Abstract
Rifampin penetrated biofilms formed by Staphylococcus epidermidis but failed to effectively kill the bacteria. Penetration was demonstrated by a simple diffusion cell bioassay and by transmission electron microscopic observation of antibiotic-affected cells at the distal edge of the biofilm.
Bacteria that adhere to implanted medical devices or damaged tissue can become the focus of persistent infections (5). These bacteria evade clearance by the host defenses and by antimicrobial chemotherapy by growing in biofilms in which they are protected. Among the most common culprits in biofilm infections are Staphylococcus aureus and Staphylococcus epidermidis (2, 4, 15). Biofilm infections that frequently involve staphylococcal strains include those associated with sutures (9), Hickman catheters (15, 20), central venous catheters (12, 14), prosthetic heart valves (10), and vascular grafts (11).
The mechanisms by which bacteria in biofilms escape being killed by antibiotics are not known (13, 18). The failure of the antibiotic to penetrate at bactericidal concentrations throughout the entire extent of the biofilm is perhaps the reason most often given for reduced rates of killing. In experimental tests, some antibiotics seemed to penetrate while others were retarded or failed to penetrate (17). Such mixed results suggest that the extent of antibiotic penetration is agent and organism specific. Only three papers have addressed antibiotic penetration into staphylococcal biofilms in particular (6, 7, 22). These reports suggest that rifampin, vancomycin, cefotiam, and ofloxacin penetrated in vitro biofilms but failed to effectively kill bacteria.
We measured the penetration of rifampin through S. epidermidis biofilms formed on membranes resting on a complex agar medium. Polycarbonate membrane filters were sterilized by exposure to UV light, placed on tryptic soy agar (TSA), and seeded with a 40-μl drop of an overnight culture of S. epidermidis ATCC 35984. The plates were inverted and incubated at 37°C for 48 h. Each “colony biofilm” was transferred to a fresh TSA plate every 12 h during this period. Further details of the colony biofilm growth procedure are described by Anderl et al. (1). After 48 h of development, the average number of bacteria per membrane was 8.00 ± 0.01 log10 CFU.
Antibiotic penetration through 48-h-old S. epidermidis colony biofilms was measured by the technique of Anderl et al. (1). This technique involved placing a second, smaller membrane on top of a bacterial colony and then placing a wetted paper disk, of the type used in zone-of-inhibition bioassays, on top of the smaller membrane. This process resulted in a primitive diffusion cell in which the colony biofilm was sandwiched between the two membranes. Up to 20 of these assemblies were prepared at one time. These assemblies were simultaneously transferred to separate TSA plates containing 0.1 μg of rifampin per ml and incubated at 37°C. Each colony biofilm sandwich was transferred to fresh plates containing antibiotic every 12 h. The antibiotic had to diffuse out of the agar, through the lower membrane, through the bacterial colony, and through the upper membrane to reach the moistened disk. Disks were removed at 1-h intervals and placed on TSA plates spread with 100 μl of an S. epidermidis suspension diluted to an optical density of 0.05. The diameter of the zone of inhibition around the disk was measured after 24 h of incubation. A dilution series of rifampin loaded onto fresh disks was used to obtain a standard relationship between the rifampin concentration and the inhibition zone. Zones of inhibition were converted to rifampin concentrations by using this standard curve.
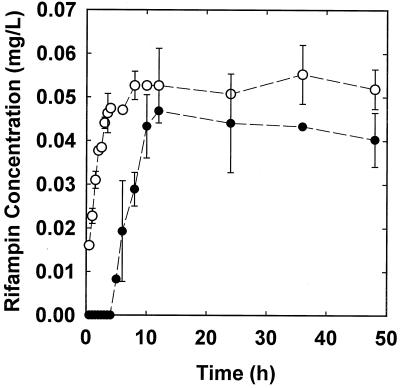
Active rifampin fully penetrated through S. epidermidis colony biofilms in about 12 h (Fig. 1). After 12 h, the concentration of rifampin measured in the disks on the distal side of the biofilm exceeded 90% of the equilibrium concentration measured in the absence of biofilm. Rifampin penetration through assemblies with biofilm was delayed by about 4 h in comparison to that in a biofilm-free control.
FIG. 1.
Penetration of 0.1 μg of rifampin per ml through membrane assemblies with (filled symbols) and without (open symbols) S. epidermidis colony biofilms. Error bars indicate the standard errors of the means.
The killing of bacteria in intact colony biofilms was measured by transferring colony biofilms to agar plates containing 0.1 μg of rifampin per ml. These plates were incubated at 37°C. Colony biofilms were transferred to fresh plates containing antibiotic every 12 h. A biofilm was sampled by placing the membrane and associated bacteria into a tube containing 9 ml of phosphate-buffered water (pH 7.2) and vortexing the tube at high speed for 1 min. The resulting cell suspension was serially diluted, and the viable bacteria were enumerated by drop plating on TSA. The number of bacteria killed was reported as a log reduction. The growth rate of bacteria in untreated control colony biofilms was calculated by regressing the natural log of cell numbers against time to compute the slope of the line.
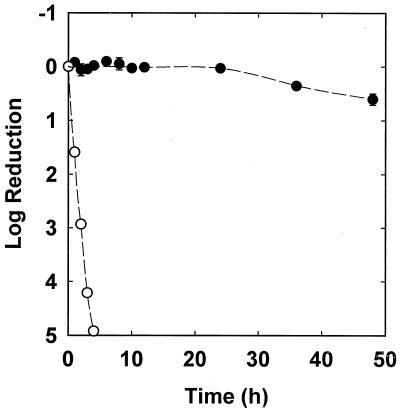
S. epidermidis in intact colony biofilms was scarcely affected by continuous exposure to 0.1 μg of rifampin per ml over 48 h (Fig. 2). After 48 h, the mean log reduction was only 0.60 ± 0.10. Planktonic bacteria were rapidly killed by treatment with the same concentration of rifampin (Fig. 2). An exponential-phase planktonic culture exposed to 0.1 μg of rifampin per ml experienced a 4.92 log reduction after 4 h. Rapid killing was also measured for suspended bacteria treated with 0.05 μg of rifampin per ml (log reduction, 3.77). The susceptibility of bacteria in suspension was measured by diluting 100 μl of a late-log-phase culture into 100 ml of tryptic soy broth containing the desired concentration of rifampin. This culture, which contained approximately 3 × 106 CFU/ml, was placed in a shaking incubator at 37°C and sampled every hour for 4 h. Culture samples were serially diluted and drop plated on TSA to enumerate viable bacteria. Additional experiments were performed with a slow-growing inoculum by diluting 100 μl of a stationary-phase culture into 100 ml of broth. The growth rate of bacteria in untreated control experiments was calculated by regressing the natural log of cell numbers against time to compute the slope of the line.
FIG. 2.
Comparison of susceptibilities of planktonic S. epidermidis (open symbols) and S. epidermidis in a 48-h colony biofilm (filled symbols) exposed to 0.1 μg of rifampin per ml. Error bars indicate the standard errors of the means.
The appearance of spontaneous rifampin-resistant mutants is well known (19). One explanation for the reduced susceptibility of bacteria in the biofilm is that the biofilm is populated by such mutants. We checked for rifampin-resistant mutants by dispersing bacteria from colony biofilms and plating them on both TSA and TSA containing 0.05 μg of rifampin per ml. No colonies were ever noted on the antibiotic plates. This indicated that resistant mutants, if present, constituted less than 0.004% of the population. This percentage is far too low to explain the poor killing rate shown in Fig. 2. Rifampin-resistant mutants were detected, by the same assay described above, when colony biofilms were exposed to rifampin for periods longer than 48 h.
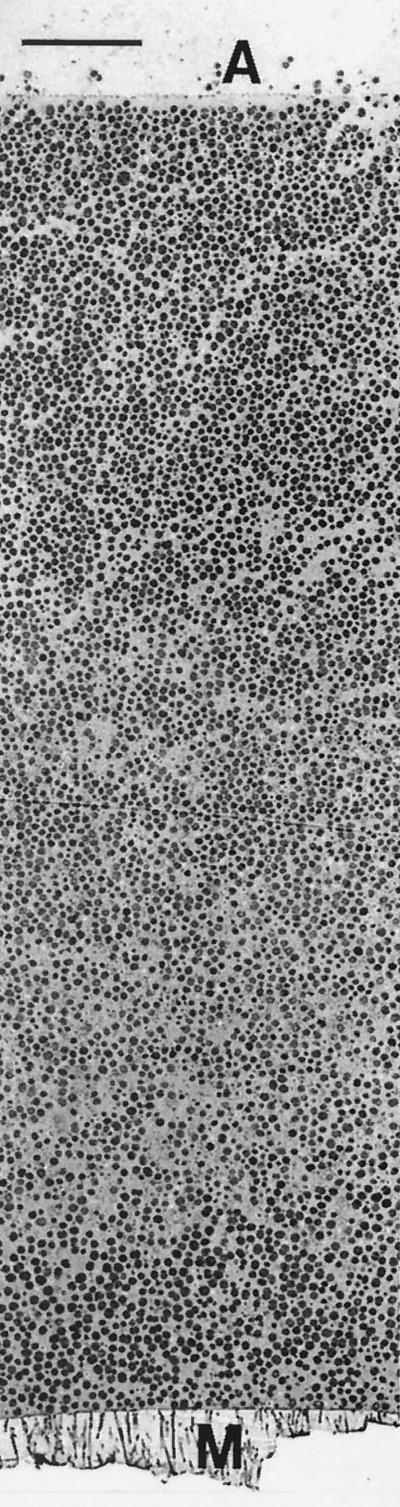
To obtain further evidence of antibiotic penetration, antibiotic-treated and untreated colony biofilms were examined by transmission electron microscopy. Three samples were prepared: a 48-h-old biofilm not exposed to antibiotic (time zero control), a 48-h-old biofilm transferred to TSA without antibiotic for an additional 48 h (48-h control), and a 48-h-old biofilm transferred to TSA containing 0.1 μg of rifampin per ml for 48 h. These biofilms were fixed with 5% glutaraldehyde, stained with osmium tetroxide (1%), and washed. Specimens were then dehydrated in an ethanol series, which included a staining step with 1% uranyl acetate-1% phosphotungstic acid. The dehydrated samples were embedded in Spurrs epoxy resin, which was polymerized for 14 h at 70°C. Thin sections were cut and examined using a Jeol JEM-100CX electron microscope. In a 48-h-old colony biofilm that was not exposed to antibiotic, the colony was approximately 83 μm thick and bacteria were evenly distributed throughout the colony (Fig. 3). These bacteria exhibited a typical coccoid morphology that did not change much with location in the biofilm. Colony biofilms from 48-h control experiments were slightly larger in diameter than were those from time zero control colonies. The 48-h control colony biofilm was also thicker (approximately 171 μm thick) than the time zero control biofilm. Bacterial cells in the 48-h control were similar in appearance to those in the time zero control. Rifampin-treated colonies were slightly smaller in diameter than time zero control colonies. The thickness of a colony biofilm treated with rifampin for 48 h was 84 μm, about the same as that of the time zero control biofilm.
FIG. 3.
Transmission electron micrograph of the untreated, time zero control S. epidermidis colony biofilm. The membrane (M) is at the bottom, and the air interface (A) is at the top. Scale bar, 10 μm.
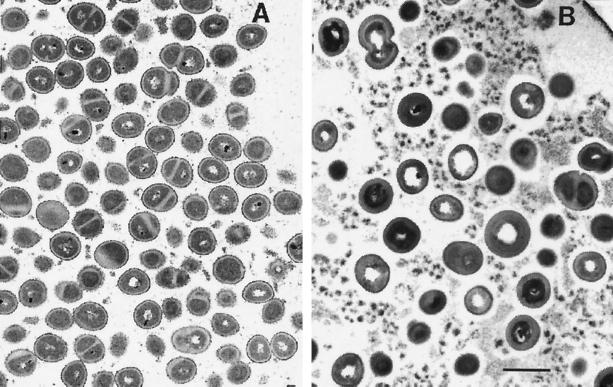
In the rifampin-treated biofilm, antibiotic action was evident, especially near the air interface (Fig. 4). Bacterial cell walls were significantly thickened throughout the antibiotic-treated specimen (Fig. 4; Table 1). Thickening of the cell wall is a common reaction to antibiotic treatment in staphylococci (8). The cell densities and frequencies of dividing bacteria were also reduced by statistically significant levels at the air interface in the rifampin-treated colony biofilms compared to what occurred in the 48-h control (Table 1). These results confirm that the antibiotic was able to penetrate through the entire extent of the colony biofilm and act on bacteria at the distal edge. Antibiotic action was apparent along the full length of the air interface of the colony biofilm, which shows that rifampin penetrated uniformly in this system, not just at isolated locations.
FIG. 4.
Transmission electron micrographs of S. epidermidis colony biofilms near the air interface. (A) Untreated 48-h control; (B) 48-h treatment with 0.1 μg of rifampin per ml. The air interface is located in the upper right-hand corners in both panels. Both images were taken at the same magnification. Scale bar, 1 μm.
TABLE 1.
Cell density, dividing-cell frequency, and cell wall thickness in different locations of 48-h control and 48-h treated colony biofilmsa
| Location | Biofilm sample | No. of cells/mm2 (105) | Dividing-cell frequency (%) | Cell wall thickness (nm) |
|---|---|---|---|---|
| Membrane | Control | 7.73 ± 1.12 | 30 ± 6 | 44 ± 11 |
| Treated | 7.78 ± 1.61 (0.85) | 27 ± 11 (0.84) | 101 ± 38 (<0.001) | |
| Air interface | Control | 10.9 ± 1.1 | 33 ± 7 | 48 ± 12 |
| Treated | 5.13 ± 1.05 (<0.001) | 18 ± 6 (0.002) | 81 ± 28 (<0.001) |
Values in parentheses are P values relative to values for the control.
We conclude that S. epidermidis in biofilms is protected from killing by rifampin, but this protection is not due to inadequate penetration of the antibiotic through the bacterial aggregate. An alternative explanation is that the biofilm harbors slow or nongrowing bacteria which are less susceptible to the antibiotic by virtue of their reduced growth rates (3, 21). There are a few reports in the literature in which the relative susceptibility of planktonic staphylococci to rifampin has been investigated as a function of growth state, and these studies support the hypothesis that slow-growing bacteria are less susceptible (16, 23). We found that stationary-phase S. epidermidis challenged with rifampin in free suspension experienced a log reduction (1.11) about one-fourth of that measured for bacteria from an exponentially growing culture (4.92). Recall that the log reduction of bacteria in the colony biofilm over a longer exposure period of 48 h was a scant 0.60. The community-averaged growth rate of bacteria in colony biofilms was 0.035 ± 0.004 h−1. This is lower than the growth rate estimated for stationary-phase bacteria in an untreated control (0.15 ± 0.06 h−1) and much lower than the growth rate of the exponential-phase bacteria (0.82 ± 0.34 h−1). These preliminary data support a correlation between growth rate and the susceptibility of S. epidermidis to rifampin and are consistent with the hypothesis that the slow growth of bacteria in biofilms is responsible for their protection from antibiotic killing.
REFERENCES
- 1.Anderl, J. N., M. J. Franklin, and P. S. Stewart. 2000. Role of antibiotic penetration limitation in Klebsiella pneumoniae biofilm resistance to ampicillin and ciprofloxacin. Antimicrob. Agents Chemother. 44:1818-1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andreoli-Pinto, T. J., and K. U. Graziano. 1999. Important aspects of the colonization of central venous catheter. Boll. Chim. Farm. 138:19-23. [PubMed] [Google Scholar]
- 3.Brown, M. R. W., D. G. Allison, and P. Gilbert. 1988. Resistance of bacterial biofilms to antibiotics: a growth-rate related effect? J. Antimicrob. Chemother. 22:777-783. [DOI] [PubMed] [Google Scholar]
- 4.Cacoub, P., P. Leprince, P. Nataf, P. Hausfater, R. Dorent, B. Wechsler, V. Bors, A. Pavie, J. C. Peitte, and I. Gandjbakhch. 1998. Pacemaker infective endocarditis. Am. J. Cardiol. 82:480-484. [DOI] [PubMed] [Google Scholar]
- 5.Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. [DOI] [PubMed] [Google Scholar]
- 6.Darouiche, R. O., A. Dhir, A. J. Miller, G. C. Landon, I. I. Raad, and D. M. Musher. 1994. Vancomycin penetration into biofilm covering infected prostheses and effect on bacteria. J. Infect. Dis. 170:720-723. [DOI] [PubMed] [Google Scholar]
- 7.Dunne, W. M., Jr., E. O. Mason, Jr., and S. L. Kaplan. 1993. Diffusion of rifampin and vancomycin through a Staphylococcus epidermidis biofilm. Antimicrob. Agents Chemother. 37:2522-2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Felice, G., G. Carnevale, P. Lanzarini, F. Castelli, C. Zappala, P. Menghini, and C. De Rysky. 1986. Alterations due to ampicillin and rifampicin in S. sanguis and S. aureus isolated from dental plaque. An electron microscopic study. Chemioterapia 5:3-6. [PubMed] [Google Scholar]
- 9.Gristina, A. G., J. L. Price, C. D. Hobgood, L. X. Webb, and J. W. Costerton. 1985. Bacterial colonization of percutaneous sutures. Surgery 98:12-19. [PubMed] [Google Scholar]
- 10.Hyde, J. A., R. O. Darouiche, and J. W. Costerton. 1998. Strategies for prophylaxis against prosthetic valve endocarditis: a review article. J. Heart Valve Dis. 7:313-315. [PubMed] [Google Scholar]
- 11.Kaebnick, H. W., D. F. Bandyk, T. M. Bergamini, and J. B. Towne. 1987. The microbiology of explanted vascular prostheses. Surgery 102:756-762. [PubMed] [Google Scholar]
- 12.Khardori, N., and M. Yassien. 1995. Biofilms in device-related infections. J. Ind. Microbiol. 15:141-147. [DOI] [PubMed] [Google Scholar]
- 13.Lewis, K. 2001. Riddle of biofilm resistance. Antimicrob. Agents Chemother. 45:999-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Passerini, L., K. Lam, J. W. Costerton, and E. G. King. 1992. Biofilms on indwelling vascular catheters. Crit. Care Med. 20:665-673. [DOI] [PubMed] [Google Scholar]
- 15.Ray, S., R. Stacey, M. Imrie, and J. Filshie. 1996. A review of 560 Hickman catheter insertions. Anaesthesia 51:189-190. [DOI] [PubMed] [Google Scholar]
- 16.Schierholz, J. M., J. Beuth, and G. Pulverer. 1998. Killing effects of antibiotics and two-fold antimicrobial combinations on proliferating and non growing staphylococci. Zentbl. Bakteriol. 288:527-539. [DOI] [PubMed] [Google Scholar]
- 17.Stewart, P. S. 1996. Theoretical aspects of antibiotic diffusion into microbial biofilms. Antimicrob. Agents Chemother. 40:2517-2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stewart, P. S., and J. W. Costerton. 2001. Antibiotic resistance of bacteria in biofilms. Lancet 358:135-138. [DOI] [PubMed] [Google Scholar]
- 19.Svensson, E., H. Hanberger, M. Nilsson, and L. Nilsson. 1997. Factors affecting development of rifampicin resistance in biofilm-producing Staphylococcus epidermidis. J. Antimicrob. Chemother. 39:817-820. [DOI] [PubMed] [Google Scholar]
- 20.Tenney, J. H., M. R. Moody, K. A. Newman, S. C. Schimpff, J. C. Wade, J. W. Costerton, and W. P. Reed. 1986. Adherent microorganisms on lumenal surfaces of long-term intravenous catheters. Importance of Staphylococcus epidermidis in patients with cancer. Arch. Intern. Med. 146:1949-1954. [PubMed] [Google Scholar]
- 21.Xu, K. D., G. A. McFeters, and P. S. Stewart. 2000. Biofilm resistance to antimicrobial agents. Microbiology 146:547-549. [DOI] [PubMed] [Google Scholar]
- 22.Yasuda, H., Y. Ajiki, T. Koga, and T. Yokota. 1994. Interaction between clarithromycin and biofilms formed by Staphylococcus epidermidis. Antimicrob. Agents Chemother. 38:138-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zimmerli, W., R. Frei, A. F. Widmer, and Z. Rajacic. 1994. Microbiological tests to predict treatment outcome in experimental device-related infections due to Staphylococcus aureus. J. Antimicrob. Chemother. 33:959-967. [DOI] [PubMed] [Google Scholar]