Abstract
Derivatives of the cytotoxic peptide dermaseptin S4 have recently emerged as potential antimicrobial agents. Here, we report on the antibacterial properties of three derivatives with improved toxicity profiles: a 28-residues K4K20-S4 and two shorter versions, K4-S4(1-16) and K4-S4(1-13). The range of MICs of K4K20-S4 against clinical isolates of Staphylococcus aureus, Pseudomonas aeruginosa, and Escherichia coli were, respectively, 1 to 4, 1 to 4, and 1 to 16 μg/ml. MICs of the short derivatives were rather similar or two to fourfold higher. Each of the three peptides was rapidly bactericidal in vitro, reducing the number of viable CFU of either E. coli or S. aureus by 6 log units in 30 min or less. Compared with MSI-78 or PG-1, K4-S4(1-13) was at least as potent against bacteria (assessed at two MIC multiples) but displayed lesser toxicity against human erythrocytes. Serial passage in subinhibitory concentrations led to emergence of resistance to commercial antibiotics but not to the l- or d isomer of either of the dermaseptin derivatives. The short derivatives were further investigated for antibacterial activity in vivo, using a peritonitis model of mice infected with P. aeruginosa. Naive mice in the vehicle control group exhibited 75% mortality, compared to 18 or 36% mortality in mice that received a single intraperitoneal injection (4.5 mg/kg) of K4-S4(1-16) or K4-S4(1-13), respectively. In vivo bactericidal activity was confirmed in neutropenic mice, where intraperitoneal administration of K4-S4(1-16) reduced the number of viable CFU in a dose-dependent manner by >3 log units within 1 h of exposure, and this was sustained for at least 5 h. Overall, the data suggest that dermaseptin S4 derivatives could be useful in treatment of infections, including infections caused by multidrug-resistant bacteria.
In the past 20 years, over 400 cationic antimicrobial peptides have been identified from extremely diverse sources from unicellular organisms to mammals, including humans (2, 16, 24, 26). Presently, cationic antimicrobial peptides are believed to represent an important, though poorly understood, component of the innate host defenses. Antimicrobial peptides display a large heterogeneity in primary and secondary structures but share common features such as amphipathy and net positive charge. These features seem to form the basis for their cytotoxic function. The facts that some antimicrobial peptides are active against a large spectrum of microorganisms and that isomers composed of d amino acids display potency identical to that of the l counterparts are consistent with the hypothesis that cytotoxic activity is not mediated by interaction with a chiral center. Although their precise mechanism of action is not fully understood, a large body of data indicates that antimicrobial peptides kill target cells by interacting with and destabilizing the ordered structure of the cell membrane(s) (9, 28). These findings imply that peptide-based antimicrobials could escape the mechanisms involved in multidrug resistance.
In addition to their direct membrane-disrupting activity, cationic antimicrobial peptides are reportedly able to activate cells of the innate immunity, such as leukocytes and monocytes/macrophages. Thus, the frog antimicrobial peptide dermaseptin S1 was shown to stimulate microbicidal activities of rat and human leukocytes (1), and more recently, the insect-derived peptide CEMA (a cecropin-melittin hybrid) was reported to induce the expression of 35 genes in macrophages (27). Moreover, an increasing number of cationic peptides and proteins, including lactoferrin (3, 22), bactericidal/permeability increasing protein (8), synthetic antiendotoxin peptides (7), dermaseptins (unpublished data), and CEMA (14), are reportedly endowed with lipopolysaccharide (LPS) binding activity. LPS is a potent activator of macrophages and is responsible for sepsis caused by gram-negative bacteria. By binding LPS, these peptides were shown to block the interaction of LPS with LPS-binding protein, suppress the ability of LPS to stimulate the production of inflammatory cytokines by macrophages, and protect animals from lethal endotoxic shock.
Thus, cationic antibacterial peptides have in recent years been attracting increasing interest of both the scientific community and the pharmaceutical industry for their potential as new therapeutic agents. However, it is widely believed that this family of agents lacks specificity and might be too toxic for systemic treatment (15, 24). Therefore, topical use has been chosen for various antimicrobial peptides that are currently undergoing clinical trials (4, 12). In this study, we report an evaluation of the in vitro antibacterial properties of three dermaseptin S4 derivatives and present data demonstrating in vivo safety and efficacy.
Dermaseptins are a large family of antimicrobial peptides (28 to 34 amino acids) expressed in amphibian skin. These linear polycationic peptides are unstructured in polar media but readily switch to an amphipathic α-helix in apolar solvents. Dermaseptins display cytolytic activity in vitro generally against a broad spectrum of host-free microorganisms, including bacteria, protozoa, yeasts, and filamentous fungi (5, 6, 10, 18, 20, 25), as well as against intracellular parasites (13, 21). Unlike most dermaseptin members, the 28-residue dermaseptin S4 is highly toxic to erythrocytes. This toxicity is probably related to its high hydrophobicity, as both nuclear magnetic resonance and fluorescence methods have indicated that the peptide is in a high aggregation state in aqueous solutions (13), whereas recent data (10) revealed that N-terminal domain interaction between dermaseptin S4 monomers is responsible for the peptide's oligomerization in solution. Aggregation in solution is probably further responsible for limiting its spectrum of potential target cells. Thus, bell-shaped dose-response profiles obtained with bacteria but not with protozoa or red blood cells (RBC) (which lack a cell wall) implied that peptide aggregation in solution is an important factor affecting selective activity (10). Tampering with the composition of the hydrophobic domains by reducing hydrophobicity or by increasing the net positive charge of native dermaseptin S4 resulted in a number of analogs that displayed enhanced antibacterial activity and reduced hemolytic activity. Among these, K4K20-S4 was two- to threefold more potent than native dermaseptin S4 against protozoa and RBC, yet K4K20-S4 was more potent by 2 orders of magnitude against bacteria. Also, a 16-mer version, K4-S4(1-16), displayed antibacterial activity against Escherichia coli that was more potent by 2 orders of magnitude (50% inhibitory concentration, 0.4 μM) than that of native dermaseptin S4 (50% inhibitory concentration, 40 μM). In contrast, this analog displayed reduced hemolytic activity (50% lethal concentration, 20 μM compared to 1.4 μM). An even shorter dermaseptin S4 derivative, K4-S4(1-13), displayed a twofold-reduced antibacterial activity and a twofold-increased relative selectivity ratio due to its low hemolytic activity (10, 21). In addition, when injected intravenously into rats, K4-S4(1-13) was well tolerated at the high dose of 10 mg of peptide/kg of body weight (11). These peptides were selected for this study to undergo a further assessment of their potential as antibacterial agents.
MATERIALS AND METHODS
Peptides.
The peptides were synthesized by the solid-phase method, applying 9-fluorenylmethoxy carbonyl active ester chemistry. After removal of the 9-fluorenylmethoxy carbonyl from the N-terminal amino acid, the peptide was cleaved from the resin with an 85:5:5:5 mixture of trifluoroacetic acid (TFA)-para-cresol-H2O-thioanisole (10 mg of resin-bound peptide in a 1-ml mixture). The TFA was then evaporated, and the peptide was precipitated with ether followed by six ether washes. The crude peptides were extracted from the resin with 30% acetonitrile in water and purified to chromatographic homogeneity in the range of 98 to >99% by reverse-phase high-pressure liquid chromatography (HPLC) (Alliance-Waters). HPLC runs were performed on preparative and then on analytical C4 columns (Vydac) using a linear gradient of acetonitrile in water (1%/minute), with both solvents containing 0.1% TFA. Purification and refolding of PG-1, which contains cysteine residues, was performed basically according to the procedure described by Harwig et al. (17). Briefly, after cleavage from the resin, the peptide was reduced with dithiothreitol, using a fivefold excess relative to thiol groups in 0.1 M ammonium bicarbonate. After overnight incubation at 25°C, the mixture was acidified (with acetic acid) then purified by HPLC. Folding of the reduced peptide was accomplished in 0.1 M Tris buffer (pH 7.7) containing 20% dimethyl sulfoxide. The mixture was air oxidized for 24 to 26 h at 25°C and repurified by HPLC as described above, and the β-sheet content was confirmed by circular dichroism. All purified peptides were subjected to amino acid and mass spectrometry analyses in order to confirm their composition. Peptides were stocked as lyophilized powder at −20°C. Prior to being tested, fresh solutions were prepared in water, vortexed, sonicated, centrifuged, and then diluted in the appropriate medium.
Reagents.
All reagents for peptide synthesis and cell cultures were analytical grade. Buffers were prepared using mQ double-distilled water (Millipore). Antibiotic solutions were prepared in 0.1 M phosphate buffer from the following diagnostic powders of known potency: ciprofloxacin (Bayer Corp., Frankfurt, Germany), gentamicin (Biogal Pharmaceutical Works Ltd., Teva Group, Debrecen, Hungary), penicillin G and rifampin (Biochemie GmbH, Kundl, Austria), ceftazidime (Glaxo Group Ltd., Worthing, United Kingdom), and piperacillin (Stragen, Geneva, Switzerland).
Bacterial strains.
All strains were epidemiologically nonrelated clinical isolates collected at Tel Aviv Medical Center. Gram-negative isolates used in the susceptibility studies were multidrug-resistant isolates that were resistant to at least three agents from distinct classes of antibiotics (e.g., gentamicin, piperacillin, and ciprofloxacin). For studies of emergence of resistance and for in vivo studies, clinical blood isolates were used.
In vitro assays.
Unless otherwise stated, antibacterial activity was assessed using a rapid screening assay for growth inhibition in 2× TY culture medium (16 g of tryptone per liter, 10 g of yeast extract per liter, 5 g of NaCl per liter [pH 7.4]) as follows. Inocula of 106 bacteria/ml at the exponential phase of growth were used. The cell populations were estimated by optical density measurements at 620 nm referred to a calibration curve. A 100-μl bacterial suspension at the exponential phase of growth was added to 100 μl of culture medium containing no test compound or various test compound concentrations (serial twofold dilutions of an initial concentration of 32 μg/ml) in 96-well plates (Nunc). The MIC was determined from the lowest concentration that induced 100% inhibition. Inhibition of proliferation was assessed by optical density measurements (620 nm) after an incubation period of 4 h with shaking at 37°C.
For the resistance studies, Pseudomonas aeruginosa strain B35225 and Staphylococcus aureus strain B38302 were used as representatives of gram-negative and gram-positive bacteria, respectively. Bacteria at the exponential phase of growth were exposed to an antimicrobial agent for MIC determination basically as described above. Following incubation overnight, bacteria were harvested from wells that displayed near 50% growth inhibition, washed and diluted in fresh medium, grown overnight, and subjected again to MIC determination for up to 15 similar serial passages. In parallel, MIC evolution during these subcultures was compared concomitantly with each new generation, using bacteria harvested from control wells (wells cultured without antimicrobial agent) from the previous generation. The relative MIC was calculated for each experiment from the ratio of MIC obtained for a given subculture to that obtained for first-time exposure. Statistical data for each of these experiments were obtained from at least two repeats performed in duplicate.
For the kinetics studies, 100-μl stock solutions of peptides prepared in culture medium to yield a final concentration of 20 μM [corresponding to 57, 36, 30, 50, and 43 μg/ml, respectively, for K4K20-S4, K4-S4(1-16), K4-S4(1-13), MSI-78, and PG-1] were added to Eppendorf tubes containing 100 μl of bacteria at the exponential phase of growth. Bacteria used were S. aureus strain 15886 and E. coli strain U16318. After 0, 5, 30, and 60 min of exposure to the peptides at 37°C, cultures were subjected to serial 10-fold dilution (up to 1/10,000) by adding 20 μl of sample to 180 μl of cold PBS (50 mM sodium phosphate, 150 mM NaCl [pH 7.3]), from which 50-μl aliquots were plated on TyE agar plates (15 g of agar per liter, 10 g of tryptone per liter, 5 g of yeast extract per liter, 8 g of NaCl per liter [pH 7.5]) for CFU counts after additional overnight incubation at 37°C.
For the hemolysis assay, human blood was washed three times in PBS by centrifugation for 1 min at 2,700 × g, and then 2.5 × 108 RBC suspended in 50 μl of PBS were added to Eppendorf test tubes containing 200 μl of peptide solutions (serial twofold dilutions in PBS), PBS alone (for baseline values), or distilled water (for 100% hemolysis). After incubation (4 h with agitation at 37°C), samples were centrifuged and the hemolytic activity was assessed as a function of hemoglobin leakage by measuring the absorbance of 200 μl of supernatant (405 nm). Statistical data were obtained from at least two independent experiments performed in duplicate.
In vivo studies.
Experiments were conducted using naive male BALB/c mice 8 to 10 weeks of age (weighing 22 to 24 g) and female neutropenic ICR mice 5 weeks old (weighing 23 to 26 g). Animals were obtained from Harlan Laboratories, Ltd., Jerusalem, Israel, and were allowed to acclimatize to our animal facility for at least 5 days before experiments were conducted.
Acute toxicity was examined by intraperitoneal (i.p.) injection of the tested dermaseptin to groups of 10 BALB/c mice. Each mouse was injected with a 0.5-ml solution of freshly prepared dermaseptin derivative in PBS. The doses of peptide administered per mouse were 0, 125, 250, 500, and 750 μg (corresponding to 0, 5.4, 10.9, 21.8, and 32.6 mg/kg of body weight). Animals were directly inspected for adverse effects for 4 h, and mortality was monitored for 7 days thereafter. Differences between groups were analyzed using the Fisher exact test. All tests were two sided, and differences were considered to be statistically significant when the P value was <0.05.
Infection was induced by i.p. injection of 106 CFU in 0.2 ml of a logarithmic-phase culture of P. aeruginosa (B35225, a clinical blood isolate). A bacterial inoculum was prepared on brain heart infusion (BHI) broth (Becton-Dickinson) with agitation (180 rpm) at 37°C for 5 to 6 h. Cells were harvested when the culture reached an optical density of 0.8 (at 620 nm), and then the cells were centrifuged and resuspended in sterile BHI broth. The number of viable cells was verified by plating serial dilutions of the injected inocula onto BHI agar plates. For induction of infection in naive BALB/c mice, inocula were prepared with 5% (wt/vol) mucin as an adjuvant (mucin bacteriological; Becton-Dickinson).
In order to decrease the influence of the host immune system and to reduce the variability in bacterial counts, models were developed with neutropenic mice. Female neutropenic ICR mice were used to evaluate in vivo the antibacterial effect of K4-S4(1-16). ICR mice were rendered neutropenic by i.p. injection of cyclophosphamide at 150 and 100 mg/kg on days 0 and 3, respectively. The procedure was confirmed to result in severe neutropenia by day 4, at which time infection was induced. Groups of mice (n = 6) were infected i.p. with a logarithmic-phase culture of P. aeruginosa (106 CFU in 0.2 ml) prepared as described above. After inoculation, K4-S4(1-16) was injected i.p. at various doses. Mice were sacrificed 0, 1, or 5 h later, and peritoneal washes (with 2 ml of sterile saline) were performed immediately to determine the bacterial count. Peritoneal fluids (approximately 1.5 ml) were collected from each mouse separately and kept on ice. The number of viable bacteria was determined by plating samples of fluids on Mueller-Hinton agar plates.
RESULTS
In vitro activity.
The susceptibility of bacteria to dermaseptin S4 derivatives was assessed by measuring the peptide MICs against 66 clinical isolates, including the multidrug-resistant gram-negative bacteria P. aeruginosa and E. coli as well as the gram-positive bacterium S. aureus. The resulting data are summarized in Table 1. The 28-residue dermaseptin derivative K4K20-S4 displayed potent activity against all clinical isolates regardless of their class (gram negative or positive), with MICs ranging between 1 and 4 μg/ml for most of the strains tested. Statistical analysis, as exemplified by the MICs for 50 and 90% of bacteria tested, revealed remarkably low fluctuations, including among different bacterial types. Consistent with the peptide's theoretic mode of action, the low level of fluctuation reflects the peptide's insensitivity to the diverse mechanisms that bacteria have developed for becoming resistant to antibiotics. These findings confirm the high antibacterial activity previously observed for three other gram-negative bacteria (10) and, combined with other studies (11, 21), establish K4K20-S4 as a potent broad-spectrum antibacterial agent.
TABLE 1.
Amino acid sequences of the dermaseptin S4 derivatives and their antibacterial activities against 66 multidrug-resistant clinical isolates
| Peptide | Amino acid sequencea | MIC (μg/ml)b
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
S. aureus (n = 23)
|
P. aeruginosa (n = 17)
|
E. coli (n = 26)
|
||||||||||
| 50% | 90% | Range | 50% | 90% | Range | 50% | 90% | Range | ||||
| K4K20-S4 | ALWKTLLKKVLKAAAKAALKAVLVGANA | 1 | 2 | 1-4 | 2 | 2 | 1-4 | 4 | 8 | 1-16 | ||
| K4-S4(1-16) | ALWKTLLKKVLKAAAKa | 2 | 4 | 0.5-8 | 4 | 4 | 1-8 | 2 | 4 | 1-8 | ||
| K4-S4(1-13) | ALWKTLLKKVLKAa | 4 | 8 | 1-16 | 8 | 8 | 2-16 | 4 | 8 | 2-16 | ||
a, amide.
50% and 90%, MICs (based on amino acid analysis) at which 50 and 90% of the tested clinical isolates were inhibited, respectively.
Replacing the C-terminal 12 residues of K4K20-S4 by a carboxamide resulted in a similar profile of activity, where potency was slightly enhanced against E. coli and somewhat reduced against S. aureus and P. aeruginosa. Broad-spectrum antibacterial activity was maintained after further reduction of the C-terminal end of K4-S4(1-16) by three residues. The resulting 13-mer analog still had considerable potency, displaying MICs that were higher by up to fourfold for S. aureus and P. aeruginosa (Table 1).
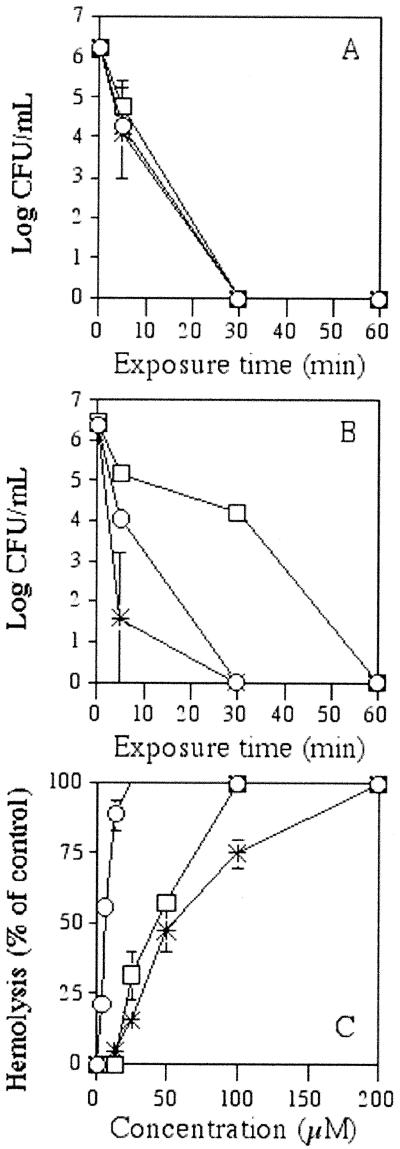
The kinetics of bactericidal activity against both gram-negative and gram-positive bacteria (represented by E. coli and S. aureus, respectively) were investigated by enumerating CFU after 0, 5, 30, and 60 min of exposure to dermaseptin peptides or to other antimicrobial peptides with reportedly rapid bactericidal activity, such as the 22-residue magainin derivative MSI-78 (31) and the 18-residue protegrin PG-1 (29). In addition, the peptides were compared with respect to hemolytic activity. Figure 1 summarizes the outcome from the three assays for the shortest dermaseptin derivative. K4-S4(1-13) was rapidly bactericidal against both gram-negative and gram-positive bacteria. At a peptide concentration of 20 μM (corresponding to 30 μg/ml, i.e., 2 multiples of the MIC), K4-S4(1-13) reduced the number of viable CFU of both E. coli (Fig. 1A) and S. aureus (Fig. 1B) by 6 log units in 30 min or less. Both longer derivatives were more rapidly bactericidal, displaying the same outcome but in 5 min or less (data not shown). Under similar conditions, PG-1 displayed kinetics identical or similar to those of K4-S4(1-13), whereas MSI-78 displayed identical kinetics against E. coli and slower kinetics against S. aureus. When the peptides were compared with respect to toxicity towards human erythrocytes (Fig. 1C), PG-1 displayed the highest hemolytic activity, MSI-78 was substantially less hemolytic, and K4-S4(1-13) displayed the least hemolytic activity. Both longer dermaseptin S4 derivatives were more hemolytic than K4-S4(1-13), by 5- and 100-fold, respectively, for K4-S4(1-16) and K4K20-S4 (10).
FIG. 1.
Bactericidal kinetics and hemolytic activities of K4-S4(1-13), MSI-78, and PG-1. A peptide concentration of 20 μM [corresponding to 30, 50, and 43 μg/ml, respectively, for K4-S4(1-13) (asterisks), MSI-78 (squares), and PG-1 (circles)] was used to treat cultures of E. coli (A) and S. aureus (B). After 0, 5, 30, and 60 min of exposure time at 37°C, aliquots were diluted (serial 10-fold dilutions) and plated for CFU counts after overnight incubation at 37°C. The peptide hemolytic activity (C) was assessed against human erythrocytes (1% hematocrit). Hemolysis was determined by measuring absorbance (405 nm) of the supernatants after 4 h of incubation at 37°C in the presence of water (100% hemolysis), PBS (baseline), or serial twofold dilutions of peptides in PBS starting from 200 μM [corresponding to 300, 500, and 430 μg/ml, respectively, for K4-S4(1-13), MSI-78, and PG-1]. Plotted values correspond to the means ± standard deviations.
Resistance studies.
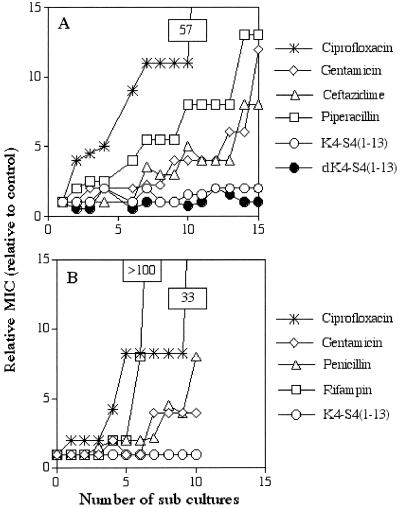
To assess emerging resistance that could develop following multiple exposures of bacteria to a given antimicrobial agent, two clinical isolates of P. aeruginosa and S. aureus were used as representatives of gram-negative and gram-positive bacteria, respectively. Bacteria were cultured for up to 15 consecutive generations in the presence of one of the dermaseptin S4 derivatives or in presence of one of four classical antibiotics. Figure 2 shows the outcome for the shorter dermaseptin S4 derivative, K4-S4(1-13). As shown in Fig. 2A, after 15 subcultures following the initial exposure, the relative MIC of K4-S4(1-13) against P. aeruginosa remained stable. A similar result was obtained when using the peptide's d isomer (composed of amino acids of the d configuration) or when using any one of the K4-S4(1-16) or K4K20-S4 isomers (data not shown). In contrast, during the same period of time, the MIC gradually increased for all other agents, reflecting emergence of resistant bacteria. Thus, at the 15th generation the MICs increased by 57-fold for ciprofloxacin, by 13-fold for gentamicin, by 12-fold for ceftazidime, and by 8-fold for piperacillin. Moreover, when the 15th generation of each of the bacteria that had developed resistance to antibiotics was tested against the dermaseptin S4 derivatives, all bacteria were found to be susceptible, with MICs similar to those at the initial exposure.
FIG. 2.
Evolution of MICs after successive exposures of bacteria to sublytic concentrations of the specified antimicrobial agent. (A and B) Evolution of MICs after 15 and 10 serial passages of P. aeruginosa and S. aureus, respectively. The relative MIC is the normalized ratio of the MIC obtained for a given subculture to the MIC (determined concomitantly) obtained for first-time exposure.
Similar results were observed when a representative clinical isolate of S. aureus was cultured for 10 consecutive generations. Figure 2B shows that the MIC of the dermaseptin S4 derivative remained stable at 10 subcultures following the initial exposure but that the MIC of ciprofloxacin increased by 33-fold, that of gentamicin increased by 4-fold, that of penicillin increased by 8-fold, and that of rifampin increased by >100-fold. This demonstrated that bacterial resistance is less prone to develop in dermaseptin-treated gram-negative or gram-positive bacteria, whether they are sensitive or resistant to conventional antibiotics.
In vivo efficacy studies.
The peptide efficacy in vivo was assessed for the three derivatives. At first, acute toxicity was examined after i.p. injection of a single dose of each peptide to groups of BALB/c mice (n = 10 mice/group). Mild fur erection was noticed for doses of above 125 μg/animal (5.4 mg/kg) minutes after injection and was resolved in all cases within 4 h or less. No immediate adverse events were noticed for the short peptides with doses of up to 250 μg (11 mg/kg), whereas K4K20-S4 caused 30% mortality at 11 mg/kg (data not shown). No mortality was observed in animals injected with up to 250 μg of either of the short peptides (corresponding to 11 mg of peptide/kg). Injection of higher doses of K4-S4(1-16), i.e., 500 and 750 μg/mouse [corresponding to 21.7 and 32.6 mg of K4-S4(1-16)/kg], resulted in mortality rates of 60 and 100%, respectively. All cases of mortality occurred within 4 days after peptide injection.
Next, we performed protection studies. Since K4K20-S4 was suspected to be toxic , only the two short dermaseptin derivatives were investigated. Also, we chose to study P. aeruginosa because of its high virulence, high propensity for emergence of resistance, and limited treatment options. Thus, BALB/c mice were infected with inoculum of 106 CFU of P. aeruginosa per mouse. K4-S4(1-16) and K4-S4(1-13) were administered i.p., immediately after the bacteria, at a single dose of 100 μg (4.5 mg/kg) to 16 animals. All cases of mortality occurred within the first 24 h after injections. Mortality in K4-S4(1-16)-treated mice was lower than that in the control group (18.7 versus 75%, respectively; P = 0.004). Mortality among K4-S4(1-13)-treated animals was also significantly lower than that among controls (36.6 versus 75%; P = 0.025).
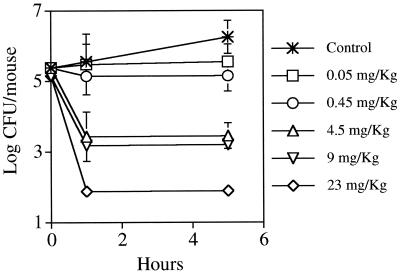
To evaluate the peptide's antibacterial effect in vivo, models were developed in neutropenic mice in order to decrease the influence of the host immune system and to reduce the variability in bacterial counts. K4-S4(1-16) was injected i.p. at doses of 0.05, 0.45, 4.5, 9, and 23 mg/kg immediately after induction of infection. Peritoneal washes were performed at 0, 1, and 5 h later and were subjected to quantitative cultures. Figure 3 summarizes the results obtained for the peritoneal washes. K4-S4(1-16) was found to reduce the bacterial counts by >3 log CFU per mouse at the nontoxic levels (up to 9 mg/kg). A dose-response effect was evident, with a further reduction of the log CFU at the dose of 23 mg/kg.
FIG. 3.
In vivo bactericidal activity of K4-S4(1-16). Neutropenic mice infected i.p. with P. aeruginosa were treated i.p. with PBS alone or with PBS containing the specified peptide doses. Animals (six mice/group) were sacrificed at 0, 1, and 5 h after bacterial infection, and peritoneal fluids were removed as described in Materials and Methods. Viable bacterial counts were determined (in triplicate) by plating diluted peritoneal fluids on Mueller-Hinton agar plates. The results shown correspond to the means ± standard deviations.
DISCUSSION
Emergence of antimicrobial resistance is a growing problem and a public health threat. To overcome infections caused by multidrug-resistant strains and strains resistant to all available antimicrobials, new classes of antimicrobials should be developed. This has been recognized by the U.S. Congress Office of Technology Assessment (30) and by the United Kingdom House of Lords (19). Antimicrobial peptides represent a promising class of new agents. These agents are rapidly bactericidal, and since their action relates to physical properties, it is all the more difficult for bacteria to develop resistance to such peptides. Moreover, it is possible to engineer their structure with relative ease, since peptide chemistry allows a multitude of modifications that are relatively time- and cost-effective. Nevertheless, most of the agents undergoing clinical trials lack specificity and are examined only for topical uses (15, 24).
Here, we describe the activities of three related synthetic antimicrobial agents derived from the natural peptide dermaseptin S4. These agents were highly active against a series of multidrug-resistant gram-negative clinical isolates as well as against gram-positive strains. In contrast to the conventional antibiotics tested, the dermaseptin derivatives did not have increased MICs upon serial passages in sublytic drug concentrations. Both K4-S4(1-16) and K4-S4(1-13) improved the survival in a P. aeruginosa-mouse peritonitis model and showed a dose response for in vivo bacterial killing. In this work, we also document that these agents caused less hemolysis than other tested peptides and did not cause acute toxicity in mice at clinically used doses. The antibacterial activity demonstrated for the dermaseptin S4 derivatives might offer significant advantages over conventional antimicrobial agents, which often act on intracellular targets. Both the speed of action and the inability of bacteria to develop resistance to dermaseptin peptides are consistent with their theorized target and mode of action, i.e., disruption of the membrane structure. Accordingly, it would be expected for d isomers to display the same activity (as was indeed observed), since they present the same physicochemical properties. Thus, by acting on a surface target, antimicrobial peptides circumvent the known mechanisms that bacteria develop in order to resist the action of antibiotic compounds. The fact that activity is not mediated by interaction with a chiral center (receptor or enzyme, etc.) combined with the external location of the target may be two solid and sufficient reasons for the lack of the development of bacterial resistance observed for conventional antimicrobial agents, which often need to reach internal targets and require longer exposure periods.
This study established the dermaseptin S4 derivatives as potent broad-spectrum antibacterial agents. Among the three derivatives, K4K20-S4 was found to be the most potent but also the most toxic, both for mice and for human erythrocytes in vitro. Toxicity is probably due to the peptide's particular physicochemical properties, which combine both high hydrophobicity and high charge content, as discussed previously (10) and as is often observed with a variety of antimicrobial peptides. Accordingly, by reduction of its hydrophobicity (by deletion of the C-terminal hydrophobic stretch), the peptide became more selective in vitro and less toxic in vivo but conserved a considerable broad-spectrum antimicrobial potency. Thus, whereas K4K20-S4 might be useful in nonsystemic antimicrobial applications, the short derivatives seem to be more adapted to a systemic use as well. Although dermaseptin derivatives have improved toxicity profiles, further perfection is desirable to reach the safety of some conventional antibiotics.
After a single administration of a nontoxic dose, the short dermaseptin S4 derivatives were efficient in treating peritoneal infections. The intraperitoneal route of administration is sometimes preferred, such as for antimicrobial therapy of peritonitis in patients receiving chronic ambulatory dialysis (23). Our results were obtained when peptides were administrated immediately after a high-inoculum infection was introduced. Further studies will determine the effectiveness of delayed treatment and other routes of administration. Intravenous administration, for instance, was well tolerated at high a dose (10 mg/kg) of K4-S4(1-13) in rats (11).
In conclusion, these studies demonstrate that short dermaseptin S4 derivatives are safe and potent antibacterial agents, with in vivo activity against clinically relevant antibiotic-resistant bacteria.
Acknowledgments
This research was supported by the MAGNETON project, administrated by the Office of the Chief Scientist at the Ministry of Industry and Trade, Israel.
REFERENCES
- 1.Ammar, B., A. Perianin, A. Mor, G. Sarfati, M. Tissot, P. Nicolas, J. P. Giroud, and M. R. Arveiller. 1998. Dermaseptin, a peptide antibiotic, stimulates microbicidal activities of polymorphonuclear leukocytes. Biochem. Biophys. Res. Commun. 247:870-875. [DOI] [PubMed] [Google Scholar]
- 2.Boman, H. G. 1995. Peptide antibiotics and their role in innate immunity. Annu. Rev. Immunol. 13:61-92. [DOI] [PubMed] [Google Scholar]
- 3.Chapple, D. S., D. J. Mason, C. L. Joannou, E. W. Odell, V. Gant, and R. W. Evans. 1998. Structure-function relationship of antimicrobial synthetic peptides homologous to a helical surface region on human lactoferrin against Escherichia coli serotype O111. Infect. Immun. 66:2434-2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen, J., T. J. Falla, H. Liu, M. A. Hurst, C. A. Fujii, D. A. Mosca, J. R. Embree, D. J. Loury, P. A. Radel, C. C. Chang, L. Gu, and J. C. Fiddes. 2000. Development of protegrins for the treatment and prevention of oral mucositis: structure-activity relationships of synthetic protegrin analogues. Biopolymers 55:88-98. [DOI] [PubMed] [Google Scholar]
- 5.Coot, P. J., C. D. Holyoak, D. Bracey, D. P. Ferdinando, and J. A. Pearce. 1998. Inhibitory action of the amphibian skin peptide dermaseptin S3 on Saccharomyces cerevisiae. Antimicrob. Agents Chemother. 42:2160-2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Lucca, A. J., J. M. Bland, T. J. Jacks, C. Grimm, and T. J. Walsh. 1998. Fungicidal and binding properties of the natural peptides cecropin B and dermaseptin S1. Med. Mycol. 36:291-298. [PubMed] [Google Scholar]
- 7.Dimitri, M. T., M. Velucchi, L. Bracci, A. Rustici, M. Porro, P. Villa, and P. Ghezzi. 1996. Inhibition of LPS-induced systemic and local TNF production by a synthetic anti-endotoxin peptide (SAEP-2). J. Endotoxin Res. 3:445-454. [Google Scholar]
- 8.Elsbach, P., and J. Weiss. 1998. Role of the bactericidal/permeability increasing protein in host defence. Curr. Opin. Immunol. 10:45-49. [DOI] [PubMed] [Google Scholar]
- 9.Epand, M. E., Y. Shai, J. P. Segrest, and G. M. Anantharamaiah. 1995. Mechanisms for the modulation of membrane bilayer properties by amphipathic helical peptides. Biopolymers 37:319-338. [DOI] [PubMed] [Google Scholar]
- 10.Feder, R., A. Dagan, and A. Mor. 2000. SAR study of antimicrobial dermaseptin S4 showing the consequences of peptide oligomerization on selective cytotoxicity. J. Biol. Chem. 275:4230-4238. [DOI] [PubMed] [Google Scholar]
- 11.Feder, R., R. Nechushtai, and A. Mor. 2001. Affinity-driven molecular transfer from erythrocyte membrane to target cells. Peptides 22:1683-1690. [DOI] [PubMed] [Google Scholar]
- 12.Ge, Y., D. MacDonald, M. M. Henry, H. I. Hait, K. A. Nelson, B. A. Lipsky, M. A. Zasloff, and K. J. Holroyd. 1999. In vitro susceptibility to pexiganan of bacteria isolated from infected diabetic foot ulcers. Diagn. Microbiol. Infect. Dis. 35:45-53. [DOI] [PubMed] [Google Scholar]
- 13.Ghosh, J. K., D. Shaool, P. Guillaud, L. Ciceron, D. Mazier, I. Kustanovich, Y. Shay, and A. Mor. 1997. Selective cytotoxicity of dermaseptin S3 towards intraerythrocytic Plasmodium falciparum and the underlying molecular basis. J. Biol. Chem. 267:6502-6509. [DOI] [PubMed] [Google Scholar]
- 14.Gough, M., R. E. W. Hancock, and N. M. Kelly. 1996. Antiendotoxin activity of cationic peptide antimicrobial agents. Infect. Immun. 64:4922-4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gura, T. 2001. Ancient system gets new respect. Science 291:2068-2071. [DOI] [PubMed] [Google Scholar]
- 16.Hancock, R. E., and R. Lehrer. 1998. Cationic peptides: a new source of antibiotics. Trends Biotechnol. 16:82-88 [DOI] [PubMed] [Google Scholar]
- 17.Harwig, S. S. L., A. Waring, H. J. Yang, Y. Cho, L. Tan, and R. I. Lehrer. 1996. Intramolecular disulfide bonds enhance the antimicrobial and lytic activities of protegrins at physiological NaCl concentrations. Eur. J. Biochem. 240:352-357. [DOI] [PubMed] [Google Scholar]
- 18.Hernandez, C., A. Mor, F. Dagger, P. Nicolas, A. Hernandez, E. L. Benedetti, and I. Dunia. 1992. Functional and structural damages in L. mexicana exposed to the cationic peptide dermaseptin. Eur. J. Cell Biol. 59:414-424. [PubMed] [Google Scholar]
- 19.House of Lords, United Kingdom. 1998. Science and technology 7th report: resistance to antibiotics and other antimicrobial agents. HL Paper 81-II, session 1997-98. House of Lords, London, United Kingdom.
- 20.Jouenne, T., Mor, A., H. Bonato, and G. A. Junter. 1998. Antibacterial activity of synthetic dermaseptins against growing and nongrowing Escherichia coli cultures. J. Antimicrob. Chemother. 42:87-90. [DOI] [PubMed] [Google Scholar]
- 21.Krugliak, M., R. Feder, Y. Z. Zolotarev, L. Gaidukov, A. Dagan, H. Ginsburg, and A. Mor. 2000. Antimalarial activity of dermaseptin S4 derivatives. Antimicrob. Agents Chemother. 44:2442-2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee, W. J., J. L. Farmer, M. Hilty, and Y. B. Kim. 1998. The protective effects of lactoferrin feeding against endotoxin lethal shock in germfree piglets. Infect. Immun. 66:1421-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levison, M. E., and L. M. Bush. 1995. Peritonitis and other intra-abdominal infections, p. 705-740. In G. L. Mandell, J. E. Bennett, and R. Dolin (ed.), Principles and practice of infection diseases, 4th ed. Churchill Livingstone, New York, N.Y.
- 24.Levy, O. 2000. Antimicrobial proteins and peptides of blood: templates for novel antimicrobial agents. Blood 96:2664-2672. [PubMed] [Google Scholar]
- 25.Mor, A., K. Hani, and P. Nicolas, P. 1994. The vertebrate peptide antibiotics dermaseptins have overlapping structural features but target specific microorganisms. J. Biol. Chem. 269:31635-31641. [PubMed] [Google Scholar]
- 26.Nicolas, P., and A. Mor. 1995. Peptides as weapons against microorganisms in the chemical defense system of vertebrates. Annu. Rev. Microbiol. 4:277-304. [DOI] [PubMed] [Google Scholar]
- 27.Scott, G. M., M. C. Rosenberg, R. M. Gold, B. B. Finlay, and R. E. W. Hancock. 2000. An α-helical cationic antimicrobial peptide selectively modulates macrophage responses to lipopolysaccharide and directly alters macrophage gene expression. J. Immunol. 165:3358-3365. [DOI] [PubMed] [Google Scholar]
- 28.Shai, Y. 1995. Molecular recognition between membrane-spanning polypeptides. Trends Biochem. Sci. 20:460-464. [DOI] [PubMed] [Google Scholar]
- 29.Steinberg, D. A., M. A. Hurst, C. A. Fuji, A. H. C. Kung, J. F. Ho, F. C. Cheng, D. J. Loury, and J. C. Fiddes. 1997. Protegrin-1: a broad-spectrum, rapidly microbicidal peptide with in vivo activity. Antimicrob. Agents Chemother. 41:1738-1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.U.S. Congress, Office of Technology Assessment. 1995. Impacts of antibiotic resistant bacteria. Office of Technology Assessment, Washington, D.C.
- 31.Yigong, G. E., D. L. MacDonald, K. J. Holroyd, C. Thornsberry, H. Wexler, and M. Zasloff. 1999. In vitro antibacterial properties of Pexiganan, an analog of magainin. Antimicrob. Agents Chemother. 43:782-788. [DOI] [PMC free article] [PubMed] [Google Scholar]