Abstract
The susceptibility of Leptospira interrogans serovar icterohaemorrhagiae strain Verdun to selected antibiotics used in medical practice (ampicillin, doxycycline, and ofloxacin) was evaluated in a Syrian hamster model, to determine the efficacy of these antibiotics during the course of the disease. A quantitative PCR assay was used to monitor the density of leptospires in blood and in target organs (liver, kidney, lung, heart, and spleen). Our results demonstrated the ability of ampicillin at a high dose (100 mg/kg of body weight) to clear leptospires from the host, except from kidneys and heart, where 102 leptospires/g remained at day 6. Ofloxacin (30 mg/kg) was unable to clear bacteria from blood or kidneys. With doxycycline (10 mg/kg), the clearance of leptospires occurred in 2 days in all the target organs studied, with the exception of liver, which required 3 days. Our data demonstrate the value of monitoring the kinetics of experimental leptospiral infection in order to accurately evaluate the efficacy of antibiotics. We have demonstrated the potential value of doxycycline for the treatment of leptospirosis cases, except in circumstances where it is contraindicated. This experimental model could be used to define better therapeutic strategies for human leptospirosis, by testing associations or new formulations of antibiotics.
Leptospirosis is a zoonosis of worldwide distribution, affecting humans as well as domestic animals and wild fauna (9). The human disease is an acute febrile illness characterized by a broad range of clinical forms, the severities of which vary from mild to rapidly fatal (10).
For many decades there has been controversy about the efficacy of antibiotics in the treatment of human leptospirosis, because few controlled studies have been conducted. In anicteric leptospirosis, oral doxycycline was reported to significantly reduce the course of the disease and to prevent leptospiruria (14). Intravenous penicillin G, in one study, was found to cause clinical improvement in acute leptospirosis with renal failure (Weil's disease), even if administration was delayed (25). However, a contrary result was obtained in another comprehensive investigation (5).
A few studies have evaluated the in vitro susceptibility of Leptospira spp. to antimicrobial agents (6, 17, 20). These showed a high degree of efficacy of a broad range of antibiotics. Among those clinically usable for human treatment, the lowest MICs were obtained with ampicillin, penicillin, tetracycline, and ciprofloxacin.
Using PCR (3, 15) to follow up patients treated with standard antibiotic regimens (10) it was shown that leptospires persist for up to 1 year in urine (3) and for up to 40 days in blood (15). In a hamster model, leptospires were seen in intercellular locations (24). Leptospires were also able to invade Vero cells in vitro (16).
There remains reason for concern about the ability of usual antibiotic treatments to remove leptospires from locations where they may be protected from the immune response. Here, we report in vivo data on the efficacy of ampicillin, doxycycline, and ofloxacin against Leptospira interrogans serovar icterohaemorrhagiae strain Verdun in a hamster model of leptospirosis. These antibiotics were selected because they are used clinically for human treatment (10) and because of their different pharmacokinetics within the host. After treatment, the density of leptospires was monitored in the main target organs (liver, kidneys, lungs, heart, and spleen) and in blood, by using a quantitative PCR assay.
MATERIALS AND METHODS
Leptospira strain.
Virulent L. interrogans serovar icterohaemorrhagiae strain Verdun was obtained from the Reference Collection of the Pasteur Institute in Paris, France. Virulence was maintained by deep-freeze preservation and iterative lethal passages in Syrian hamsters, Lak:LVG(SYR)BR (Charles River). Leptospires were grown in EMJH medium (7) at 30°C to a density of about 108 bacteria per ml, estimated using a Petroff-Hausser chamber. First-passage cultures, derived from cardiac punctures of infected animals, were used for all experimental infections.
Experimental infections.
Syrian hamsters weighing 45 to 55 g were sublethally infected subcutaneously with 108 virulent leptospires (day 0). For each antibiotic tested, the treated group included 18 animals (three hamsters tested per day) in order to perform a 6-day kinetic study. Antimicrobial agents were given using a 4-day regimen (days 3 to 6). Untreated infection controls (18 hamsters) were inoculated under the same conditions. Both treated and untreated hamsters were sacrificed each day from days 1 to 6 after inoculation. Each set of experiments, including control animals, was conducted in duplicate. At necropsy, blood and target organs (liver, kidney, spleen, lung, and heart) were collected for quantitative PCR testing.
Therapeutic trials.
Drugs tested were standard injectable formulations of ampicillin (Bristol-Myers Squibb), doxycycline (Pfizer), and ofloxacin (Roussel Diamant), ready to use or diluted in sterile water for injection. Drug dilutions were freshly prepared before use in order to deliver the appropriate dose in a volume of 0.15 to 0.30 ml. Antimicrobial agents were given intramuscularly once daily from day 3 to day 6 in the cranial part of the thigh. Ampicillin and ofloxacin were used at doses of 40 or 100 mg/kg of body weight and 15 or 30 mg/kg, respectively. Doxycycline was used at a single dose of 10 mg/kg.
Quantitative PCR testing.
For estimation of leptospiremia, the maximum amount of blood was collected by cardiac puncture from each sacrificed animal. Selected organs were carefully washed, after collection, in sterile phosphate-buffered saline (bioMerieux), to minimize the presence of leptospires due to contamination with blood. Tissue specimens (0.07 to 0.90 g) of liver, kidney, spleen, heart, and lungs were mechanically disrupted in a mortar using 1 ml of sterile phosphate-buffered saline buffer. Suspensions were centrifuged to remove cellular debris (10 min at 1,300 × g), and the supernatants (500 μl) containing leptospires were subjected to DNA extraction. Purification of DNA, amplification, and hybridization in a microplate assay were performed as previously described (23). Briefly, leptospiral DNA was purified using silica particles and guanidium thiocyanate lysis buffer, and 10 μl of eluted DNA in TE buffer (10 mM Tris, pH 7.6; 1 mM EDTA) was used for subsequent amplifications (15). The biotin-labeled amplified product (331-bp fragment of the rrs gene) was hybridized with a complementary capture probe (289 bp) covalently linked onto aminated polystyrene wells (Covalink NH microplates). The hybrid molecules were detected by extravidine conjugated with alkaline phosphatase and a chromogenic substrate.
Samples negative in the quantitative microplate assay were confirmed as negative using a highly sensitive dot filter hybridization test (15).
Statistical analysis.
Mean leptospiral densities (each with standard deviation [SD]) were calculated for each organ and each day for each duplicated set of results (three hamsters per result). Efficacies of antibiotics were evaluated using Student's t test, comparing treated and untreated animals. Results were considered significant when P was <0.05.
RESULTS
Untreated controls.
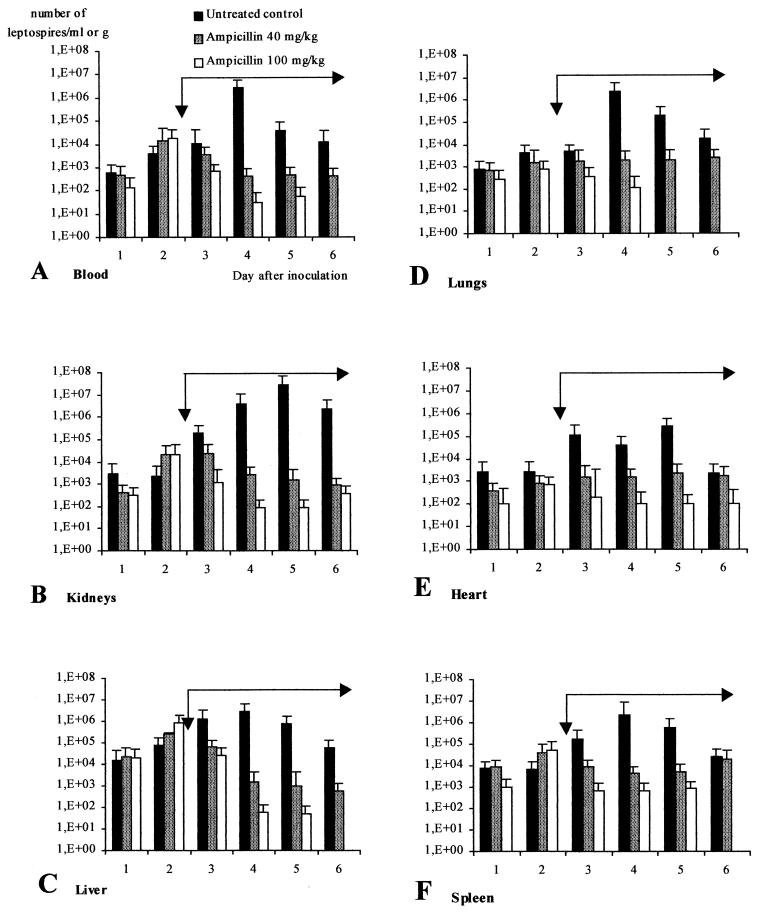
Clinically, untreated infected hamsters showed prostration during the last 2 days of the 6-day experiment. When hamsters were sacrificed at day 6, discrete signs of jaundice and hemorrhages were seen at autopsy. Leptospires were present in blood and other selected tissues as early as 24 h postinfection (p.i.) in all animals. Figure 1 shows the change over time in the densities of virulent leptospires. Bacteremia was at a low level during the first 3 days of infection, averaging 104 leptospires/ml, before reaching a peak of more than 106 leptospires/ml at 4 days p.i. (Fig. 1A). The liver (Fig. 1C), lungs (Fig. 1D), and spleen (Fig. 1F) followed the same profile with a maximum at 4 days p.i (at least 106 leptospires/g). The leptospiral density in the heart (Fig. 1E) was always less than 106 leptospires/g. The highest density was observed in the kidneys (Fig. 1B), with more than 107 leptospires/g at 5 days p.i. These data illustrate the importance of the kidney as a reservoir organ.
FIG. 1.
Efficacy of ampicillin (40 and 100 mg/kg) for treating leptospirosis in hamsters (three hamsters per day in duplicate). Ampicillin was administered once daily in a 4-day regimen (long black arrow) from day 3 (short black arrow) to day 6. Results are mean values of leptospiral density + SDs (error bars).
Ampicillin treatment.
Figure 1 shows changes with time in the density of virulent leptospires after treatment with 40 and 100 mg of ampicillin/kg. Twenty-four hours after starting the 100-mg/kg ampicillin treatment (day 3 p.i.), a significant decrease in the leptospiral density was observed in all tissues. At day 4 p.i., that decrease was significant for both ampicillin dosages (P < 0.05). The dose of 40 mg/kg was unable to clear leptospires from the host during the 4 days of treatment: at least 103 leptospires per g or per ml were detected in target organs. With the 100-mg/kg treatment, blood (Fig. 1A), liver (Fig. 1C), and spleen (Fig. 1F) were cleared of leptospires at day 6 p.i. (P < 0.05). However, persistent leptospires were detected in the kidneys (102 to 103 leptospires/g [Fig. 1B]) and in the heart (102 leptospires/g [Fig. 1E]).
Doxycycline treatment.
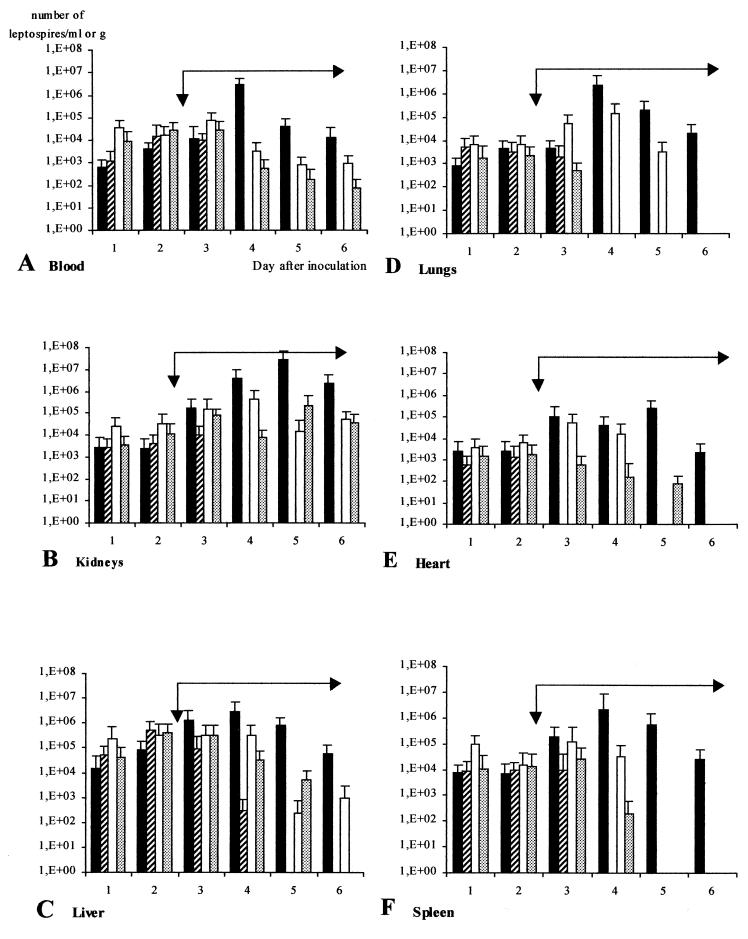
Figure 2 shows changes with time in the densities of virulent leptospires after treatment with doxycycline at 10 mg/kg (striped bars). Two days after the outset of the treatment, leptospires were cleared from blood (Fig. 2A), kidneys (Fig. 2B), lungs (Fig. 2D), heart (Fig. 2E), and spleen (Fig. 2F) (P < 0.05). Clearance from liver (Fig. 2C) did not occur until day 5, after 3 days of treatment.
FIG. 2.
Efficacies of doxycycline (10 mg/kg [striped bars]) and ofloxacin (15 and 30 mg/kg [open and shaded bars, respectively]) for treating leptospirosis in hamsters (three hamsters per day in duplicate). Antibiotics were administered once daily in a 4-day regimen (long black arrow) from day 3 (short black arrow) to day 6. Results are mean values of leptospiral density + SDs (error bars). Solid bars, untreated controls.
Ofloxacin treatment.
Figure 2 shows the time course of the densities of virulent leptospires after treatment with ofloxacin at doses of 15 and 30 mg/kg. A decrease in leptospiremia was observed 2 days after treatment (day 4), irrespective of the dose (Fig. 2A). However, even with the 30-mg/kg treatment, leptospires were still detected in blood at day 6. In the kidneys (Fig. 2B), the density of leptospires always remained between 104 and 105/g, regardless of the regimen applied (5.4 × 104 to 1.5 × 105 per g with the 15-mg/kg treatment and 3.8 × 104 to 7.6 × 104 per g with the 30-mg/kg treatment). The liver (Fig. 2C) was negative only at day 6 with the 30-mg/kg dose. Conversely, the lungs were cleared with the higher dose 2 days after beginning the treatment (P < 0.05). Heart and spleen showed a similar time course; both doses cleared detectable leptospires from these tissues between days 5 and 6.
For the three therapeutic trials, samples negative by the quantitative microplate assay were confirmed as negative using the filter hybridization test.
DISCUSSION
Various serovars of pathogenic leptospires have been reported to be susceptible in vitro to all of the commonly used antibiotics (6, 17, 20).
Since the 1950s, however, there has been controversy about the efficacy of antibiotics for treating human leptospirosis. This is essentially because of differences between the clinical trials (timing of the treatment, differences in doses, and absence of control groups). Studies with homogeneous groups of patients are difficult because of the broad range of clinical forms of the disease. Three controlled randomized studies have been designed to clarify the situation. McClain et al. (14) concluded that oral doxycycline reduced the clinical expression of anicteric systemic leptospirosis and prevented leptospiruria. Conflicting results have been reported for penicillin. Watt et al. (25) reported that penicillin had a similar impact on these two parameters in patients with severe leptospirosis (with icterus and/or renal insufficiency). On the other hand, Edwards et al. (5) concluded that penicillin has little effect on clinical outcome in icteric leptospirosis. In these studies, leptospires in blood and urine were detected by culture, which is known to have poor efficiency (9). For the mild forms of human leptospirosis, there are no clinical trials to support the antibiotic therapies currently prescribed (10), which are based on ampicillin, amoxicillin, or doxycycline.
The efficacy of antibiotics has also been evaluated in two studies using laboratory animals. Alexander and Rule (2), in a study of hamsters infected with serovar bataviae, reported that seven beta-lactams and two cyclins were effective when animals were treated early in the course of the disease. Furthermore, ampicillin and doxycycline prevented the occurrence of leptospiruria in survivors. Another study showed that ciprofloxacin was effective in hamsters infected intraperitoneally with serovar budapest (20). In these two studies, the efficacy of antibiotic therapy was assessed from the survival of challenged animals and by culture of leptospires from target organs.
In our study, we used a sensitive quantitative PCR to monitor leptospiral density in target organs, in order to monitor the course of the disease and the effectiveness of the treatment. In contrast to the previous studies with experimental animals (2, 20), we tried to define experimental conditions as close as possible to those in a natural infection: (i) the infecting serovar was icterohaemorrhagiae, which is involved in the majority of human infections (9), and (ii) the subcutaneous route of infection was selected instead of the intraperitoneal one. The in vivo efficacy of the selected antibiotics in hamsters was evaluated by comparing leptospiral density in target organs between control and treated animals. The choice of antibiotics and their respective doses was based on previous experimental studies in hamsters (2, 20) and on current recommendations for the treatment of the human disease (10). Antibiotics were administered 3 days after challenge with leptospires, this being a compromise between early and delayed treatments investigated in previous studies (2, 20). The timing of the initiation of antibiotic treatment in human leptospirosis has been reported as critical to success in preventing severe disease manifestations (25).
In our model, ampicillin (both 40 and 100 mg/kg) reduced the number of leptospires in all the target organs tested (Fig. 1). A dose of 40 mg/kg was unable to clear the leptospires from any of the organs studied; a dose of 100 mg/kg was more effective, although leptospires remained detectable in kidneys and heart. Indeed, the susceptibility of leptospires to ampicillin in vitro (17) did not predict in vivo efficacy in our model. This seems consistent with a reported study of leptospiruria in hamsters treated orally or subcutaneously with ampicillin (2). Possibly the administration of ampicillin only once daily in our study might be inappropriate for eradication of leptospires from all tissues.
Conversely, doxycycline is effective in vivo early in the course of the disease: clearance of leptospires was observed in all the target organs on the second day of treatment, except in liver, which required an additional day. With a daily administration, both ofloxacin regimens were ineffective in blood and kidneys. Lungs, spleen, liver, and heart were cleared of leptospires with the dose of 30 mg/kg.
The high capacity of some quinolones to diffuse in cerebrospinal fluid and in aqueous humor could lead to their use in neurologic (9) or ophthalmologic (4) forms of leptospirosis; this possibility was not explored in our model. It would thus be of interest to monitor the leptospiral density in target organs with various administration schedules of different quinolones.
It is difficult to demonstrate conclusively that quantitative PCR data indicate the presence of viable leptospires in target organs, and the clinical relevance of that is difficult to state (3, 15). However, leptospires have a very fragile outer membrane which is subject, during the course of the disease, to an antibody immune response linked with the complement system (1, 9). The consequent elimination of dead leptospires by the immune system is extremely efficient (9). Classically, in the acute phase of the human disease, which lasts about 10 days, leptospires can be cultured from blood or cerebrospinal fluid (9). When a specific antibody response is detected, leptospires usually disappear from the blood. During the second clinical phase, which may last up to several weeks, bacteriuria is intermittent (9). However, patients treated for leptospirosis caused by some serovars may have a positive PCR for months when urine is tested (3) or up to 40 days when blood is tested (15), without any evident signs of clinical illness. In contrast, an experimental infection study of serovar hardjo in cows (11) and a clinical study of cattle naturally infected with hardjo (12) both showed correlation between negative PCR, cessation of urinary shedding, and clinical recovery. These data emphasize the complexity of the physiopathology of leptospirosis in both humans and animals and the differences in the pathogenicity of leptospires belonging to various species and serovars (9).
The persistence of leptospires in humans after the initial clinical period, in spite of treatment with beta-lactams (3, 15), suggests that these antibiotics are ineffective in clearing leptospires that are located in protected sites. Invasive phenotypes of virulent leptospires, which have been demonstrated in vitro (16), may be able to invade such sites. Our data validate previous evidence that doxycycline has therapeutic and prophylactic value in leptospirosis (14, 21). Unlike the natural cyclins known for their nephrotoxicity, doxycycline has the potential for use in leptospirosis; its intestinal elimination is modulated according to the level of renal insufficiency (19). Our results suggest a second potential value for doxycycline in the treatment of leptospiral pneumonia, a severe manifestation of the disease which is being detected more frequently (9, 13, 22, 26). Nevertheless, doxycycline cannot be recommended for exclusive use, as it is absolutely contraindicated, for example, in children and in pregnancy. Additional experimental studies are therefore needed to validate alternative therapeutic strategies using other antibiotics.
In our model, eradication of leptospires in all tested tissues was only observed with doxycycline (10 mg/kg). On the other hand, other treatments selectively eradicated leptospires from some organs. Eradication is not, however, always necessary for an antibiotic to be considered efficacious. The timing of the antibody response is critical to the control of acute leptospiral infection in rodents (9). BALB/c mice are able to resist peritoneal inoculation with 1010 serovar pomona leptospires by producing circulating antibodies as early as 2 days after infection (1). It appears that, when leptospiral density is below a critical level, the immune system can overcome the infection. In humans, this hypothesis is supported by the finding that, during acute icterohemorrhagic leptospirosis, a leptospiremia under the threshold of 104/ml predicted recovery of the patients (23).
In conclusion, our study demonstrates the value of modeling antibiotic treatment of leptospirosis in laboratory animals. The combination of an animal model and a sensitive PCR-based method for measuring bacterial density in the target organs allows objective evaluation of the efficacy of treatment. Longer-term studies could be performed to investigate persistent leptospirosis; this would require the use of adapted animal models, such as the horse for ocular disorders (8) or the squirrel monkey for Weil's disease (18). Studies of other antibiotic regimens, including associations of antibiotics, are also required to determine the most-suitable antibiotic therapies for human leptospirosis.
Acknowledgments
This work was supported by the Institut Pasteur, Paris (International Network of Pasteur Institutes and Associated Institutes).
Warm thanks are due to Rod Chappel (National Serology Laboratory, Melbourne, Australia) for editorial contributions and to Guy Baranton (Institut Pasteur, Paris) for scientific support.
REFERENCES
- 1.Adler, B., and S. Faine. 1976. Susceptibility of mice treated with cyclophosphamide to lethal infection with Leptospira interrogans serovar pomona. Infect. Immun. 14:703-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexander, A. D., and P. L. Rule. 1986. Penicillins, cephalosporins, and tetracyclines in treatment of hamsters with fatal leptospirosis. Antimicrob. Agents Chemother. 30:835-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bal, A. E., C. Gravekamp, R. A. Hartskeerl, J. De Meza-Brewster, H. Korver, and W. J. Terpstra. 1994. Detection of leptospires in urine by PCR for early diagnosis of leptospirosis. J. Clin. Microbiol. 32:1894-1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chu, K. M., P. Rathinam, P. Namperumalsamy, and D. Dean. 1998. Identification of Leptospira species in the pathogenesis of uveitis and determination of clinical ocular characteristics in South India. J. Infect. Dis. 177:1314-1321. [DOI] [PubMed] [Google Scholar]
- 5.Edwards, C. N., G. D. Nicholson, T. A. Hassell, C. O. Everard, and J. Callender. 1988. Penicillin therapy in icteric leptospirosis. Am. J. Trop. Med. Hyg. 39:388-390. [DOI] [PubMed] [Google Scholar]
- 6.Ellinghausen, H. C. 1983. Growth, cultural characteristics, and antibacterial sensitivity of Leptospira interrogans serovar hardjo. Cornell Vet. 73:225-239. [PubMed] [Google Scholar]
- 7.Ellinghausen, H. C., and W. G. McCullough. 1965. Nutrition of Leptospira pomona and growth of 13 other serotypes: fractionation of oleic albumin complex and a medium of bovine albumin and polysorbate 80. Am. J. Vet. Res. 26:45-51. [PubMed] [Google Scholar]
- 8.Faber, N. A., M. Crawford, R. B. Lefebvre, N. C. Buyukmihci, J. E. Madigan, and N. H. Willits. 2000. Detection of Leptospira spp. in the aqueous humor of horses with naturally acquired recurrent uveitis. J. Clin. Microbiol. 38:2731-2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faine, S., B. Adler, C. Bolin, and P. Perolat. 1999. Leptospira and leptospirosis, 2nd ed. MediSci, Armadale, Australia.
- 10.Farr, R. W. 1995. Leptospirosis. Clin. Infect. Dis. 21:1-8. [DOI] [PubMed] [Google Scholar]
- 11.Gerritsen, M. J., M. J. Koopmans, and T. Olyhoek. 1993. Effect of streptomycin treatment on the shedding of and the serologic responses to Leptospira interrogans serovar hardjo subtype hardjobovis in experimentally infected cows. Vet. Microbiol. 38:129-135. [DOI] [PubMed] [Google Scholar]
- 12.Gerritsen, M. J., M. J. Koopmans, T. C. Dekker, M. C. De Jong, A. Moerman, and T. Olyhoek. 1994. Effective treatment with dihydrostreptomycin of naturally infected cows shedding Leptospira interrogans serovar hardjo subtype hardjobovis. Am. J. Vet. Res. 55:339-343. [PubMed] [Google Scholar]
- 13.Kim, J. S., C. W. Lee, D. K. Oh., S. D. In, Y. H. Lee, and W. H. Cho. 1985. An epidemiological study on the identification of cause for epidemic pulmonary hemorrhagic fever. J. Korean Med. Assoc. 28:77-87. [Google Scholar]
- 14.McClain, J. B. L., W. R. Ballou, S. M. Harrison, and D. L. Steinway. 1984. Doxycycline therapy for leptospirosis. Ann. Int. Med. 100:696-698. [DOI] [PubMed] [Google Scholar]
- 15.Merien, F., G. Baranton, and P. Perolat. 1995. Comparison of polymerase chain reaction with microagglutination test and culture for diagnosis of leptospirosis. J. Infect. Dis. 172:281-285. [DOI] [PubMed] [Google Scholar]
- 16.Merien, F., G. Baranton, and P. Perolat. 1997. Invasion of Vero cells and induction of apoptosis in macrophages by pathogenic Leptospira interrogans are correlated with virulence. Infect. Immun. 65:729-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oie, S., K. Hironaga, A. Koshiro, H. Honishi, and Z. Yoshii. 1983. In vitro susceptibilities of five Leptospira strains to 16 antimicrobial agents. Antimicrob. Agents Chemother. 24:905-908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perolat, P., J. P. Poingt, J. C. Vie, C. Jouaneau, G. Baranton, and J. Gysin. 1992. Occurrence of severe leptospirosis in a breeding colony of squirrel monkeys. Am. J. Trop. Med. Hyg. 46:538-545. [DOI] [PubMed] [Google Scholar]
- 19.Saivin, S., and G. Houin. 1988. Clinical pharmacokinetics of doxycycline and minocycline. Clin. Pharmacokinet. 15:355-366. [DOI] [PubMed] [Google Scholar]
- 20.Shalit, I., A. Barnea, and A. Shahar. 1989. Efficacy of ciprofloxacin against Leptospira interrogans serogroup icterohaemorrhagiae. Antimicrob. Agents Chemother. 33:788-789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takafuji, E. T., J. W. Kirkpatrick, R. N. Miller, J. J. Karwacki, P. W. Kelley, M. R. Gray, K. M. McNeill, H. L. Timboe, R. E. Kane, and J. L. Sanchez. 1984. An efficacy trial of doxycycline chemoprophylaxis against leptospirosis. N. Engl. J. Med. 23:497-500. [DOI] [PubMed] [Google Scholar]
- 22.Trevejo, R. T., J. G. Rigau-Pérez, D. A. Ashford, E. M. McClure, C. Jarquin-González, J. J. Amador, J.O. de los Reyes, A. Gonzalez, S. R. Zaki, W. Ju Shieh, R. G. McLean, R. S. Nasci, R. S. Weyant, C. A. Bolin, S. L. Bragg, B. A. Perkins, and R. A. Spiegel. 1998. Epidemic leptospirosis associated with pulmonary hemorrhage-Nicaragua, 1995. J. Infect. Dis. 178:1457-1463. [DOI] [PubMed] [Google Scholar]
- 23.Truccolo, J., O. Serrais, F. Merien, and P. Perolat. 2001. Follow-up of human leptospirosis: evidence of a critical threshold for the vital prognosis using a PCR quantitative assay. FEMS Microbiol. Lett. 204:317-321. [DOI] [PubMed] [Google Scholar]
- 24.Van den Ingh, T. S. G. A. M., and E. G. Hartman. 1986. Pathology of acute Leptospira interrogans serotype Icterohaemorrhagiae infection in the Syrian hamster. Vet. Microbiol. 12:367-376. [DOI] [PubMed] [Google Scholar]
- 25.Watt, G., L. P. Padre, M. L. Tuazon, C. Calubaquib, E. Santiago, C. P. Ranoa, and L. W. Laughlin. 1988. Placebo-controlled trial of intravenous penicillin for severe and late leptospirosis. Lancet 27:433-435. [DOI] [PubMed] [Google Scholar]
- 26.Yersin, C., P. Bovet, F. Merien, J. Clément, M. Laille, M. Van Ranst, and P. Perolat. 2000. Pulmonary haemorrhage as a predominant cause of death in leptospirosis in Seychelles. Trans. R. Soc. Trop. Med. Hyg. 94:71-76. [DOI] [PubMed] [Google Scholar]