Abstract
Human respiratory syncytial virus (RSV), a paramyxovirus, is a major cause of acute upper and lower respiratory tract infections in infants, young children, and adults. RFI-641 is a novel anti-RSV agent with potent in vitro and in vivo activity. RFI-641 is active against both RSV type A and B strains. The viral specificity and the large therapeutic window of RFI-641 (>100-fold) indicate that the antiviral activity of the compound is not due to adverse effects on normal cells. The potent in vitro activity of RFI-641 can be translated to efficacy in vivo: RFI-641 is efficacious when administered prophylactically by the intranasal route in mice, cotton rats, and African green monkeys. RFI-641 is also efficacious when administered therapeutically (24 h postinfection) in the monkey model. Mechanism of action studies indicate that RFI-641 blocks viral F protein-mediated fusion and cell syncytium formation.
Human respiratory syncytial virus (RSV), a member of the Paramyxoviridae family (18), is a major cause of acute upper and lower respiratory tract infections in infants, young children, and adults. Serological evidence indicates that approximately 95% of children have been exposed to RSV by 2 years of age, and 100% of children have been exposed by the time they reach adulthood (8). In a given year, around 91,000 infants are hospitalized with RSV infection in the United States. These infections are responsible for 40 to 50% of hospitalizations for pediatric bronchiolitis and 25% of hospitalizations for pediatric pneumonia (8, 15). Since the immune response to RSV infection is not protective, RSV infections reoccur throughout adulthood. In adults and older children, RSV infection has been associated with upper respiratory infection, tracheobronchitis, and otitis media. However, RSV in the institutionalized elderly can be more serious and is characterized by severe pneumonia and mortality rates of up to 20 and 78%, respectively (5, 6). Adults with a previous history of heart or lung conditions are at a high risk for RSV infection. The infection has been linked to exacerbation of patients with chronic obstructive pulmonary disease. Significant mortality has been observed in immunocompromised patients, particularly those undergoing bone marrow transplantation. Regular outbreaks of RSV are well characterized and predictable, occurring between October and May each year with peak occurrences in January and February. Ribavirin is the only commercially available agent used to treat RSV infection (2). The utilization of ribavirin is limited due to efficacy and toxicity concerns as well as the very long treatment regimen required for its delivery by aerosol inhalation (19). Protective antibodies (14, 27), indicated for prophylaxis in high-risk children, are administered intravenously (RespiGam) or intramuscularly (Synagis). A number of small-molecule inhibitors of RSV have been identified, but to date none are clinically approved (7, 23). RFI-641 is the result of a chemical optimization of CL387626 (1, 4, 28), a compound that inhibits RSV fusion and demonstrates antiviral activity in vitro and in vivo. We report here on the in vitro activity, mechanism, and in vivo activity of RFI-641.
MATERIALS AND METHODS
Viral strains.
RSV strains A2 and Long (American Type Culture Collection, Rockville, Md.) were grown in human foreskin fibroblast (HFF) cells to contain approximately 107 PFU/ml. Cultures were aliquoted and kept frozen at −70°C until required. Recent human isolates, collected from 1992 to 1995 (Baylor College of Medicine, Houston, Tex.), were received at passage no. 2, expanded to a larger stock (passage no. 3), and tested for sensitivity to RFI-641. The cold-passaged RSV deletion mutant cp-52 was previously described (3, 10).
Compound.
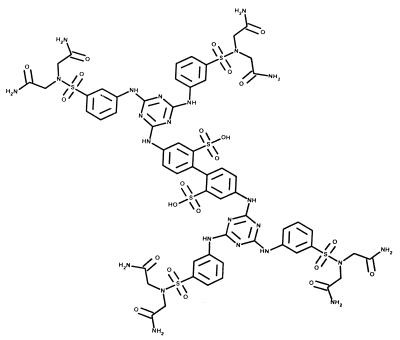
RFI-641 (4,4"-bis-{4,6-bis-[3-(bis-carbamoylmethyl-sulfamoyl)-phenylamino]-(1,3,5) triazin-2-ylamino}-biphenyl-2,2"-disulfonic acid) (Fig. 1) was synthesized at Wyeth-Ayerst Research, Pearl River, N.Y. (18a). The compound was solubilized in water at a concentration appropriate to the dose to be administered.
FIG. 1.
Structure of RFI-641 (4,4"-bis-{4,6-bis-[3-(bis-carbamoylmethyl-sulfamoyl)-phenylamino]-(1,3,5)triazin-2-ylamino}-biphenyl-2,2"-disulfonic-acid). Molecular mass, 1,684 Da.
Antiviral activity and cytotoxicity assays.
The antiviral activity of RFI-641 was evaluated by measuring the amount of RSV protein with an enzyme-linked immunosorbent assay (ELISA). Vero or HFF cells were infected with RSV at a multiplicity of infection (MOI) of 0.004, and 50% inhibitory concentrations (IC50s) were determined over a range by using 5 to 10 concentrations of the compound. Infected cells were incubated for 4 days before the cells were fixed by treatment with 50% methanol-50% acetone, washed with buffer, and developed by an ELISA with antibody to F protein. Cytotoxicity assays were performed with the same cell line incubated with serially diluted RFI-641 for 4 days. At the end of the incubation period, cell viability was determined by the addition of CellTiter 96 Aqueous One solution (Promega) containing the tetrazolium compound MTS [3-(4,5-dimethylithiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfonyl)-2 H-tetrazolium]. The relative cytotoxic effect of a compound is assessed by the decrease in cellular reduction of MTS into its formazan product as read on a spectrophotometer at 490-nm absorbance (30).
To test the sensitivity of other viruses to RFI-641, standard virological assays were used. Using a recombinant human cytomegalovirus (hCMV) stably expressing the β-glucuronidase reporter gene, HFF cells were infected at an MOI of 0.004 and the reduction in reporter gene expression was measured 4 days postinfection. For herpes simplex virus type 1 (HSV-1) and HSV-2, HFF cells were infected at an MOI of 0.006 and an ELISA for HSV glycoprotein D was performed 48 h postinfection. For influenza A and B cells, an ELISA for nucleocapsid protein was performed 22 h postinfection (MOI, 0.001 to 0.01). For human parainfluenza virus type 3 (hPIV3) an ELISA was performed for hPIV3 hemagglutinin 48 h after infection (MOI, 0.03).
To rule out the possibility that RFI-641 has a nonspecific or viricidal activity, the compound (3 μg/ml) was incubated with 1.5 × 105 PFU of RSV strain A2 in a water bath (37°C for 0.5, 1.0, or 2.0 h). After incubation, samples were serially diluted in media and titers were determined on Vero cells as described below.
Yield reduction assay.
The antiviral activity of RFI-641 was evaluated in a yield reduction assay, a direct measurement of the amount of virus produced during a single round of viral infection. Vero cells were infected with 1.5 × 105 PFU (MOI = 0.3) of RSV in medium (Dulbecco's minimal essential medium containing 2% fetal calf serum) and freshly prepared RFI-641 at either 0, 0.05, 0.1, 0.3, 1.0, or 3.0 μg/ml (final concentration). After a 90-min adsorption period at room temperature, the inoculum was removed and replaced with fresh medium containing RFI-641. After 24 h at 37°C, an equal volume of RSV freezing medium (1.36× minimal essential medium, 0.02 M HEPES, 10% fetal calf serum, 7.6 mM KH2PO4, 14.4 M K2HPO4, 10 mM glutamate, 0.44 M sucrose) was added to each well and the plates were stored overnight at −70°C. The cells were thawed, scraped with a rubber policeman, transferred to microcentrifuge tubes, and sonicated for one minute (Branson model 1200 water bath sonicator). Cellular debris was pelleted for 10 min at 80 × g at 4°C. The titers of the virus-containing supernatant were determined by serial dilution in Vero cells. After adsorption, the inoculum was removed and the cells were washed with phosphate-buffered saline (PBS), overlaid with methylcellulose, and incubated at 37°C. After 5 days, the cells were fixed with methanol-acetone (1:1) and viral plaques were visualized by immunoperoxidase staining. The primary antibody was mouse anti-RSV (1:1,000 in 1× PBS), and the secondary antibody was peroxidase-conjugated anti-mouse immunoglobulin G (1:1,000 dilution in 1× PBS; Sigma). 3,3"-Diaminobenzidine-peroxide solution was used for development of plaques.
Plaque reduction assay.
Monolayer cultures of Vero cells in six-well plates were infected at room temperature with approximately 230 PFU of RSV in medium containing RFI-641 (as indicated). After adsorption for 1.5 h, the inoculum was removed and replaced with medium containing 0.5% agarose and RFI-641. After 5 days, the agarose medium was removed and the plaques were visualized and quantified after immunoperoxidase staining as described above.
Time of addition and temperature shift assays.
Monolayer cultures of CV-1 cells were infected with 4.8 × 104 PFU (MOI = 0.3) of RSV wild-type strain A2 in the absence or presence of RFI-641. After 2 h of adsorption at room temperature (i.e., 2 h postinfection), the infection inoculum was removed and fresh medium was added for subsequent incubation at 37°C. RFI-641 (final concentration, 2 μg/ml) was added to the plates at either 0, 3, 5, 7, or 9 h postinfection. At 48 h postinfection, freezing medium was added to each well and the plates were frozen at −70°C. Viral titers were determined by plaque assay as described above. In temperature shift assays, Vero cells were exposed to RSV wild-type strain A2 (MOI = 1.0) at 4°C, either in the presence or in the absence of RFI-641 (final concentration, 2 μg/ml). After 1 h, the inoculum was removed and replaced with fresh medium at 37°C. RFI-641 (2 μg/ml) was added at the time of temperature shift or after the shift, as indicated. At 30 h after the temperature shift, cells were exposed to methionine-cysteine-free medium for 1 h and then cell proteins were metabolically labeled with [35S]methionine-cysteine for 4 h. Radiolabeled proteins were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and autoradiography either with or without prior immunoprecipitation. For immunoprecipitation, either anti-RSV virion or anti-RSV N protein antibodies (both from Chemicon International, Inc.) were used.
Viral syncytium assay.
Monolayer cultures of CV-1 cells in six-well dishes were infected with RSV wild-type strain A2 (MOI = 0.3) at room temperature. After a 2-h adsorption period, the monolayers were washed with PBS and overlaid with fresh medium. RFI-641 was added to a final concentration of 2 μg/ml at 8 h postinfection. At 40 h postinfection, cell monolayers were examined microscopically for syncytium formation.
Animals.
Female BALB/c mice, 4 to 6 weeks old, 14 to 20 g, were obtained from Charles River Laboratories. Cotton rats, 50 to 100 g and 4 to 12 weeks old, of either sex were used in these studies. Cotton rats were obtained from either descendants of a breeding pair obtained from the Small Animal Section of the Veterinary Research Branch, Division of Research Services, National Institutes of Health, or from a colony maintained at Virion Systems, Rockville, Md. African green monkeys (Cercopithecus aethiops), 2 to 4 kg, of either sex were obtained from New Iberia Primate Research Center, New Iberia, La. All animals were housed and cared for in accordance with National Institutes of Health guidelines. All experimental models were approved by and conducted in accordance with protocols reviewed by the appropriate Institutional Animal Care and Use Committees.
Animal models.
Mice (5 animals per group) were anesthetized with a solution of ketamine HCl (40 mg per kg of body weight) and xylazine (6 mg per kg of body weight) in 0.1 ml of PBS injected intraperitoneally. While mice were under the effects of anesthesia, 60 μl of RFI-641 was instilled into the nostrils by using a micropipette, allowing the mouse to inhale the solution. An equal volume of water was administered to the placebo (untreated) control group. Two hours after treatment, mice were again anesthetized and 40 μl of the virus stock (5 × 107 PFU/ml) solution in PBS was instilled into the nose of each animal (20). Each mouse was allowed to recover from the effects of the anesthesia and returned to its cage. Mice were euthanatized by CO2 inhalation at 5 days postinfection for the determination of viral titers. The lungs were removed aseptically and analyzed for viral titer by plaque assay.
Protocols for the cotton rat model (11, 21) were performed at Virion Systems (G. Prince) and Baylor College of Medicine (P. Wyde). Animals were anesthetized by either CO2 or methoxyflurane inhalation, and 100 μl of RFI-641 (50 μl per nostril) was instilled into the nares of each cotton rat. Viral infection followed 2 h after prophylactic treatment in similarly anesthetized rats by intranasal instillation of 100 μl of viral suspension to yield an approximate infection of 106 PFU per animal. Cotton rats were euthanatized by CO2 inhalation at 4 days postinfection for the determination of viral titers. The lungs were removed aseptically and analyzed for viral titer by plaque assay.
African green monkeys were sedated with ketamine (10 mg per kg of body weight, intramuscularly). The compound was administered intranasally in a volume of 0.6 ml/monkey (0.3 ml/nostril) by using a 1-ml tuberculin syringe fitted with a murine feeding needle at the selected concentrations. The virus was instilled in a similar manner in a volume of 0.5 ml (0.25 ml/nostril) of a 107-PFU/ml stock solution. In the case of prophylactic treatment, RFI-641 was administered 2 h preinfection. Therapeutic efficacy was determined by treatment initiated 24 h postinfection and continued once per day for 8 days. The virus in the nasal passage was sampled by washing on days 1 to 11 (prophylactic regimen) following infection. To do this, anesthetized monkeys were placed in a prone position on a table with the head off the table and tilted back. Sterile saline (1.5 ml) was drawn into a 3-ml syringe attached to a 3.5-in. French Tom Cat catheter (Sherwood Medical). The catheter was inserted into the area of the nasal turbinate, and the saline was administered. The fluid was promptly aspirated, and about 0.5 to 0.7 ml was usually recovered. The fluid was added to a tube containing 0.5 ml of viral transport medium. The virus in the throat was sampled on days 1 to 11 following infection by using a Dacron-tipped swab. The animal was positioned as before, and the upper throat was swabbed just behind the uvula, rotating the swab 3 to 4 times. The swab was then placed in a tube of viral transport medium, mixed, and broken off in the tube. The virus in the lung was sampled by bronchoalveolar lavage (BAL). Ten milliliters of PBS was introduced via a bronchoscope into the right lung and promptly aspirated, and 5 to 7 ml was usually recovered. Animals intended for BAL sampling were treated with ketamine (10 mg/kg, intramuscularly) and xylazine (0.6 mg/kg, subcutaneously) for chemical restraint and to inhibit the cough reflex. All samples were quickly frozen (ethanol-dry ice) after sampling and kept at −70°C.
Tissue viral titer determinations.
Viral titers in all samples recovered from the primate model were determined by viral plaque assay. Briefly, to determine virus titers, aliquots were serially diluted (10-fold) in Dulbecco's minimal essential medium containing 2% serum. The diluted sample (0.4 ml) was used to infect Vero cells. The infected cells were incubated for 90 min at room temperature. After incubation, the medium was removed and cells were then overlaid with 2 ml of methylcellulose. After incubation for 5 days, viral plaques were visualized by immunoperoxidase staining as described above. For statistical analysis, the data were analyzed and compared via Student's t test, Fisher's least significant difference comparisons, and analysis of variance.
RESULTS
Antiviral activity.
RSV isolates are classified into two groups according to differential interactions with panels of monoclonal antibodies based on the RSV G protein. RFI-641 is a potent inhibitor of laboratory strains and clinical isolates of both RSV A and B strains (Table 1). Based on the ELISA, the average IC50s against all tested RSV A and B subtypes are 0.055 and 0.018 μg/ml, respectively. The concentration that inhibited 90% of the clinical isolates tested by 50% was 0.03 μg/ml. In a more stringent measure of antiviral activity, a yield reduction assay, RFI-641 was a potent inhibitor of new virion formation. The mean IC90s for RSV A and B clinical isolates were 0.22 μg/ml and 0.12 μg/ml, respectively (data not shown). The antiviral activity of RFI-641 is independent of the cell line used in the assay as the IC50 for RFI-641 against RSV A2 strains was similar on HFF, Vero, and CV-1 cells (0.18, 0.37, and 0.28 μg/ml, respectively) (data not shown). A yield reduction assay was performed to determine the efficacy of RFI-641 against RSV strain A2 over an MOI ranging from 0.03 to 3.0. The IC90s increased a modest 4.8-fold from 0.19 to 0.92 μg/ml over the 100-fold increase in MOI.
TABLE 1.
Inhibitory effect of RFI-641 on RSV laboratory strains and recent isolatesa
| RSV strain | Mean IC50 (μg/ml) | IC50 range (μg/ml) | Therapeutic index (fold) |
|---|---|---|---|
| Laboratory strains | |||
| Type A (n = 3) | 0.08 | 0.025-0.18 | >417 |
| Type B (n = 3) | 0.018 | 0.01-0.03 | >2,500 |
| Recent isolates | |||
| Type A (n = 9) | 0.03 | 0.013-0.07 | >1,071 |
| Type B (n = 9) | 0.019 | 0.003-0.04 | >1,875 |
RSV laboratory strains were tested on HFF cells by an RSV ELISA. Recent isolates were tested on Vero cells. The therapeutic index was determined by dividing the concentration that reduced the MTS signal by 50% (LC50) by the IC50 of the least sensitive isolate. The LC50 for RFI-641 on both Vero and HFF cells was >75 μg/ml.
Selectivity and therapeutic indices.
RFI-641 shows little or no activity against other viruses as measured by a virus-specific ELISA (Table 2). Activity (IC50) against influenza strains A and B and hPIV3 was >10 μg/ml. Relatively poor activity (4.3 to 13 μg/ml) was observed against hCMV and HSV. To confirm the specificity of RFI-641's antiviral activity, cell viability in the presence of RFI-641 was measured using the common MTS assay. For consistency, these cytotoxicity assays were performed on all cell lines with conditions similar to the antiviral assays (i.e., 4 days, confluent cells). RFI-641 had a 50% lethal concentration (LC50) of >75 μg/ml for Vero and HFF cells. The therapeutic index for all RSV isolates tested was calculated based on their IC50s and LC50s. The narrowest therapeutic index was 417-fold, but the range was as great as >2,500-fold (Table 1). Furthermore, RFI-641 does not act nonspecifically as a viricidal agent like a detergent or bleach. To test this, RFI-641 (3 μg/ml) was incubated with RSV at 37°C for 2 h before serial dilution to bring the drug concentrations below therapeutic levels. There was no effect on viral titers (data not shown). Taken together these data strongly suggest that the anti-RSV activity of RFI-641 is specific and is not due to adverse effects on the cells in vitro.
TABLE 2.
Effect of RFI-641 on non-RSV viruses
| Virus and strain | Cell type | IC50 (μg/ml) |
|---|---|---|
| hCMV AD169 | HFF | 4.3 |
| HSV-1 Patton | HFF | 13 |
| HSV-2 333 | HFF | 7 |
| Influenza virus A/W S/N | MDCK | >10 |
| Influenza virus B G1 | MDCK | >10 |
| hPIV3 | Vero | >10 |
Time of addition and temperature shift experiments.
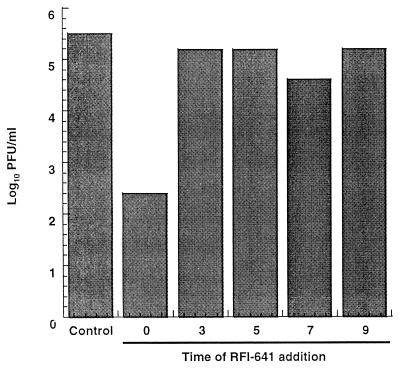
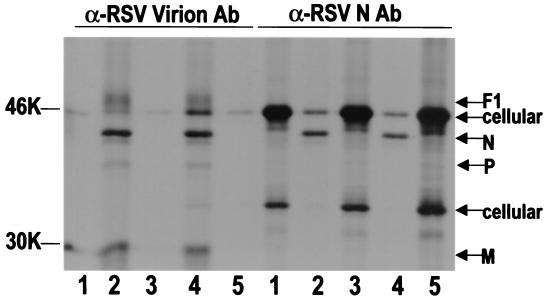
To determine at which point during the virus replication cycle RFI-641 exerts its antiviral effect, the inhibitor was added at various times and viral yield was quantitated at 48 h postinfection (Fig. 2). The reduction in yield when the compound was present at the time of infection was 3.1 log10. When RFI-641 was added later, 3 to 9 h postinfection, there were no significant reductions in virus yield. In a modification of this experiment, the production of viral proteins was analyzed (Fig. 3). Cells were exposed to virus at 4°C for a 1-h adsorption period in the presence or absence of RFI-641. At 4°C, RSV binds to cells but does not fuse with cellular membranes. After inoculum removal, the cells were shifted to 37°C and RFI-641 was added at the indicated time; after 30 h, the cells were metabolically radiolabeled as described. To obtain evidence for viral infection, radiolabeled cell extracts were subjected to immunoprecipitation with antibodies reactive with either RSV virion proteins or RSV N protein (Fig. 3). RSV proteins (i.e., F1, N, P, and M proteins) were only detected when RFI-641 was either not added or added after the temperature shift. However, several cellular proteins, which are immunoprecipitated with the promiscuous RSV N antibody, are observed in equal abundance in the uninfected cells or in infected cells when the inhibitor was present prior to, or coincident with, the temperature shift. In infected cells, either in the absence of inhibitor or when inhibitor was added 2 h after the temperature increase, levels of these cellular proteins were similarly reduced. It is important to note that substantial antiviral effect was observed when the compound was added after viral adsorption but coincident with the initiation of viral fusion.
FIG. 2.
Effect of time of addition of RFI-641 on RSV progeny production. CV-1 cells were infected with RSV at an MOI of 0.3, and RFI-641 was added to a final concentration of 2 μg/ml at various times after infection (in hours) as described in Materials and Methods. RFI-641 was not added to the control infection. Data represent the means of duplicate determinations.
FIG. 3.
Temperature shift and time of addition assay immunoprecipitation. Radiolabeled Vero cell extracts were immunoprecipitated with antibodies reactive with either RSV virion proteins (α-RSV Virion Ab) or RSV N protein (α-RSV N Ab). Lanes 1, uninfected Vero cells; lanes 2 to 5, Vero cells exposed to RSV strain A2. Lanes 1 and 2 contained no inhibitor. RFI-641 was present at the time of RSV exposure and after temperature shift in lanes 3, RFI-641 was added 2 h after temperature shift in lanes 4, and RFI-641 was present beginning at the time of temperature shift in lanes 5. The positions of the RSV F1, N, and M proteins are indicated. Also indicated are two cellular proteins (cellular).
Inhibition of syncytium formation.
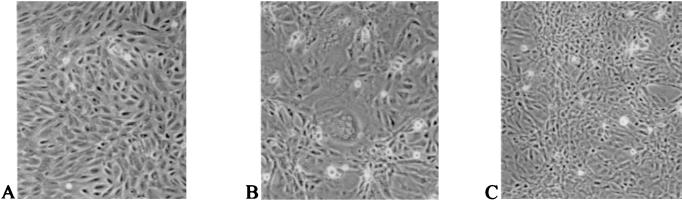
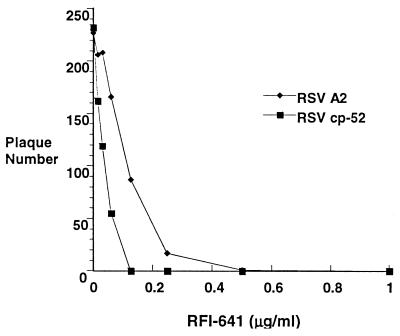
To study the effects of inhibitor on syncytium formation, RFI-641 (2 μg/ml) was added at 8 h postinfection to CV-1 cells infected at a low multiplicity with RSV wild-type strain A2. As shown in Fig. 4, RFI-641 added at 8 h postinfection did block syncytium formation. Thus, RFI-641 appears to block two F glycoprotein-mediated fusion events: (i) fusion of the virion envelope with the cellular plasma membrane and (ii) syncytium formation. In support of this conclusion, an RSV mutant designated cp-52, which expresses the F envelope glycoprotein, but not the SH and G envelope glycoproteins (3, 10), was tested in the ELISA. RFI-641 inhibited the cp-52 virus with an IC50 of 0.03 μg/ml (Fig. 5).
FIG. 4.
Morphology of CV-1 cells treated with RFI-641. (A) Noninfected, nontreated CV-1 cells; (B) RSV-infected, untreated CV-1 cells; (C) RSV-infected, RFI-641 (>8 h)-treated CV-1 cells.
FIG. 5.
Sensitivity of RSV strains A2 and cp-52 to RFI-641 as determined by plaque reduction assay.
Animal models.
The in vivo efficacy of RFI-641 was assessed in three animal model systems. In the mouse infection model, prophylactic intranasal administration of RFI-641 at doses ranging from 0.04 to 1.3 mg/kg at 2 h prior to RSV challenge resulted in a statistically significant reduction in viral lung titers at day 5 (0.63 to 1.53 log10 PFU/ml) compared to untreated controls at all doses (Table 3).
TABLE 3.
Prophylactic efficacy of RFI-641 against strain A2 n the RSV mouse model
| Treatment | Dose (mg/kg) | Mean titer ± SD (log10 PFU/lung) | Log10 reduction |
|---|---|---|---|
| Placebo | 3.68 ± 0.09 | ||
| RFI-641 | 0.04 | 3.05 ± 0.29 | 0.63a |
| 0.08 | 2.47 ± 0.05 | 1.21a | |
| 0.16 | 2.54 ± 0.35 | 1.14a | |
| 0.32 | 2.29 ± 0.35 | 1.39a | |
| 0.65 | 2.24 ± 0.38 | 1.44a | |
| 1.30 | 2.15 ± 0.23 | 1.53a |
Significance at a P value of <0.05 by Student's t test based on 5 mice per group.
Efficacy of RFI-641 in the cotton rat model (Table 4) was determined at Virion Systems (study 1) and Baylor College of Medicine (studies 2 and 3). In this model, the efficacy was determined by quantitation of viral titers in the lungs 4 days after infection. Prophylactic administration 2 h before RSV challenge of a low dose of RFI-641 (0.2 mg/kg) resulted in a slight decrease (0.6 log10) in lung titers compared to placebo-treated controls from study 1. However, prophylactic administration of RFI-641 over the dose range of 1 to 10 mg/kg resulted in a statistically significant reduction of at least 3.2 log10 PFU/lung compared to placebo-treated control animals (study 2). In the third study, infection was with the RSV Long strain. RFI-641 administered prophylactically at a single dose of 10 mg/kg resulted in a 2.6 log10 reduction of lung titers.
TABLE 4.
Intranasal prophylactic efficacy of RFI-641 in cotton rats
| Study no. | Strain | Mean control titer ± SD (log10 PFU/lung) | Dose (mg/kg) | Log10 reduction |
|---|---|---|---|---|
| 1 | A2 | 4.3 ± 0.23 | 0.2 | 0.6 |
| 2 | A2 | 3.9 ± 0.24 | 1.0 | 3.2a |
| 3.3 | 3.2a | |||
| 10 | 3.2a | |||
| 3 | Long | 4.6 ± 0.45 | 10 | 2.6a |
Significance at a P value of <0.05 by Student's t test based on 5 mice per group.
In the African green monkey model, prophylactic administration of RFI-641 reduced the viral nasal titers by 1.5 log10 or greater for days 3 to 10 post-RSV challenge (Table 5). On the day of peak nasal titers in the control group (day 8) RFI-641-treated monkeys exhibited a 3.4 log10 reduction in viral load. At the 6-mg dose, viral titers were significantly lower on days 4 to 9 (peak period of viral load) with a 1.8 to 3.4 log10 reduction. Similar to those in the nasal samples, viral titers were reduced in the throat samples. Throat sample titers were 1.4 to 2.1 log10 lower than those of the controls for days 6 to 10 (peak of throat viral load). Prophylactic treatment with RFI-641 also reduced the viral spread to the lungs. The viral load in the lung was significantly reduced in the controls on days 6 (1.99 log10 reduction) and 8 (1.56 log10 reduction).
TABLE 5.
Prophylactic efficacy of RFI-641 after intranasal administration (6 mg) at 2 h preinfection in African green monkeys
| Day | Log10 titer reduction in samples from:
|
||
|---|---|---|---|
| Nasal passage | Throat | BAL fluid | |
| 3 | 0.2 | 0.43 | |
| 4 | 1.87a | 0.7 | 0.39 |
| 6 | 2.32a | 1.81a | 1.99a |
| 7 | 2.26a | 1.75a | |
| 8 | 3.38a | 1.4a | 1.56a |
| 9 | 3.33a | 1.39 | |
| 10 | 1.46 | 2.17a | |
Significant by Fisher's least significant difference comparison at a P value of <0.05.
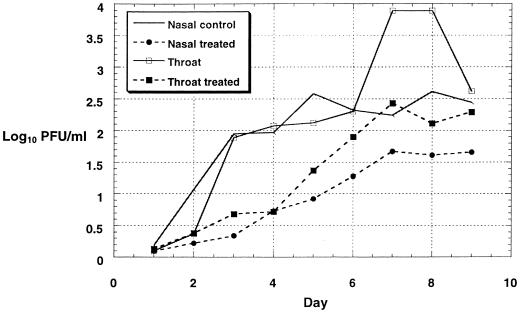
Daily therapeutic intranasal administration of RFI-641, initiated 24 h after RSV infection in monkeys, reduced viral titers in both nasal and throat samples (Fig. 6). In the nasal samples, viral loads were 0.57 to 1.66 log10 lower than those of untreated controls throughout all of the days of the study. At day 5, when control titers had peaked at 2.58 log10 PFU/ml, the titers in the RFI-641-treated group were 0.92 log10 PFU/ml. Viral titers in throat samples from RFI-641-treated animals were 0.33 to 1.84 log10 lower than in those of the control group of animals (days 3 to 9).
FIG. 6.
Therapeutic efficacy of RFI-641 after intranasal administration to RSV-infected African green monkeys.
DISCUSSION
RSV virion envelopes are composed of a membrane-derived lipid bilayer and three viral transmembrane surface glycoproteins: G, SH, and F. G is a heavily glycosylated viral attachment protein and initiates infection via interaction with an unknown cellular receptor(s) (9). Heparin sulfate moieties on cell surfaces have been implicated in viral attachment (12). The function of SH is unknown. The F proteins in the RSV virion have a disulfide linkage between two F domains, F1 and F2. Critical to the structural conformation of F are two interacting heptad repeat domains, HR1 and HR2. The association of three HR1 domains into a coiled-coil complex is thought to be necessary for the formation of mature F protein trimers on virion or infected cell membranes (16, 17, 29). While the biophysical steps of viral fusion are not completely delineated, homology to human immunodeficiency virus gp41 suggests a common fusion mechanism for these two enveloped viruses. F is believed to exist in two conformations and undergoes a structural change to a fusogenic state after virus binding. This conformational change exposes the fusion peptide within F (25). Recent data suggest that F may then interact specifically with a cellular protein such as RhoA to facilitate pH-independent fusion to cellular membranes (20).
The RSV F protein has an essential role in the virus lifecycle. In addition to directing virus-cell membrane fusion, F protein also causes cell-to-cell fusion (syncytium formation) when expressed on the surface of infected cells. Lambert et al. (13) have shown that peptides derived from the HR2 region of RSV, hPIV3, and measles virus could block the infection of tissue culture cells by preventing the fusion of viral envelopes and cell membranes. These peptides were highly active and selective for their parental virus. Antiviral properties have also been reported for analogous peptides derived from the HR2 region of human immunodeficiency virus gp41 (26). A possible mechanism is that HR2 peptides may block or alter a structural rearrangement in F proteins during the transition from an inactive to a fusogenic conformation (13). Further development of synthetic peptides as antiviral inhibitors has been hampered by delivery and stability problems.
To develop new small-molecule inhibitors of RSV, we initiated a screen of 20,000 compounds in a whole-virus cell-based assay. A specific inhibitor of RSV, CL387626, was identified and subsequently shown to have activity in the nanomolar range against a wide variety of RSV clinical and laboratory strains (1, 4). That compound demonstrated an excellent safety profile in tissue culture cells (selective index of 1,500 in proliferating HEP-2 and Vero cells) and showed encouraging in vivo activity in RSV-infected cotton rats (28). Chemical synthesis of CL387626 analogs demonstrated a clear structure activity relationship for activity against RSV. RFI-641 is a more-potent analog of CL387626, and the present study examines the activity of RFI-641.
Mechanism studies indicate that RFI-641 interacts specifically with the viral F protein and inhibits F function. Biophysical studies with earlier members of the series (4) and with RFI-641 (22) indicate that this series of inhibitors interacts directly with F. Consistent with an interaction with RSV F, RFI-641 is not viricidal and exerts its effects only in the context of virus-cell interactions. Importantly, RFI-641 is active against a panel of both type A and B RSV strains but does not inhibit other viruses, even the phylogenetically related parainfluenza virus. This suggests that the target for RFI-641 activity is well conserved in RSV. Time of addition studies indicate that RFI-641 must be present early in infection before F-mediated fusion. RFI-641 does not block viral attachment when a 4°C temperature shift is utilized, suggesting that inhibition is at the level of viral fusion. Finally, addition of RFI-641 during viral infection causes an inhibition of viral syncytia as observed microscopically.
Compelling evidence that the viral fusion protein is the target of RFI-641 comes from the use of an RSV mutant designated cp-52, which expresses the F envelope glycoprotein but does not express the SH and G envelope glycoproteins (due to deletion of their coding sequences from the viral genome) (3, 10). This virus grows well in tissue culture but is attenuated in vivo. When compared to RSV strain A2, strain cp-52 demonstrated a similar sensitivity to RFI-641. Thus, RFI-641 inhibits RSV in the context of a virus that contains only F on its surface, strongly implicating that protein as the target for antiviral activity.
Due to the specificity of action, RFI-641 is a highly potent inhibitor of laboratory and recent clinical isolates of RSV with IC50s of 0.01 to 0.18 μg/ml. This antiviral activity was also observed in three animal models of RSV infection. RFI-641 and several other compounds of this series were evaluated by an intravenous route in the mouse model of RSV (24). None of the compounds exhibited efficacy, and therefore, this route of administration was not pursued. RFI-641 was shown to be efficacious by intranasal administration in the mouse model of RSV infection and replication at doses as low as 0.04 mg/kg. Reductions of the viral load in the lungs of greater than 1 log10 were observed with administration of 0.08 to 1.3 mg of RFI-641/kg. The efficacy of the fusion inhibitor is further supported by the observed reduction of viral titers in the lungs of cotton rats. Significant reductions (>2 log10 PFU/lung) were observed with doses as low as 1 mg/kg against infections with two different strains of virus. In the African green monkey model, intranasal application of RFI-641 reduced viral titers in nasal and throat samples at a dose of 6 mg. Viral titers in BAL fluids (days 6 and 8) were also reduced by greater than 1 log10 at the 6-mg dose. Persistence of RFI-641 in the respiratory tract was not observed when BAL samples were examined. In this model, virus first replicates in nasal and throat epithelial cells and passes rapidly into the lungs, possibly through aspiration. Since the intranasal form of RFI-641 would not provide good exposure to the deep lung, an inhaled formulation of the compound has been developed. Efficacy studies with inhaled RFI-641 also demonstrated efficacy in the African green monkey model (unpublished data).
Of note are two recent reports of small-molecule inhibitors of RSV. Both the Janssen Research Foundation (K. Andries, M. Moeremans, T. Gevers, R. Willebrords, J. Lacrampe, and F. Janssens, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1160, 2000) and Viropharma, Inc. (D. Pevear, T. Tull, R. Direnzo, N. Ma, T. Nitz, and M. Collett, Abstr 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1854, 2000), have presented data on compounds that target the RSV F protein. Interestingly, both were discovered in whole-virus cell-based screens similar to that leading to the development of RFI-641.
Acknowledgments
We acknowledge the assistance of Geraldine Bebernitz, Jeanette Fairhurst, Stephen Johann, Shi-Wu Lee, Eileen Lenoy, Todd Littell, and Jennifer Obregon for technical assistance and Fred Immermann for statistical analysis of the titer data. We thank Valerie Randolph for providing the RSV cp-52 virus and mouse anti-RSV antibodies.
REFERENCES
- 1.Aulabaugh, A., W. Ding, G. Ellestad, A. Gazumyan, C. Hess, G. Krishnamurthy, B. Mitsner, and J. Zacardi. 2000. Inhibition of respiratory syncytial virus by a new class of chemotherapeutic agents. Drugs Future 25:287-294. [Google Scholar]
- 2.Aylward, B., and D. Burdge. 1991. Ribavirin therapy of adult respiratory syncytial virus pneumonitis. Arch. Intern. Med. 151:2303-2304. [PubMed] [Google Scholar]
- 3.Bukreyev, A., S. Whitehead, B. Murphy, and P. Collins. 1997. Recombinant respiratory syncytial virus from which the entire SH gene has been deleted grows efficiently in cell culture and exhibits site-specific attenuation in the respiratory tract of the mouse. J. Virol. 71:8973-8982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ding, W., B. Mitsner, G. Krishnamurthy, A. Aulabaugh, C. Hess, J. Zaccardi, M. Cutler, B. Feld, A. Gazumyan, Y. Raifeld, A. Nikitenko, S. Lang, Y. Gluzman, B. O'Hara, and G. Ellestad. 1998. Novel and specific respiratory syncytial virus inhibitors that target virus fusion. J. Med. Chem. 41:2671-2675. [DOI] [PubMed] [Google Scholar]
- 5.Dowell, S., L. Anderson, H. Gray, D. Erdman, J. Plouffe, T. File, B. Marston, and R. Breiman. 1996. Respiratory syncytial virus is an important cause of community-acquired lower respiratory infection among hospitalized adults. J. Infect. Dis. 174:456-462. [DOI] [PubMed] [Google Scholar]
- 6.Falsey, A., and E. Walsh. 2000. Respiratory syncytial virus infection in adults. Clin. Microbiol. Rev. 13:371-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gazumyan, A., B. Mitsner, and G. Ellestad. 2000. Novel anti-RSV dianionic dendrimer-like compounds: design, synthesis and biologic evaluation. Curr. Pharm. Des. 6:525-546. [DOI] [PubMed] [Google Scholar]
- 8.Hall, C. 1999. Respiratory syncytial virus: a continuing culprit and conundrum. J. Pediatr. 135:2-7. [PubMed] [Google Scholar]
- 9.Heminway, B., Y. Yu, Y. Tanaka, K. Perrine, E. Gustafson, J. Bernstein, and M. Galinski. 1994. Analysis of respiratory syncytial virus F, G, and SH proteins in cell fusion. Virology 200:801-805. [DOI] [PubMed] [Google Scholar]
- 10.Karron, R., D. A. Buonagurio, A. Georgiu, S. Whitehead, J. Adamus, M. Clements-Mann, D. Harris, V. Randolph, S. Udem, B. Murphy, and M. Sidhu. 1997. Respiratory syncytial virus SH and G proteins are not essential for viral replication in vitro: clinical evaluation and molecular characterization of a cold-passaged, attenuated RSV subgroup B mutant. Proc. Natl. Acad. Sci. USA 94:13961-13986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kimpen, J. 1996. Experimental models for respiratory syncytial virus infections. Rev. Med. Microbiol. 7:115-122. [Google Scholar]
- 12.Krusat, T., and H.-J. Streckert. 1997. Heparin-dependent attachment of respiratory syncytial virus (RSV) to host cells. Arch. Virol. 142:1247-1254. [DOI] [PubMed] [Google Scholar]
- 13.Lambert, D., S. Barney, A. Lambert, K. Gutherie, R. Medinas, D. Davis-Rhodes, T. Bucy, J. Erickson, G. Merutka, and T. Matthews. 1996. Peptides from conserved regions of paramyxovirus fusion (F) proteins are potent inhibitors of viral fusion. Proc. Natl. Acad. Sci. USA 93:2186-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.LaVia, W., M. Marks, and H. Stutman. 1992. Respiratory syncytial virus puzzle: clinical features, pathophysiology, treatment and prevention. J. Pediatr. 121:503-510. [DOI] [PubMed] [Google Scholar]
- 15.LaVia, W., S. Grant, H. Stutman, and M. Marks. 1993. Clinical profile of pediatric patients hospitalized with respiratory syncytial virus infection. Clin. Pediatr. 8:450-454. [DOI] [PubMed] [Google Scholar]
- 16.Lawless-Delmedico, M., P. Sista, R. Sen, N. Moore, J. Antczak, J. White, R. Greene, K. Leanza, T. Matthews, and D. Lambert. 2000. Heptad-repeat regions of respiratory syncytial virus F1 protein form a six-membered coiled-coil complex. Biochemistry 39:11684-11695. [DOI] [PubMed] [Google Scholar]
- 17.Matthews, J., T. Young, S. Tucker, and J. Mackay. 2000. The core of the respiratory syncytial virus fusion protein is a trimeric coiled coil. J. Virol. 74:5911-5920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McIntosh, K. 1997. Respiratory syncytial virus, p. 691-705. In A. Evans and R. Kaslow (ed.), Viral infections of humans, 4th ed. Plenum Medical Book Company, New York, N.Y.
- 18a.Nikitenko, A., Y. Raifeld, and T. Wang. 2001. The discovery of RFI-641 as a potent and selective inhibitor of the respiratory syncytial virus. Bioorg. Med. Chem. Lett. 11:1041-1044. [DOI] [PubMed] [Google Scholar]
- 19.Ohmit, S., F. Moler, A. Monto, and A. Khan. 1996. Ribavirin utilization and clinical effectiveness in children hospitalized with respiratory syncytial virus infection. J. Clin. Epidemiol. 49:963-967. [DOI] [PubMed] [Google Scholar]
- 20.Pastey, M., T. Gower, P. Spearman, P. Crowe, and B. Graham. 2000. A RhoA-derived peptide inhibits syncytium formation induced by respiratory syncytial virus and parainfluenza virus type 3. Nat. Med. 6:35-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prince, G., B. Jenson, R. Horswood, E. Camargo, and R. Chanock. 1978. The pathogenesis of respiratory syncytial virus infection in cotton rats. Am. J. Pathol. 93:771-783. [PMC free article] [PubMed] [Google Scholar]
- 22.Razinkov, V., A. Gazumyan, A. Nikitenko, G. Ellestad, and G. Krishnamurthy. 2001.. RFI-641 inhibits entry of respiratory syncytial virus via interactions with fusion protein. Chem. Biol. 8:645-659. [DOI] [PubMed] [Google Scholar]
- 23.Shigeta, S. 2000. Recent progress in antiviral chemotherapy for respiratory syncytial virus infections. Exp. Opin. Investig. Drugs 9:221-235. [DOI] [PubMed] [Google Scholar]
- 24.Sudo, K., W. Wantanabe, S. Mori, K. Konno, S. Shigeta, and T. Yokota. 1999. Mouse model of respiratory syncytial virus infection to evaluate antiviral activity in vivo. Antivir. Chem. Chemother. 10:135-139. [DOI] [PubMed] [Google Scholar]
- 25.Walsh, E. E., and J. Hruska. 1983. Monoclonal antibodies to respiratory syncytial virus proteins: identification of the fusion protein. J. Virol. 47:171-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wild, C., T. Greenwell, and T. Mathews. 1993. A synthetic peptide from HIV-1 GP-41 is a potent inhibitor of virus mediated cell-cell fusion. AIDS Res. Hum. Retrovir. 9:1051-1053. [DOI] [PubMed] [Google Scholar]
- 27.Wyde, P. 1998. Respiratory syncytial virus (RSV) disease and prospects for its control. Antivir. Res. 39:63-79. [DOI] [PubMed] [Google Scholar]
- 28.Wyde, P., D. Moore-Poveda, B. O'Hara, W. Ding, B. Mitsner, and B. Gilbert. 1998. CL387626 exhibits marked and unusual antiviral activity against respiratory syncytial virus in tissue culture and in cotton rats. Antivir. Res. 38:31-42. [DOI] [PubMed] [Google Scholar]
- 29.Zhao, X., M. Singh, V. N. Malashkevich, and P. Kim. 2000. Structural characterization of the human respiratory syncytial virus fusion protein core. Proc. Natl. Acad. Sci. USA 97:14172-14177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zolnai, A., E. Toth, R. Wilson, and V. Frenyo. 1998. Comparison of 3H-thymidine incorporation and CellTiter 96 aqueous colorimetric assays in cell proliferation of bovine mononuclear cells. Acta Vet. Hung. 46:191-197. [PubMed] [Google Scholar]