Abstract
An international interlaboratory quality control program for measurement of antiretroviral drugs was initiated. The first round was confined to protease inhibitors and showed large variability in the performance of participating laboratories. The results demonstrate the need for and utility of an ongoing quality control program in this area of bioanalysis.
There has been increasing interest in bioanalysis of protease inhibitors (PIs) and nonnucleoside reverse transcriptase inhibitors (NNRTIs) since the advent of these drugs for treatment of human immunodeficiency virus infection. Numerous analytical methods have been published, describing the quantitation of PIs and NNRTIs in human plasma and other body fluids (1). These methods have been used to study the pharmacokinetics and interactions of these drugs (1, 4). Furthermore, it has been suggested that analysis and interpretation of plasma levels can be applied to individualize drug dosage of antiretrovirals, especially PIs (2, 3). Randomized clinical trials have been started to determine the value of therapeutic drug monitoring (TDM) for these drugs (P. Clevenbergh et al., Abstr. 8th Conf. Retrovir. Opport. Infect., abstr. 260B, 2001; D. M. Burger et al., Abstr. 2nd Int. Workshop Clin. Pharmacol. HIV Ther., abstr. 6.2, 2001). Anticipating the final results of these trials, many laboratories already offer a TDM service for antiretroviral drugs.
The wide application of analytical methods for antiretroviral drugs requires quality control (QC) procedures to ensure that these methods have sufficient accuracy, precision, and specificity. Such procedures usually include intralaboratory (internal) method validation, intralaboratory QC procedures, and participation in an interlaboratory (external) QC program. So far there has been no interlaboratory QC program for antiretroviral drugs. Therefore, such a program was initiated in order to enable laboratories to assess and improve their performance with respect to measurement of these drugs (6, 7).
The first round of the program was confined to measurement of PIs. First, QC samples were prepared by spiking drug-free plasma from human immunodeficiency virus-negative volunteers with indinavir, nelfinavir, ritonavir, and saquinavir. All PIs were obtained from pharmaceutical industries and had a very high (>99%) and specified purity. Drug-free human plasma was obtained from the regional blood bank.
PIs were weighed out on an independently calibrated balance. They were dissolved in methanol and diluted with blank plasma to obtain three different QC samples. Each of the three samples contained all four PIs in variable concentrations. For every PI, there was a sample with a low concentration, a sample with an intermediate concentration, and a sample with a high concentration (Table 1).
TABLE 1.
Concentrations in QC samples (expressed as free base and in milligrams per liter)
| Drug | Low concn | Intermediate concn | High concn |
|---|---|---|---|
| Indinavir | 0.15 | 1.98 | 8.49 |
| Nelfinavir | 0.20 | 2.86 | 8.00 |
| Ritonavir | 0.20 | 2.87 | 11.04 |
| Saquinavir | 0.087 | 2.06 | 4.80 |
The QC samples were dispensed in polypropylene vials that were kept at −20°C. Stability under these and other conditions had been assessed before (5).
All weighed-in concentrations were considered true values. Three vials of every QC sample were analyzed with our own validated high-performance liquid chromatography (HPLC) method (5) as a confirmative check (<5% deviation from true concentrations) before the samples were released for the QC program.
Nine laboratories from six Western European countries and one North American country participated in the first round of the program. They were asked to analyze the samples and to return their results (with concentrations expressed as free base) within 6 weeks after dispatch.
Descriptive statistics were calculated after standardization of all laboratory results to percentages with reference to the true value. By subtraction of 100% from these percentages, the percentage bias from the true concentration (inaccuracy) was calculated. Twenty percent limits around the true values were considered to be appropriate threshold values for satisfactory measurements.
Multifactorial analysis of variance was used to evaluate the simultaneous effect of two factors, the PI to be measured and the concentration level, on the absolute inaccuracy. Results for different concentration levels of the same PI were considered to be related to each other, and the concentration level was therefore included as a within-subjects (repeated-measure) factor. The PI to be analyzed was a between-subjects factor.
All participants were informed about their performance within 2 months after reporting of their results. All results were interpreted briefly in words.
Five of the nine participating laboratories were able to measure all four PIs. Three laboratories were not able to determine nelfinavir. One laboratory measured indinavir only. All laboratories measuring more than one PI used an assay for simultaneous determination of PIs.
Six laboratories used HPLC with UV detection to quantify the PIs and three laboratories used liquid chromatography with mass (or tandem mass) spectrometry detection (LC-MS or LC-MS/MS). Because of the small number of participants in this first round of the program, no valid comparison could be performed between the HPLC-UV and LC-MS/MS methods.
Two laboratories reported being unable to measure some low concentrations of PIs with sufficient accuracy, since these concentrations were below the lower limits of quantitation of their methods. Results for these measurements were not included in the analyses.
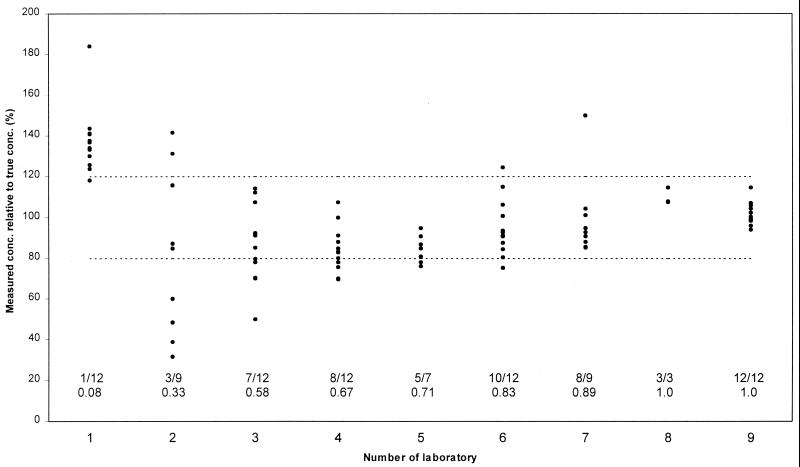
Table 2 and Fig. 1 summarize the results for the participating laboratories. Four laboratories used analytical methods that appeared to have a large systematic error in one direction, as all measured concentrations of at least one PI were either above or below the assigned 20% threshold for acceptable measurements.
TABLE 2.
Measurements of QC samples, subdivided by drug and concentration levela
| Drug | No. of measurements | Concn level | Measured concn relative to true value (%) (median [min-max]) | Absolute inaccuracy (%)b (median [min-max]) | No. and % of measurements with acceptable accuracyc | |
|---|---|---|---|---|---|---|
| Indinavir | 7 | Low | 107 (96-142) | 7 (0.7-42) | 5/7 | 80% |
| 9 | Intermediate | 106 (85-133) | 12 (4-33) | 7/9 | ||
| 9 | High | 106 (91-124) | 9 (0.2-24) | 8/9 | ||
| Nelfinavir | 6 | Low | 94 (50-150) | 28 (7-50) | 2/6 | 67% |
| 6 | Intermediate | 86 (70-126) | 18 (12-30) | 4/6 | ||
| 6 | High | 92 (83-118) | 12 (6-18) | 6/6 | ||
| Ritonavir | 6 | Low | 84 (48-184) | 30 (1-84) | 1/6 | 36% |
| 8 | Intermediate | 88 (32-134) | 18 (2-68) | 4/8 | ||
| 8 | High | 85 (39-144) | 22 (5-61) | 3/8 | ||
| Saquinavir | 7 | Low | 100 (60-138) | 15 (0-40) | 5/7 | 74% |
| 8 | Intermediate | 86 (69-137) | 15 (2-37) | 6/8 | ||
| 8 | High | 89 (75-142) | 13 (4-42) | 6/8 | ||
Abbreviations: min, minimum value; max, maximum value.
Inaccuracy is percentage bias from the true concentration, i.e., inaccuracy = (100 × measured concentration/true concentration) − 100%
Acceptable measurements are within 20% limits from the true concentration.
FIG. 1.
Performance of individual laboratories. Diagram shows the results for all measurements (all four PIs and three concentration [conc.] levels combined), arranged by laboratory. Results for individual measurements are depicted by points (note that some points are superimposed). Accuracy (y axis) is expressed as percentage relative to the true concentration (100%). The dotted lines represent the thresholds for measurements with acceptable accuracy (80 to 120%). Proportions of measurements with acceptable accuracy are placed on the x axis, above the number of each laboratory.
Mean absolute inaccuracies for measurement of the four PIs (in percentage deviation from true values) were not significantly different: F(3,22) = 1.40, P = 0.27. This may be due to the small number of laboratories and measurements in this first round of the program and/or the large variability in results. However, conversion of the results to a dichotomous scale (acceptable accuracy or not) suggested a much worse performance for ritonavir (Table 2, last column). This could be attributed to a relatively large number of ritonavir measurements with an inaccuracy of just more than 20%.
A significant effect due to the concentration level to be analyzed was assessed: F(2,44) = 5.04, P = 0.01. Mean inaccuracy over all PIs was 25.3% for low concentrations and decreased to 17.1% for high concentrations. The PI-by-concentration level interaction was not statistically significant.
The initial results of this program show large variability in the performance of laboratories with regard to measurement of PIs in plasma. The magnitude of observed inaccuracies may have important implications for the interpretation of pharmacokinetic studies and may lead to inappropriate dose adjustments in TDM or to advice not to adjust doses where adjustment might actually be desirable. For example, if it was assumed that the small number of participants represented all laboratories for PI analysis and that 20% deviations from true concentrations could negatively affect patient management, then a physician would have 35% probability to receive such an incorrect result after submission of a random sample to a random laboratory. If 50% inaccuracy would be considered relevant within the context of TDM, the probability of receiving such a result would still be 6%.
Fortunately, by participating in the program, laboratories were alerted to possible inaccuracies and to previously undetected problems, such as systematic errors and high limits of quantitation that restrict the applicability of analytical methods. Such information may enable and encourage them to optimize their methods and intralaboratory QC procedures; this would confirm the role of interlaboratory QC testing as a means to achieve improvement in laboratory performance (6, 7).
Accordingly, the first round of this program highlights both the need for and utility of an ongoing QC program. The program will be extended to measurement of more PIs (amprenavir and lopinavir) and to analysis of NNRTIs (efavirenz and nevirapine) and will be open for more laboratories to participate.
Acknowledgments
We are indebted to Merck Sharp & Dohme, Agouron, Hoffmann-La Roche, and Abbott for the supply of indinavir, nelfinavir, saquinavir, and ritonavir, respectively.
REFERENCES
- 1.Aarnoutse, R. E., C. P. W. G. M. Verweij-van Wissen, W. J. M. Underberg, J. Kleinnijenhuis, Y. A. Hekster, and D. M. Burger. 2001. High-performance liquid chromatography of HIV protease inhibitors in human biological matrices. J. Chromatogr. B 764:363-384. [DOI] [PubMed] [Google Scholar]
- 2.Acosta, E. P., T. N. Kakuda, R. C. Brundage, P. L. Anderson, and C. V. Fletcher. 2000. Pharmacodynamics of human immunodeficiency virus type 1 protease inhibitors. Clin. Infect. Dis. 30:S151-S159. [DOI] [PubMed] [Google Scholar]
- 3.Back, D. J., S. H. Khoo, S. E. Gibbons, M. G. Barry, and C. Merry. 2000. Therapeutic drug monitoring of antiretrovirals in human immunodeficiency virus infection. Ther. Drug. Monit. 22:122-126. [DOI] [PubMed] [Google Scholar]
- 4.Barry, M. G., F. Mulcahy, C. Merry, S. Gibbons, and D. J. Back. 1999. Pharmacokinetics and potential interactions amongst anti-retroviral agents used to treat patients with HIV infection. Clin. Pharmacokinet. 36:289-304. [DOI] [PubMed] [Google Scholar]
- 5.Hugen, P. W. H., C. P. W. G. M. Verweij-van Wissen, D. M. Burger, E. W. Wuis, P. P. Koopmans, and Y. A. Hekster. 1999. Simultaneous determination of the HIV-protease inhibitors indinavir, nelfinavir, saquinavir and ritonavir in human plasma by reversed-phase high-performance liquid chromatography. J. Chromatogr. B 727:139-149. [DOI] [PubMed] [Google Scholar]
- 6.Laessig, R. H., and S. S. Ehrmeyer. 1988. Proficiency testing programs--promises, progress, and problems. A 40-year prospective. Arch. Pathol. Lab. Med. 112:329-333. [PubMed] [Google Scholar]
- 7.Shahangian, S. 1998. Proficiency testing in laboratory medicine: uses and limitations. Arch. Pathol. Lab. Med. 122:15-30. [PubMed] [Google Scholar]