Abstract
We recently described a screening system designed to detect neurotoxicity of artemisinin derivatives based on primary neuronal brain stem cell cultures (G. Schmuck and R. K. Haynes, Neurotoxicity Res. 2:37-49, 2000). Here, we probe possible mechanisms of this brain stem-specific neurodegeneration, in which artemisinin-sensitive neuronal brain stem cell cultures are compared with nonsensitive cultures (cortical neurons, astrocytes). Effects on the cytoskeleton of brain stem cell cultures, but not that of cortical cell cultures, were visible after 7 days. However, after a recovery period of 7 days, this effect also became visible in cortical cells and more severe in brain stem cell cultures. Neurodegeneration appears to be induced by effects on intracellular targets such as the cytoskeleton, modulation of the energy status by mitochondrial or metabolic defects, oxidative stress or excitotoxic events. Artemisinin reduces intracellular ATP levels and the potential of the inner mitochondrial membrane below the cytotoxic concentration range in all three cell cultures, with these effects being most dominant in the brain stem cultures. Surprisingly, there were substantial effects on cortical neurons after 7 days and on astrocytes after 1 day. Artemisinin additionally induces oxidative stress, as observed as an increase of reactive oxygen species and of lipid peroxidation in both neuronal cell types. Interestingly, an induction of expression of AOE was only seen in astrocytes. Here, manganese superoxide dismutase (MnSOD) expression was increased more than 3-fold and catalase expression was increased more than 1.5-fold. In brain stem neurons, MnSOD expression was dose dependently decreased. Copper-zinc superoxide dismutase and glutathione peroxidase, two other antioxidant enzymes that were investigated, did not show any changes in their mRNA expression in all three cell types after exposure to artemisinin.
Chemically induced neurodegeneration is characterized by different patterns of neuronal cell death, gliosis, swollen or destroyed axons, or destruction of the myelin sheet. Effects on biochemical targets possibly precede manifestation of these morphologic endpoints. Therefore, not only cell viability but also integrity of the cytoskeleton and neuronal energy state should be considered (6). Primary neuronal cell cultures derived from the embryonal rat cortex have been used as a model for neuron-specific toxicity of various neurotoxicants, whose neurotoxic mode of action has been well characterized (36). The neurotoxicants include chemicals that primarily target the cytoskeleton, such as the organophosphate mipafox and β,β-iminodipropionitrile, and substances that primarily impair the cellular energy supply, such as potassium cyanide and 3-nitropropionic acid. Also included were compounds such as acrylamide and 2,5-hexanedione, which act on both cytoskeleton and cellular energy production, and the well-known excitotoxin N-methyl-d-aspartate, which indirectly affects mitochondria.
Artemisinin, isolated from the traditional Chinese herb Artemisia annua, is a representative of a new class of antimalarial drugs (22). However, studies with dogs and rats indicated that these compounds have considerable neurotoxic potency (4, 5, 33). Neurological findings included gait disturbance, loss of spinal and pain response reflexes, and prominent loss of brain stem and eye reflexes. Prominent neuropathic lesions were restricted primarily to the pons and medulla. In vivo toxicity studies with rats and monkeys also revealed neuropathies in the caudal brain stem (13, 14, 29, 30).
In humans treated with the artemisinin drugs, adverse affects directly ascribed to neurotoxicity have yet to be unambiguously demonstrated but may possibly be due to neurodegeneration (3.3% of the cases) (15, 25, 31, 32, 44).
Recently, we described a new in vitro screening method using primary brain stem cell cultures from the rat (37). We demonstrated that the brain stem cells were selectively sensitive against artemisinin, in contrast to cells from other brain regions, such as the cortex. Endpoints in this in vitro assay were cytotoxicity measured by the viability assay (live/dead assay) and a neuronal cytoskeleton marker, the 200-kDa neurofilaments. Specifically, artemisinin diminished the amount of nonphosphorylated neurofilaments below the cytotoxic concentration range, therefore indicating that the cytoskeleton may be the target associated with this specific neurodegeneration in the brain stem. Comparable effects were shown with three other derivatives, dihydroartemisinin, artemether, and artesunate. The ranking of these four compounds in vitro was comparable to that in the in vivo situation (4, 5).
The peroxide bridge in the artemisinin molecule may induce oxidative stress. Tanaka and coworkers (41, 42) showed that reaction of the endoperoxide bridge with intraparasitic heme irons generates O and C radicals.
To prevent injury from reactive oxygen species (ROS), cells have developed defense systems. Beside scavenger molecules such as glutathione or α-tocopherol, specific enzymes, the antioxidant enzymes (AOE), fulfill this task. Included among the AOE are catalase, glutathione peroxidases (GPxs), copper-zinc superoxide dismutase (CuZnSOD) and manganese superoxide dismutase (MnSOD). Catalase and GPx convert H2O2 to H2O, and the superoxide dismutases (SODs) catalyze the dismutation of the superoxide radical anion. The expression of AOE can be regulated by oxidative stress itself (32, 37, 39, 43), which could also be shown for, among others, astroglial cell cultures (34). It has been demonstrated that primary neuronal cell culture systems are suitable for detection of oxidative stress evoked by paraquat using specific endpoints such as ROS production, lipid peroxidation, and AOE expression (38).
In this study, we compared artemisinin with known neurotoxins in a well-characterized in vitro model by using the energy supply, oxidative stress, and cytoskeleton as sensitive targets to identify possible modes of action.
MATERIALS AND METHODS
Culture of primary cells.
The cell culture technique used has been described in detail elsewhere (36, 37). Briefly, the cortex and brain stem were dissected from the whole brain tissue of fetal rats in the developmental stage E18-E19. The tissues were pooled in sterile cultivation medium (Opti-MEM; GIBCO-BRL, Eggenstein, Germany) containing 10 ml of B27 (Gibco) and 625 μl of a protein solution (in 2.5 ml of sterile distilled water; Seromed, Berlin, Germany). The brain stem was digested with collagenase A (150 mg/50 ml of medium; Boehringer, Ingelheim, Germany) for 30 min. Isolation of individual cells from cortex and brain stem tissues was performed by filtration of the neuronal cells through two nylon meshes with different pore diameters (135 and 22 μm). The single-cell suspension was centrifuged (500 to 700 × g), washed twice with culture medium, and cultivated in 24-well laminin-coated cell culture plates (Biocoat; Becton Dickinson, Heidelberg, Germany).
Astrocytes were isolated from the whole brain by filtration through two meshes with pore diameters of 211 and 125 μm. Cells were cultivated in Dulbecco modified Eagle medium supplemented with 10% fetal calf serum (Gibco). As neuronal cell cultures generate a permanent neuronal network within 10 days, artemisinin (Sigma, Deisenhofen, Germany) treatment was started at day 11 and was completed at day 12 or 18, respectively. After 7 days of treatment, a recovery period of 7 days was added in some cases. Astrocytes mature within 2 weeks with regard to AOE expression, and the experiments were therefore done after this period (37). The cell cultures were treated with artemisinin at 0.001 to 20 μg/ml dissolved in dimethyl sulfoxide (DMSO; stock solution, 10 mg/ml). DMSO controls were run in parallel with a nontreated control; a maximum of 0.2% DMSO had no effect on the cell cultures (data not shown). Each experiment was repeated two times with three different cell preparations.
Test compounds.
Artemisinin was a gift from R. K. Haynes (University of Hong Kong, Hong Kong, China) and has a purity of 99%. Paraquat (methylviologen) was purchased from Sigma and had a purity of 98%, and Mipafox was a gift from A. Freyberger (Bayer AG) and had a purity of 97%.
Viability assay (live/dead assay).
Cells were washed twice with phosphate-buffered saline (PBS) and then incubated in a Calcein-AM-PBS solution (1:2; Molecular Probes, Eugene, Oreg.) for 30 min in a cell incubator. Fluorescence was determined with a Fluostar spectrophotometer (SLT, Crailsheim, Germany) at excitation and emission wavelengths of 485 and 530 nm, respectively.
Enzyme-linked immunosorbent assay.
The cell culture plates were fixed in cold (4°C) methanol for 10 min and subsequently incubated for 1 h in a 0.1% human albumin-PBS solution (Sigma). The cells were then treated with detergent (0.3% Triton X-100 in PBS; Sigma) for 10 min and then washed two times with PBS (plus 0.3% gelatin; Sigma). The first antibody (nonphosphorylated mouse neurofilament [200 kDa] diluted 1:100; Roche, Ingelheim, Germany) was added, and the mixture was incubated for 2 h. After the second antibody (anti-mouse [1:500]; Roche) was added, the mixture was incubated for 1 h at 4°C. After removal of antibodies 1 and 2, the plates were washed three times with PBS (plus 0.3% gelatin). The attached two antibodies were coupled to horseradish peroxidase and quantified by a peroxidase substrate, the 2,2"-azinobis(3-ethylbenzthiazolinesulfonic acid) (ABTS) solution (Boehringer), for 30 min. The quantification of the attached antibodies was conducted at 405 nm in an enzyme-linked immunosorbent assay reader.
Mitochondrial membrane potential.
The membrane potential of the inner mitochondrial membrane was measured by the dye tetramethylrhodamine. The dye was added to the cell culture medium in a concentration of 3.3 μM, and the mixture was incubated for 30 min. The cells were washed twice, and the fluorescence was determined with a Fluorostar spectrophotometer (SLT) at excitation and emission wavelengths of 540 and 575 nm, respectively.
Intracellular ATP concentration.
The intracellular ATP concentration was determined according to a chemiluminescence reaction in a luciferin-luciferase system (ATP Lite-M; Packard, Groningen, The Netherlands). The procedure is described in detail in the kit manual.
ROS.
The cells were washed with PBS and loaded with 2,7-dichlorofluorescein diacetate (Sigma) at a concentration of 50 μM in PBS for 1 h. Afterwards, the dye was removed and the cells were treated with artemisinin at the following concentrations: 0.001, 0.01, 0.1, 1, and 10 μg/ml in PBS. 2,7-Dichlorofluorescein diacetate and ROS produced a fluorescein complex that was determined for up to 4 h (0.5, 1, 2, 3, and 4 h) in a Fluorostar spectrophotometer (SLT) at excitation and emission wavelengths of 485 and 530 nm, respectively.
Lipid peroxidation.
Intracellular production of 4-HNE (4-hydroxynonenal) was determined with the Lipid Peroxidation Assay Kit (Calbiochem, San Diego, Calif.) in accordance with the manufacturer's protocol. Results for 4-HNE are shown as percentages of the control value.
RNA isolation and analysis.
Total RNA was isolated from cells with Trizol reagent (GIBCO BRL). Five micrograms of total RNA was resolved by electrophoresis in a 1% agarose-2.25 mM formaldehyde gel in a running buffer containing 20 mM MOPS (morpholinepropanesulfonic acid, pH 7), 0.5 mM sodium acetate, and 1 mM EDTA. RNA was transferred to nylon membrane (Amersham, Arlington Heights, Ill.) as described by Maniatis et al. (26). Purified cDNAs were labeled with [α-32P]dCTP (111 TBq/mmol; Hartmann Analytic, Braunschweig, Germany) by random hexamer priming (Roche Diagnostics GmbH, Mannheim, Germany). Blots were prehybridized and hybridized with cDNAs for catalase, MnSOD, and CuZnSOD as described previously (32). Autoradiographs were made by exposing blots to X-ray film (Kodak XAR) with an intensifying screen at −80°C. Blots were stripped and reprobed with different cDNAs. Autoradiographs were analyzed by densitometric scanning using the Quantity One system from Bio-Rad, Munich, Germany.
Shuichi Furuta (Department of Biochemistry, Sinshu University, Nagano, Japan) provided the rat catalase cDNA containing plasmid, PMJ1010 (12). A 1.4-kb rat MnSOD cDNA containing plasmid pSP65-RMS (16) and a 0.6-kb rat CuZnSOD cDNA containing plasmid pUC-13-RCS (17) were obtained from Ye-Shih Ho (Institute of Chemical Toxicology, Wayne State University, Detroit, Mich.). For 18S rRNA detection, the oligonucleotide probe 5"-GCC GTG CGT ACT TAG ACA TGC ATG-3" (8) was used. PCR following reverse transcription was performed for semiquantitative determination of GPx mRNA. One microgram of total RNA was transcribed into cDNA in a 25-μl final volume of reaction buffer (50 mM Tris-HCl, 75 mM KCl, 3 mM MgCl2, 10 mM dithiothreitol, 0.5 mM each deoxynucleoside triphosphate) and 5 μM oligo(dT)16-primer, 1 U of RNase inhibitor, and 2.5 U of murine leukemia virus reverse transcriptase by incubation for 1 h at 42°C. The reaction was stopped by incubation at 99°C for 5 min. For rat GPx PCR, 100 ng of the synthesized cDNA was added to a 25-μl PCR mixture containing 10 mM Tris-HCl, 50 mM KCl, 0.1% Triton X-100, 2 mM MgCl2, 0.2 mM each deoxynucleoside triphosphate, 1 U of Taq DNA polymerase (Promega, Mannheim, Germany), and 0.2 μM each PCR primer. The primers were designed for detection of the rat GPx in accordance with the genomic sequence published by Ho et al. (18). The upstream primer was 5"-ATGTCTGCTGCTCGGCTCTC-3", and the downstream primer was 5"GTTGCTAGGCTGCTTGG ACAG-3". Amplification was initiated with 4 min of denaturation at 94°C, followed by 27 cycles of 94°C, 61°C, and 72°C for 60 s. Glyceraldehyde-3-phosphate dehydrogenase PCR was performed with 100 ng of synthesized cDNA as described elsewhere (10). The amplified PCR products were 602 bp for GPx mRNA and 450 bp for glyceraldehyde-3-phosphate dehydrogenase mRNA. The PCR conditions were set in the linear phase of amplification to allow semiquantification of mRNA content. Five microliters of each PCR product was submitted to electrophoresis in a 1% agarose gel in Tris-borate-EDTA buffer. The cDNA bands were visualized by UV illumination after staining of the gels with ethidium bromide. Gels were photographed and scanned densitometrically.
Statistics.
Statistical analysis was performed with Student's t test (Sigma Stat; Scientific, Erkrath, Germany) to analyze treated cells in relation to control cells. To analyze differences between different cell cultures, the one-way analysis of variance, followed by a Dunett test (Sigma Stat; Scientific), was performed.
RESULTS
Neurofilaments.
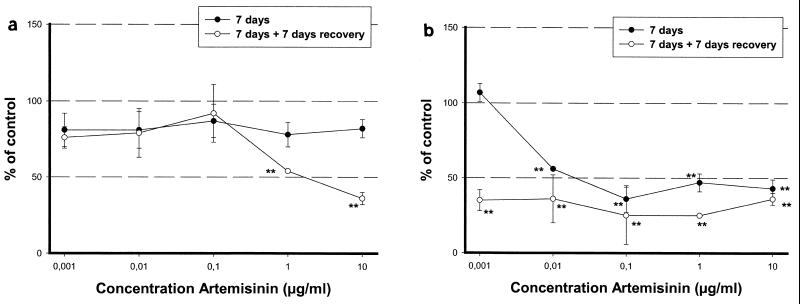
Artemisinin had no effect on the cytoskeleton (neurofilaments) in cortical neurons after 7 days of treatment. However, effects on neurofilaments became obvious during the recovery phase, probably secondary to the energy depletion. The no-effect concentration (NOEC) was 0.1 μg/ml, and the 50% effective concentration (EC50) of artemisinin was 3 μg/ml, (Fig. 1a). In the brain stem cultures, however, this effect was much more severe. At 7 days, the degradation of the neurofilaments had a NOEC of 0.001 μg/ml and artemisinin had an EC50 of 0.04 μg/ml. After the recovery period, no NOEC or EC50 could be determined (Fig. 1b).
FIG. 1.
The influence of artemisinin on the cytoskeleton was determined with neurofilament antibodies in cortical neuronal cell cultures (a) and brain stem cell cultures (b). The cells were treated for 7 days. Half of them were allowed to grow for another week in compound-free medium (recovery). Statistical analysis was done with Student's t test. ∗∗, P ≤ 0.01.
Short-term exposure.
Astrocyte, brain stem, and cortical neuronal cell cultures were exposed to artemisinin for 24 h. Afterwards, cytotoxicity, mitochondrial parameters, lipid peroxidation, and the AOE expression were measured (Table 1). For ROS determination, an exposure of 30 min was sufficient. The organophosphate mipafox and the herbicide paraquat served as control compounds. It was shown in cortical neurons that mipafox had no effect on mitochondrial functions, in contrast to paraquat (36, 38), and this was also found in brain stem cultures (Table 1).
TABLE 1.
Determination of the NOECs and EC50s of artemisinin, paraquat, and mipafox in brain stem neuronal cell cultures and artemisinin in cortical cell and astrocyte cultures after 24 h of treatmenta
| Criterion | Brain stem
|
Cortex, artemisinin
|
Astrocytes artemisinin
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Artemisinin
|
Paraquat
|
Mipafox
|
|||||||||
| NOEC | EC50 | NOEC | EC50 | NOEC | EC50 | NOEC | EC50 | NOEC | EC50 | ||
| Viability assay | 10 | >10 | 2 | 10 | 10 | >10 | 10 | >10 | 10 | >10 | |
| Tetramethylrhodamine | 0.1 | 5 | 5 | 9 | 10 | >10 | 1 | >10 | 0.1 | >10 | |
| ATP | <0.001 | 1 | 0.1 | 2 | 10 | >10 | 1 | >10 | 1 | >10 | |
Endpoints were cytotoxicity (viability assay), the inner mitochondrial membrane potential (tetramethylrhodamine), and intracellular glucose consumption. All values are in micrograms per milliliter.
Artemisinin was not cytotoxic after 24 h at up to 10 μg/ml (Table 1). However, mitochondrial functions such as the mitochondrial membrane potential and the intracellular ATP content were inhibited in all three cell cultures by artemisinin. Cortical neurons were less sensitive, with an artemisinin NOEC of 1 μg/ml, than brain stem cells, with a NOEC of 0.1 μg/ml for the inner mitochondrial membrane potential and <0.001 μg/ml for the intracellular ATP level (Table 1). In astrocytes, artemisinin had a higher potential on the inner mitochondrial membrane potential (NOEC of 0.1 μM) than on the intracellular ATP concentration (1 μM) (Table 1).
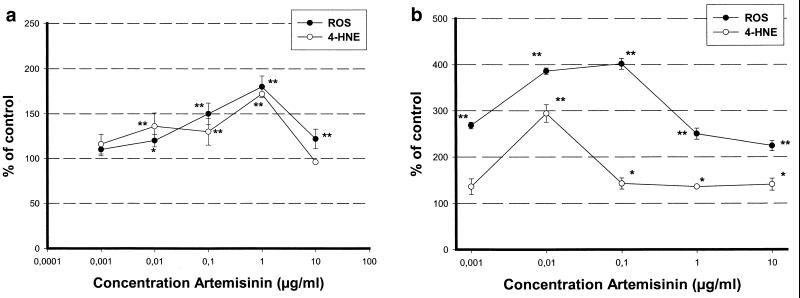
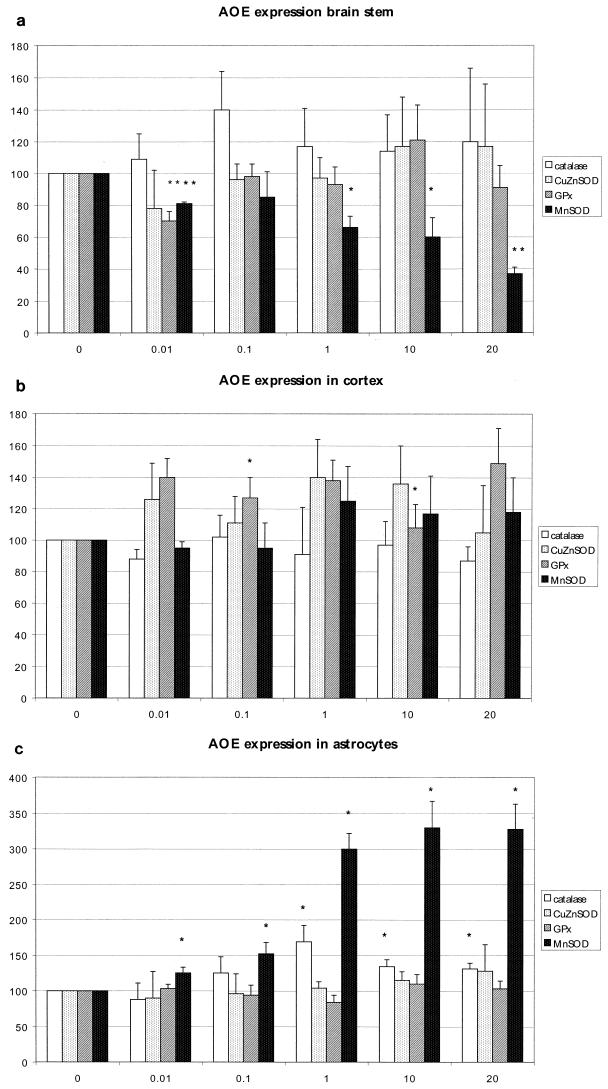
Artemisinin produced ROS very quickly (within 30 min) in cortical and brain stem neurons. However, the total amount of ROS was 400% at 0.01 μg/ml in the brain stem cultures but only 170% at 1 μg/ml in the cortical neurons (Fig. 2a and b). However, artemisinin induced no ROS in astrocytes (data not shown). Lipid peroxidation was determined by an intracellular assay of 4-HNE. Artemisinin induced lipid peroxidation in parallel with ROS production and was more dominant in brain stem cultures than in cortical cultures. (Fig. 2a and b). In astrocytes also, no lipid peroxidation could be found. The expression of AOE CuZnSOD, MnSOD, catalase, and GPx was determined by Northern blotting or PCR. Cortical neurons showed no significant dose-dependent increase in these enzymes at all (Fig. 3b), whereas the brain stem neurons showed a dose-dependent reduction of mitochondrial MnSOD (Fig. 3a). Interestingly, astrocytes, which are less sensitive to artemisinin than are brain stem neuronal cell cultures with respect to viability and oxidative stress, showed a threefold increase in MnSOD and a slight (1.5-fold) increase in catalase (Fig. 3c). GPx and CuZnSOD expression was unchanged in all cell cultures (Fig. 3a to c).
FIG. 2.
Measurement of ROS and 4-HNE production in cortical neuronal cell cultures (a) and brain stem cell cultures (b). ROS production was determined after 30 min of treatment, and 4-HNE production was determined after 24 h of treatment. Statistical analysis was done with Student's t test. ∗, P ≤ 0.05; ∗∗, P ≤ 0.01.
FIG. 3.
Determination of the expression of AOE catalase, CuZnSOD, MnSOD, and GPx in brain stem neuronal cell cultures (a), cortical cell cultures (b), and astrocyte cell cultures (c). The values are percentages of the control value. Statistical analysis was done with Student's t test. ∗, P ≤ 0.05; ∗∗, P ≤ 0.01.
Long-term exposure.
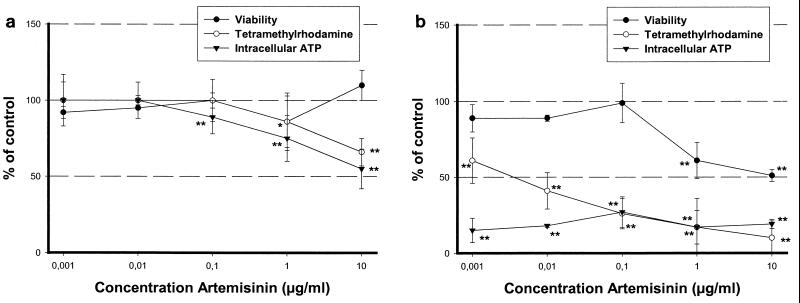
In contrast to the results obtained after the short-term exposure to artemisinin, a high cytotoxic potential was seen in primary brain stem cultures with a NOEC of 0.1 μg/ml and an EC50 of 10 μg/ml after 7 days (Fig. 4b). After 7 and 14 days, artemisinin was still not cytotoxic to cortical cells, in contrast to brain stem cells (Fig. 4a).
FIG. 4.
Determination of the cytotoxicity (viability assay) and mitochondrial functions (tetramethylrhodamine and intracellular ATP content) in cortical neuronal cell cultures (a) or in brain stem cell cultures (b) after 7 days of treatment with artemisinin. Statistical analysis was done with Student's t test. ∗, P ≤ 0.05; ∗∗, P ≤ 0.01.
After 7 days of treatment, the inner mitochondrial membrane potential was more inhibited in cortical neurons than after 1 day, with an artemisinin NOEC of 0.1 μg/ml. The brain stem cultures also increased their sensitivity with an artemisinin NOEC of <0.001 μg/ml and an EC50 of 0.006 μg/ml, respectively. The ATP level was reduced to such an extent that it was almost undetectable in brain stem cell cultures, even at the lowest artemisinin concentration used (0.001 μg/ml). In cortical neurons, the level of intracellular ATP closely correlated with the reduction of the membrane potential.
DISCUSSION
The investigations described here were performed in order to identify the basis for brain stem neurodegeneration induced by artemisinin. Differentiated cell cultures build up a complicated network of neurites, which make them more sensitive to neurodegenerative effects than undifferentiated ones or permanent cell lines. This has been shown for organophosphates and other neurotoxins like acrylamide, n-hexane, 3-nitropropionic acid, β,β-iminodipropionitrile, and N-methyl-d-aspartate (35, 36). Various mechanisms at the molecular level have been discussed as the basis of toxic neuropathies (6). These include depletion of cellular energy or, more generally, disruption of the balance between cellular energy supply and demand (9) by the toxic chemical, oxidative stress, deficiency of antioxidants (3), and alterations in the cytoskeleton of neuronal cells (1, 3, 36, 37). The primary effects, either alone or in combination, in acting on these targets may disturb the fragile homeostasis of the neuronal cell, thus leading to destruction of the long axons to yield the histopathological picture of degenerative axonopathy.
The underlying mechanism of action of artemisinin is not understood. The neurofilaments were used as a mechanistically relevant endpoint for neurodegeneration. Neurofilaments may be involved directly by degradation or cross-linking (19) or influenced indirectly by other mechanisms like energy deficit or apoptotic processes (2). However, it was demonstrated by Fishwick et al. (11) that dihydroartemisinin did not affect the neurofilaments directly. A reduction of the neurites and a parallel degradation of the neurofilaments may have occurred by other mechanisms, such as inhibition of respiratory functions or functions of the endoplasmic reticulum. McLean and Ward (27) reported that dihydroartemisinin binds to mitochondrial and endoplasmic membranes in differentiated neuronal cell cultures. It is known that inhibitors of the respiratory chain such as KCN induce delayed Parkinsonism and a basal ganglion disease that is associated with locomotor impairment (9, 20, 23). In vitro, this neurodegenerative effect is demonstrated by a decrease in the neurofilaments (35).
Experiments with primary neuronal cortical cell cultures and astrocytes from the rat brain showed an insensitivity to artemisinin with regard to cytotoxicity and the cytoskeleton for up to 7 days (37). In the brain stem cultures, the situation was completely different. Cell viability was markedly reduced after 7 days, and the cytoskeleton was profoundly affected. Effects on the cytoskeleton increased during the recovery period in brain stem neurons, which indicated an irreversible effect. Interestingly, during the recovery period, cortical neuronal cells also developed a sensitivity to artemisinin that was documented by a degradation of the cytoskeleton although it was much less prominent. This delayed effect on the cytoskeleton is also demonstrated by KCN, paraquat, and 3-nitropropionic acid and points to a secondary effect through inhibition of respiratory function (36).
It has been demonstrated that artemisinin had an effect on the inner mitochondrial membrane potential in all three cell cultures after 24 h, although to different degrees. This effect is not accompanied by a decrease in the intracellular ATP level in astrocytes and cortical neurons at this time point. After 7 days of treatment with artemisinin, effects on mitochondrial functions became more prominent in cortical and brain stem neurons. In cortical neurons, no difference between intracellular ATP content and the reduction of the inner mitochondrial membrane potential was visible; however, in brain stem cell neurons, the reduction in the ATP level was the most sensitive parameter.
Apart from inhibition of the respiratory chain, oxidative stress is a possible mechanism of neurodegeneration. Paraquat is one model compound that induces ROS by decoupling of the respiratory chain (36, 38). Like paraquat, artemisinin produced ROS in brain stem (400% of the control at 0.01 μg/ml) and cortical (175% of the control at 1 μg/ml) cell cultures. However, the degree and the time point were different from those of paraquat (37). Paraquat generates a maximum ROS production of greater than 1,100% of the control value within 2 h in cortical neurons, whereas artemisinin reaches an ROS maximum within 30 min. This points to another mechanism of ROS induction by artemisinin.
Lipid peroxidation by artemisinin, as determined by formation of the degradation product 4-HNE after 24 h, occurs in parallel with ROS production. It was shown with paraquat that 4-HNE is the prominent degradation product in neuronal cells, whereas malondialdehyde is produced predominantly by nonneuronal cells (38).
Oxidative stress is often accompanied by an increase in the expression of AOE like SODs, catalase, or GPx (7). This was not found for cortical or brain stem neurons after 24 h of treatment with artemisinin. Surprisingly, in the brain stem neurons, levels of expression of MnSOD mRNA, which is located in the mitochondria, dropped severely; this was probably related to mitochondrial dysfunction induced by artemisinin. However, a prominent increase in the MnSOD level and a less distinct increase in the catalase mRNA level were seen in astrocytes. The involvement of mitochondria in artemisinin neurotoxicity is obvious in all cell cultures, but the increase in AOE like MnSOD and catalase may be the reason for the insensitivity of astrocytes, in contrast to neurons. This may also be the reason for the missing ROS production and lipid peroxidation.
In summary, neuronal cells and astrocytes showed two effects induced by artemisinin, namely, inhibition of mitochondrial functions and induction of oxidative stress. It appears that production of radicals is the toxic mechanism in Plasmodium parasites (41, 42) and is also one toxic mechanism for neuronal cells. The sensitivity to artemisinin, as demonstrated by cytotoxicity and cytoskeleton degradation, is related to the amount of oxidative stress. Astrocytes may be protected by their ability to induce antioxidative stress enzymes like MnSOD and catalase. The second effect, inhibition of the inner membrane potential, has also been described in parasites (24). Krungkrai and coworkers showed that the respiratory chain of Plasmodium parasites is similar to that of mammals with regard to classical mitochondrial inhibitors of complex I-IV. Artemisinin and primaquine inhibited the respiratory chain of the sexual and asexual stages of parasites. The mechanism of this inhibition is unclear, but it could be related to the iron-group in the cytochrome center. Iron appears to be necessary to activate artemisinin and related compounds in the parasite (21, 28, 40).
Consideration of all of the available data together makes it seem likely that the neurotoxic mechanism of artemisinin is related to the possibility of producing radicals and, in parallel, inhibition of the respiratory chain. Neuronal cells seemed to be generally more sensitive than nonneuronal cells because their antioxidative defense system is less active (38). Interference with the respiratory chain induces irreversible degeneration, as shown with other neurotoxins. The differences between the brain areas may have a kinetic origin; the cortical cells showed clear neurotoxic effects after a longer time period.
Acknowledgments
We thank S. Ohler for excellent technical assistance.
The Hong Kong Research Grants Council of the Hong Kong SAR and HKUST are thanked for financial support (grants HKUST 6184/99P and HKUST RIG.SC03 95/96). We thank Hans-Jürgen Rosenkranz and Jörg Stetter of Bayer Central Research, Bayer AG, Leverkusen, Germany, for financial support provided by Bayer AG, Leverkusen, and Bayer China to R.K.H.
REFERENCES
- 1.Abou-Donia, M. B. 1995. Involvement of cytoskeletal proteins in the mechanisms of organophosphorus ester-induced delayed neurotoxicity. Clin. Exp. Pharmacol. Physiol. 22:358-359. [DOI] [PubMed] [Google Scholar]
- 2.Bonfoco, E., M. Leist, B. Zhivotovsky, S. Orrenius, S. A. Lipton, and P. Nicotera. 1996. Cytoskeletal breakdown and apoptosis elicited by NO donors in cerebellar granule cells require NMDA receptor activation. J. Biochem. 67:2484-2493. [DOI] [PubMed] [Google Scholar]
- 3.Bowling, A. C., and M. F. Beal. 1995. Bioenergetic and oxidative stress in neurodegenerative diseases. Life Sci. 56:1151-1171. [DOI] [PubMed] [Google Scholar]
- 4.Brewer, T. G., J. O. Peggins, S. J. Grate, J. M. Petras, B. S. Levine, P. J. Weina, J. Swearengen, M. H. Heffer, and B. G. Schuster. 1994. Neurotoxicity in animals due to artemether and arteether. Trans. R. Soc. Trop. Med. Hyg. 88(Suppl. 1):33-36. [DOI] [PubMed] [Google Scholar]
- 5.Brewer. T. G., R. F. Genovese, D. B. Newman, and Q. Li. 1998. Factors relating to neurotoxicity of artemisinin antimalarial drugs “listening to arteether.” Med. Trop. 58(Suppl.):22S-27S. [PubMed] [Google Scholar]
- 6.Cavanagh, J. B. 1984. The problems of neurons with long axons. Lancet 1284-1287. [DOI] [PubMed]
- 7.Ceballos-Picot, I. 1997. Biology of oxidative stress, p. 5-38. In I. Ceballos-Picot (ed.), The role of oxidative stress in neuronal death. Springer Verlag, New York, N.Y.
- 8.Chan, Y. L., R. Gutell, H. F. Noller, and I. G. Wool. 1984. The nucleotide sequence of a rat 18S ribosomal ribonucleic acid gene and a proposal for the secondary structure of 18S ribosomal ribonucleic acid. J. Biol. Chem. 259:224-230. [PubMed] [Google Scholar]
- 9.Dawson, R., M. F. Beal, S. C. Bondy, D. A. Di Monte, and G. E. Isom. 1995. Excitotoxins, aging and environmental neurotoxins: implications for understanding human neurodegenerative diseases. Toxicol. Appl. Pharmacol. 134:1-17. [DOI] [PubMed] [Google Scholar]
- 10.El-Bahay, C., E. Gerber, M. Horbach, Q.-H. Tran-Thi, and E. Röhrdanz. 1999. Influence of tumor necrosis factor-α and silibin on the cytotoxic action of α-amanitin in rat hepatocyte culture. Toxicol. Appl. Pharmacol. 158:2-8. [DOI] [PubMed] [Google Scholar]
- 11.Fishwick, J., G. Edwards, S. A. Ward, and W. G. McLean. 1998. Morphological and immunocytochemical effects of dihydroartemisinin on differentiating NB2a neuroblastoma cells. Neurotoxicology 19:393-404. [PubMed] [Google Scholar]
- 12.Furuta, S., H. Hayashi, M. Hijikata, S. Miyazawa, T. Osumi, and T. Hashimoto. 1986. Complete nucleotide sequence of cDNA and deduced amino acid sequence of rat liver catalase. Proc. Natl. Acad. Sci. USA 83:313-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Genovese, R. F., D.B. Newman, Q. Li, J. O. Peggins, and T. G. Brewer. 1998. Dose dependent brain stem neuropathology following repeated arteether administration in rats. Brain Res. Bull. 45:199-205. [DOI] [PubMed] [Google Scholar]
- 14.Genovese, R. F., J. M. Petras, and T. G. Brewer. 1995. Arteether neurotoxicity in the absence of deficits in behavioural performance in rats. Ann. Trop. Med. Parasitol. 89:447-449. [DOI] [PubMed] [Google Scholar]
- 15.Hien, T. T., P. J. N. Day, N. H. Phu, N. T. Hoang, T. T. H. Chau, P. P. Loc, D. X. Sinh, L. V. Cuong, H. Vinh, D. Waller, T. E. A. Peto, and N. J. White. 1996. A controlled trial of Artemether or quinine in Vietnamese adults with severe Falciparum malaria. N. Engl. J. Med. 335:76-83. [DOI] [PubMed] [Google Scholar]
- 16.Ho, Y.-S., and J. D. Crapo. 1987. Nucleotide sequences of cDNAs coding for rat manganese-containing superoxide dismutase. Nucleic Acids Res. 15:10070-10074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ho, Y.-S., and J. D. Crapo. 1987. cDNA and deduced amino acid sequence of rat copper-zinc-containing superoxide dismutase. Nucleic Acids Res. 15:6746-6750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ho, J. S., and A. J. Howard. 1992. Cloning and characterization of the rat glutathione peroxidase gene. FEBS Lett. 301:5-9. [DOI] [PubMed] [Google Scholar]
- 19.Johnson, D. J., D. G. Graham, V. Amarnath, and W. M. Valentine. 1998. Release of carbon disulfide is a contributing mechanism in the axonopathy produced by N,N-diethyldithiocarbamate. Toxicol. Appl. Pharmacol. 148:288-296. [DOI] [PubMed] [Google Scholar]
- 20.Kanthasamy, A. G., A. Rathinavelu, J. L. Borowitz, and G. E. Isom. 1994. Interaction of cyanide with the dopamine metabolite formation of a cyanohydrin adduct and its implication for cyanide-induced neurotoxicity. Neurotoxicology 15:887-896. [PubMed] [Google Scholar]
- 21.Kapetanaki, S., and C. Varotsis. 2000. Ferryl-oxo heme intermediate in the antimalarial mode of action of artemisinin. FEBS Lett. 474:238-241. [DOI] [PubMed] [Google Scholar]
- 22.Klayman, D. L. 1985. Qinghaosu, the antimalarial drug from China. Science 228:1049-1055. [DOI] [PubMed] [Google Scholar]
- 23.Krieglstein, J., and J. Nuglisch. 1992. Metabolic disorders as consequences of drug-induced energy deficits, p. 111-140. In H. Herken and F. Hucho (ed.), Selective neurotoxicity. Springer Verlag, New York, N.Y.
- 24.Krungkrai, J., D. Burat, S. Kudan, S. Krungkrai, and P. Prapunwattana. 1999. Mitochondrial oxygen consumption in asexual and sexual blood stages of the human malarial parasite Plasmodium falciparum. Southeast Asian J. Trop. Med. Public Health 30:636-642. [PubMed] [Google Scholar]
- 25.Looareesuwan, S., P. Wilairatana, S. Vanijanonta, P. Pitisuttithum, and C. Viravan. 1996. Treatment of acute, uncomplicated Falciparum malaria with oral dihydroartemisinin. Ann. Trop. Med. Parasitol. 90:21-28. [DOI] [PubMed] [Google Scholar]
- 26.Maniatis, T., E. F. Fritsch, and J. Sambrook. 1982. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 27.McLean, W. G., and S. A. Ward. 1998. In vitro neurotoxicity of artemisinin derivatives. Med. Trop. 58:28-31. [PubMed] [Google Scholar]
- 28.Olliaro, P., R. K. Haynes, B. Meunier, and Y. Yuthavong. 2001. Possible modes of action of the artemisinin-type compounds. Trends Parasitol. 17:123-126. [DOI] [PubMed] [Google Scholar]
- 29.Petras, J. M., D. E. Kyle, M. Gettayacamin, G. D. Young, R. A. Bauman, H. K. Webster, K. D. Corcoran, J. O. Peggins, M. A. Vane, and T. G. Brewer. 1997. Arteether: risks of two-week administration in Macaca mulatta. Am. J. Trop. Med. Hyg. 56:390-396. [DOI] [PubMed] [Google Scholar]
- 30.Petras, J. M., G. D. Young, R. A. Bauman, D. E. Kyle, M. Gettayacamin, H. K. Webster, K. D. Corcoran, J. O. Peggins, M. A. Vane, and T. G. Brewer. 2000. Arteether-induced brain injury in Macaca mulatta. I. The precerebellar nuclei: lateral reticular nuclei, paramedian reticular nuclei, and perihypoglossal nuclei. Anat. Embryol. 201:383-397. [DOI] [PubMed] [Google Scholar]
- 31.Phillips-Howard, P., and F. O. ter Kuile. 1995. CNS and adverse events associated with antimalarial agents. Drug Safety 12: 370-383. [DOI] [PubMed] [Google Scholar]
- 32.Price, R., M. Van Vugt, L. Phaipun, C. Luxenburger, J. Simpson, R. McGready, F. Ter Kuile, A. Kham, T. Chongsuphajaisiddhi, N. T. White, and F. Nosten. 1999. Adverse effects in patients with acute falciparum malaria treated with artemisinin derivatives. Am. J. Trop. Med. Hyg. 60:547-555. [DOI] [PubMed] [Google Scholar]
- 33.Röhrdanz, E., and R. Kahl. 1998. Alterations of antioxidant enzyme expression in response to hydrogen peroxide. Free Radical Biol. Med. 24:27-38. [DOI] [PubMed] [Google Scholar]
- 34.Röhrdanz, E., G. Schmuck, S. Ohler, Q.-H. Tran-Thi, and R. Kahl. 2001. Changes in antioxidant enzyme expression on response to hydrogen peroxide in rat astroglial cells. Arch. Toxicol. 3:150-158. [DOI] [PubMed] [Google Scholar]
- 35.Schmuck, G., and G. Schlüter. 1996. An in vitro model for toxicological investigations of environmental neurotoxins in primary neuronal cell cultures. Toxicol. Ind. Health 12:683-696. [DOI] [PubMed] [Google Scholar]
- 36.Schmuck,G., H.-J. Ahr, and G. Schlüter. 2000. Rat cortical neuron cultures: an in vitro model for differentiating mechanisms of chemically induced neurotoxicity. In vitro Mol. Toxicol. 13:37-50. [PubMed] [Google Scholar]
- 37.Schmuck, G., and R. K. Haynes. 2000. Establishment of an in vitro screening model for neurodegeneration induced by antimalarial drugs of the artemisinin-type. Neurotoxicity Res. 2:37-49. [DOI] [PubMed]
- 38.Schmuck,G., E. Röhrdanz, S. Ohler, Q.-H. Tran Thi, and R. Kahl. Oxidative stress in cortical neurons and astrocytes induced by paraquat. Neurotoxicol. Res., in press. [DOI] [PubMed]
- 39.Shull, S., N. H. Heintz, M. Periasamy, M. Manohar, Y. M. W. Janssen, J. P. Marsh, and B. T. Mossman. 1991. Differential regulation of antioxidant enzymes in response to oxidants. J. Biol. Chem. 266:24398-24403. [PubMed] [Google Scholar]
- 40.Smith, S. L., G. Maggs, G. Edwards, S. A. Ward, B. K. Park, and W. G. McLean. 1998. The role of iron in neurotoxicity: a study of novel antimalarial drugs. Neurotoxicology 19:557-560. [PubMed] [Google Scholar]
- 41.Tanaka, Y., K. Kamai, K. Otoguro, and S. Omura. 1999. Heme radical generation: possible involvement in antimalarial action of non-peroxide microbial metabolites, nanaomycin A and radicicol. J. Antibiot. 52:880-888. [DOI] [PubMed] [Google Scholar]
- 42.Tanaka, Y., F. Fang, C. G. Zhang, X. W. Zhang, and S. Omura. 1997. Heme-dependent radical generation from antimalarial fungal metabolites, radicicol and heptelidic acid. J. Antibiot. 51:451-453. [DOI] [PubMed] [Google Scholar]
- 43.Tate, D. J., Jr., M. V. Miceli, and D. A. Newsome. 1995. Phagocytosis and H2O2 induce catalase and metallothionein gene expression in human retinal pigment epithelial cells. Investig. Ophthalmol. Vis. Sci. 36:1271-1279. [PubMed] [Google Scholar]
- 44.van Hensbroek, M. B., E. Onyiorah, S. Jaffar, G. Schneider, A. Palmer, J. Frenkel, G. Enwere, S. Forck, A. Nusmeijer, S. Bennet, B. Greenwood, and D. Kwiatowski. 1996. A trial of Artemether or quinine in children with cerebral malaria. N. Engl. J. Med. 335:69-75. [DOI] [PubMed] [Google Scholar]