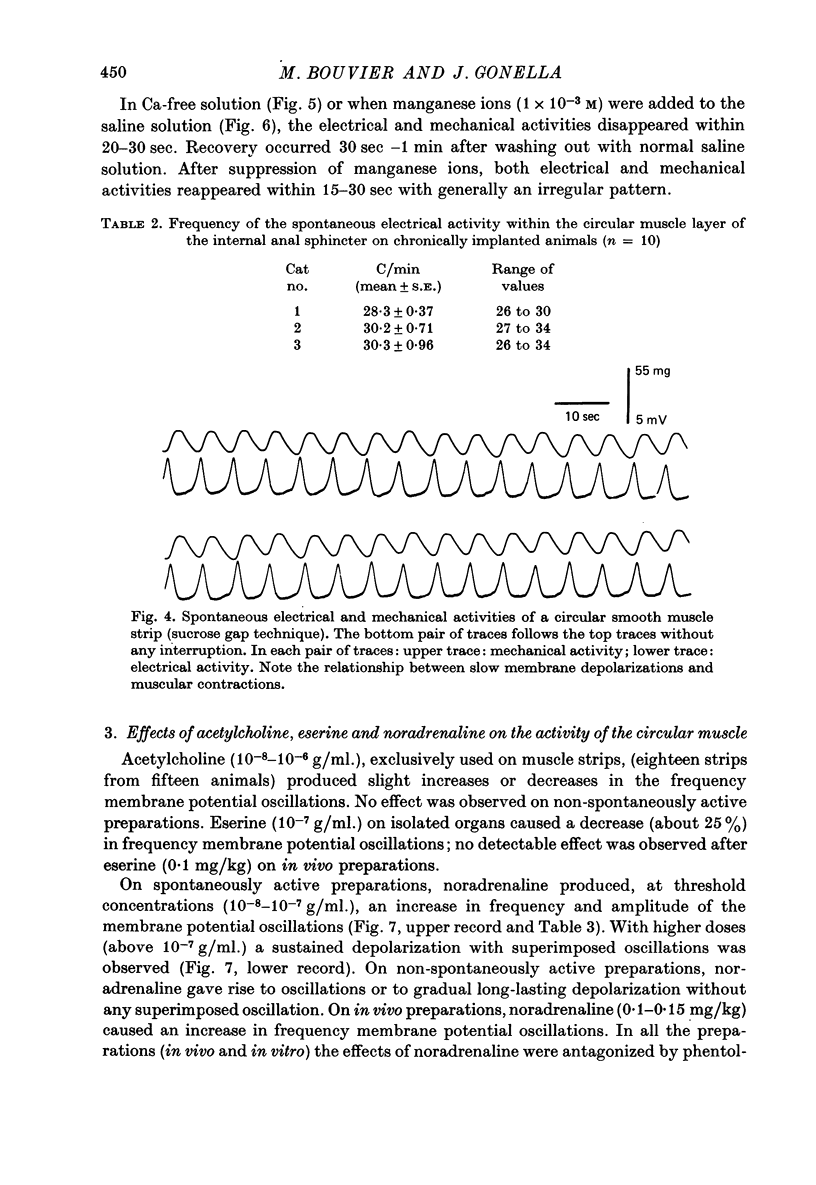


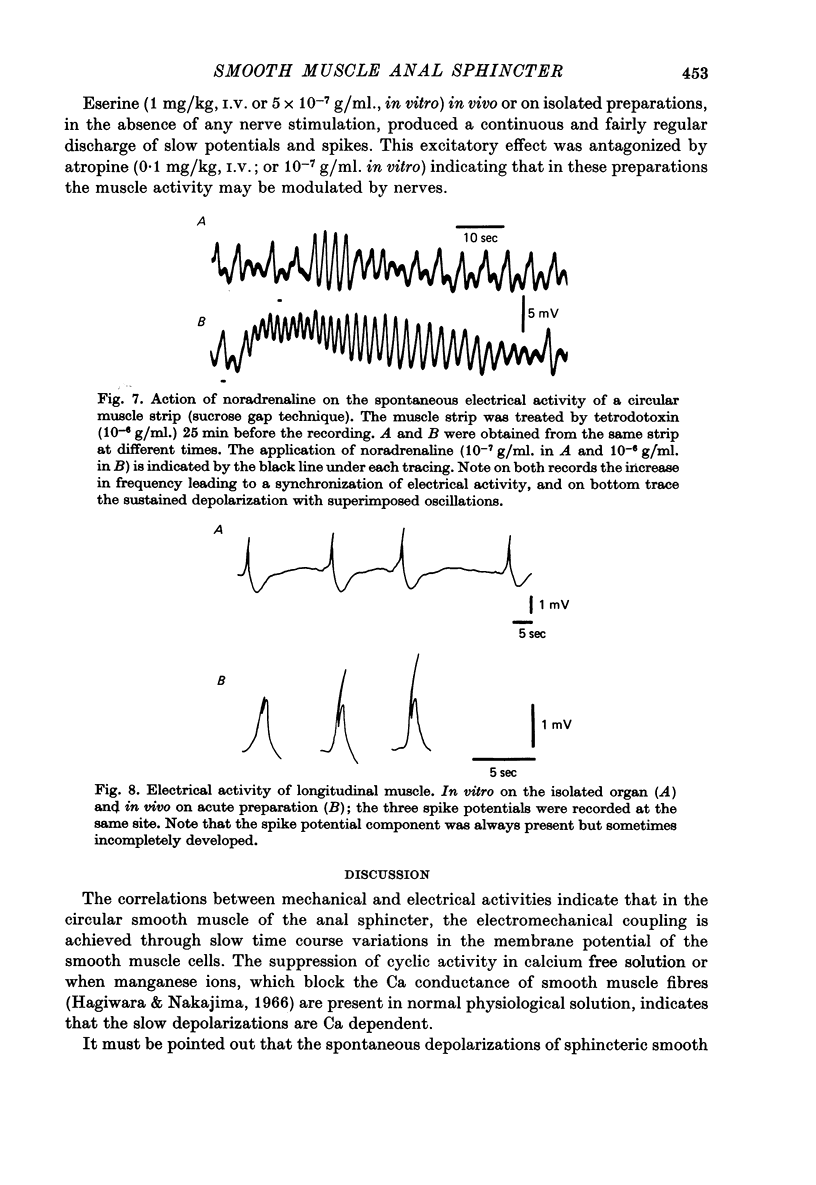



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bülbring E., Tomita T. Increase of membrane conductance by adrenaline in the smooth muscle of guinea-pig taenia coli. Proc R Soc Lond B Biol Sci. 1969 Mar 11;172(1027):89–102. doi: 10.1098/rspb.1969.0013. [DOI] [PubMed] [Google Scholar]
- Christensen J., Caprilli R., Lund G. F. Electric slow waves in circular muscle of cat colon. Am J Physiol. 1969 Sep;217(3):771–776. doi: 10.1152/ajplegacy.1969.217.3.771. [DOI] [PubMed] [Google Scholar]
- Christensen J., Daniel E. E. Effects of some autonomic drugs on circular esophageal smooth muscle. J Pharmacol Exp Ther. 1968 Feb;159(2):243–249. [PubMed] [Google Scholar]
- El-Sharkaway T. Y., Daniel E. E. Ionic mechanisms of intestinal electrical control activity. Am J Physiol. 1975 Nov;229(5):1287–1298. doi: 10.1152/ajplegacy.1975.229.5.1287. [DOI] [PubMed] [Google Scholar]
- Garrett J. R., Howard E. R., Jones W. The internal anal sphincter in the cat: a study of nervous mechanisms affecting tone and reflex activity. J Physiol. 1974 Nov;243(1):153–166. doi: 10.1113/jphysiol.1974.sp010747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonella J. Etude de l'activité électrique de la couche musculaire longitudinale du duodénum de lapin. J Physiol (Paris) 1970 Nov-Dec;62(6):447–476. [PubMed] [Google Scholar]
- Gonella J. Modifications of electrical activity of the rabbit duodenum longitudinal muscle after contractions of the circular muscle. Am J Dig Dis. 1972 Apr;17(4):327–332. doi: 10.1007/BF02231733. [DOI] [PubMed] [Google Scholar]
- Gonella J., Niel J. P., Roman C. Sympathetic control of lower oesophageal sphincter motility in the cat. J Physiol. 1979 Feb;287:177–190. doi: 10.1113/jphysiol.1979.sp012653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Nakajima S. Differences in Na and Ca spikes as examined by application of tetrodotoxin, procaine, and manganese ions. J Gen Physiol. 1966 Mar;49(4):793–806. doi: 10.1085/jgp.49.4.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerremans R. Electrical activity and motility of the internal anal sphincter: an "in vivo" electrophysiological study in man. Acta Gastroenterol Belg. 1968 Jul;31(7):465–482. [PubMed] [Google Scholar]
- Kerremans R., Penninckx F. A study in vivo of adrenergic receptors in the rectum and in the internal and sphincter of the cat. Gut. 1970 Aug;11(8):709–714. doi: 10.1136/gut.11.8.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley J. N., Anderson H. K. On the Innervation of the Pelvic and Adjoining Viscera: Part I. The Lower Portion of the Intestine. J Physiol. 1895 May 20;18(1-2):67–105. doi: 10.1113/jphysiol.1895.sp000558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papasova M. P., Nagai T., Prosser C. L. Two-component slow waves in smooth muscle of cat stomach. Am J Physiol. 1968 Apr;214(4):695–702. doi: 10.1152/ajplegacy.1968.214.4.695. [DOI] [PubMed] [Google Scholar]
- Shuba M. Mechanism of action of catecholamines and histamine on smooth muscle cells of guinea-pig ureter. J Physiol. 1975 Feb;245(2):88P–89P. [PubMed] [Google Scholar]
- Szurszewski J. H. Mechanism of action of pentagastrin and acetylcholine on the longitudinal muscle of the canine antrum. J Physiol. 1975 Nov;252(2):335–361. doi: 10.1113/jphysiol.1975.sp011147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TALBOT S. A., LILIENTHAL J. L., Jr, BESER J., REYNOLDS L. W. A wide range mechano-electronic transducer for physiological applications. Rev Sci Instrum. 1951 Apr;22(4):233–236. doi: 10.1063/1.1745898. [DOI] [PubMed] [Google Scholar]
- Ustach T. J., Tobon F., Hambrecht T., Bass D. D., Schuster M. M. Electrophysiological aspects of human sphincter function. J Clin Invest. 1970 Jan;49(1):41–48. doi: 10.1172/JCI106220. [DOI] [PMC free article] [PubMed] [Google Scholar]


