Abstract
The antibacterial activity of DQ-113, formerly D61-1113, was compared with those of antibacterial agents currently available. MICs at which 90% of the isolates tested are inhibited (MIC90s) of DQ-113 against clinical isolates of methicillin-susceptible and -resistant Staphylococcus aureus and methicillin-susceptible and -resistant coagulase-negative staphylococci were 0.03, 0.008, 0.03, and 0.06 μg/ml, respectively. Moreover, DQ-113 showed the most potent activity against ofloxacin-resistant and methicillin-resistant S. aureus, with a MIC90 of 0.25μg/ml. DQ-113 inhibited the growth of all strains of Streptococcus pneumoniae, including penicillin-resistant strains, and Streptococcus pyogenes at 0.06 μg/ml, and DQ-113 was more active than the other quinolones tested against Enterococcus faecalis and Enterococcus faecium with MIC90s of 0.25 and 2 μg/ml, respectively. Against vancomycin-resistant enterococci, DQ-113 showed the highest activity among the reference compounds, with a MIC range from 0.25 to 2 μg/ml. DQ-113 also showed a potent activity against Haemophilus influenzae, including ampicillin-resistant strains (MIC90, 0.015 μg/ml), and Moraxella catarrhalis (MIC90, 0.03 μg/ml). The activity of DQ-113 was roughly comparable to that of levofloxacin against all species of Enterobacteriaceae. The MICs of DQ-113 against ofloxacin-susceptible Pseudomonas aeruginosa ranged from 0.25 to 2 μg/ml, which were four times higher than those of ciprofloxacin. From these results, DQ-113 showed the most potent activity against gram-positive pathogens among antibacterial agents tested.
Multidrug-resistant gram-positive pathogens, including methicillin-resistant Staphylococcus aureus (MRSA), penicillin-resistant Streptococcus pneumoniae (PRSP), and vancomycin-resistant enterococci (VRE), have become a serious problem in the medical community (1-5, 7, 9, 10). There are a few therapeutic agents, such as vancomycin, quinupristin/dalfopristin, and linezolid; however, these agents showed some problems, e.g., resistance mutations and/or side effects (1, 3, 4, 6, 8, 9). These problems have been the driving force for the development of new antibacterial agents that would be applicable to infections caused by multidrug-resistant gram-positive pathogens.
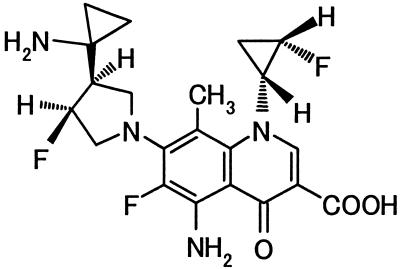
DQ-113 is a new fluoroquinolone whose chemical structure shown in Fig. 1. In this study, we compared the antimicrobial activity of DQ-113 with those of ciprofloxacin, gatifloxacin, gemifloxacin, levofloxacin, moxifloxacin, sitafloxacin, sparfloxacin, tosufloxacin, and T-3811ME (BMS284756) and other classes of antibacterial agents, such as β-lactam antibiotics, against freshly isolated bacteria.
FIG. 1.
Chemical structure of DQ-113.
DQ-113, ciprofloxacin, gatifloxacin, gemifloxacin, levofloxacin, moxifloxacin, sitafloxacin, sparfloxacin, tosufloxacin, T-3811ME (BMS284756), vancomycin, teicoplanin, oxacillin, benzylpenicillin, ampicillin, imipenem, cefaclor, cefotaxime, arbekacin, minocycline, and linezolid were used in this study. All quinolones and linezolid were synthesized at Daiichi Pharmaceutical Co. Ltd., Tokyo, Japan. The other antibiotics were purchased from their manufacturers or Sigma Aldrich Japan (Tokyo, Japan).
A total of 608 strains, which were collected by the Levofloxacin Surveillance Group in 1998 and 2000 from patients in Japan (11), were used (one isolate per patient). Twenty-six strains of VRE were collected in Europe in 1997 and 1998. The reference strains were included as internal controls throughout the study.
The MICs were determined by the standard agar dilution method with Mueller-Hinton agar (Difco Laboratories, Detroit, Mich.) (6). Mueller-Hinton agar supplemented with 2% NaCl was used for staphylococci, Mueller-Hinton agar supplemented with 5% sheep blood was used for streptococci and Moraxella catarrhalis, and Mueller-Hinton agar supplemented with 3% Fildes enrichment was used for Haemophilus influenzae. GC agar (Difco) was used for Neisseria gonorrhoeae. Drug-containing agar plates were incubated with one loopful (5 μl) of inoculum corresponding to about 104 CFU per spot and were incubated at 35°C for 18 h. N. gonorrhoeae was incubated under 5% CO2. The MIC was defined as the lowest drug concentration which prevented visible growth of bacteria.
Tables 1 and 2 compare the activity of DQ-113 against gram-positive and -negative bacteria to those of the reference drugs. The MICs at which 90% of the isolates tested are inhibited (MIC90s) of DQ-113 against methicillin-susceptible S. aureus (MSSA) and methicillin-susceptible coagulase-negative staphylococci (MSCNS) were both 0.03 μg/ml. MIC90s against ofloxacin-susceptible MRSA, ofloxacin-resistant (MIC of ofloxacin, ≥8 μg/ml) MRSA, and methicillin-resistant coagulase-negative staphylococci (MRCNS) were 0.008, 0.25, and 0.06 μg/ml, respectively. The antibacterial activity against MSSA was twofold higher than those of T-3811ME, gemifloxacin, and imipenem; fourfold higher than those of sitafloxacin, tosufloxacin, moxifloxacin, and minocycline; and at least eightfold higher than those of the other reference compounds, including vancomycin, teicoplanin, and linezolid, at MIC90 levels. Against MSCNS, DQ-113 showed activity comparable to that of imipenem, and activity was at least eightfold higher than those of the other reference compounds. Against ofloxacin-susceptible MRSA, DQ-113 activity was fourfold higher than that of T-3811ME and at least eightfold higher than those of the other reference compounds at MIC90 levels. Furthermore, DQ-113 showed the highest activity against ofloxacin-resistant MRSA among the compounds tested. Against MRCNS, the MIC90 of DQ-113 was at least eightfold lower than those of the reference compounds.
TABLE 1.
Antibacterial activities of DQ-113 and reference compounds against gram-positive bacteriaa
| Organism (no. of strains) and compound | MIC (μg/ml)
|
|
Organism (no. of strains) and compound | MIC (μg/ml)
|
||||
|---|---|---|---|---|---|---|---|---|
| Range | MIC50 | MIC90 | Range | MIC50 | MIC90 | |||
| MSSA (25) | ||||||||
| DQ-113 | ≤0.004-0.06 | 0.008 | 0.03 | |||||
| Sitafloxacin | 0.008-0.12 | 0.03 | 0.12 | |||||
| Levofloxacin | 0.12-1 | 0.25 | 0.5 | |||||
| Ciprofloxacin | 0.25-4 | 0.5 | 1 | |||||
| Sparfloxacin | 0.06-0.25 | 0.12 | 0.25 | |||||
| Tosufloxacin | 0.015-0.25 | 0.06 | 0.12 | |||||
| Gatifloxacin | 0.06-0.5 | 0.12 | 0.25 | |||||
| Moxifloxacin | 0.06-0.25 | 0.06 | 0.12 | |||||
| T-3811ME | ≤0.004-0.12 | 0.03 | 0.06 | |||||
| Gemifloxacin | 0.03-0.25 | 0.06 | 0.06 | |||||
| Imipenem | 0.03-0.06 | 0.06 | 0.06 | |||||
| Arbekacin | 0.5-4 | 4 | 4 | |||||
| Minocycline | 0.06-0.25 | 0.12 | 0.12 | |||||
| Vancomycin | 1 | 1 | 1 | |||||
| Teicoplanin | 0.25-1 | 0.5 | 0.5 | |||||
| Linezolid | 2 | 2 | 2 | |||||
| Oxacillin | 0.25-1 | 0.5 | 1 | |||||
| MRSA | ||||||||
| Ofloxacin-susceptible (24) | ||||||||
| DQ-113 | ≤0.004-0.015 | ≤0.004 | 0.008 | |||||
| Sitafloxacin | 0.008-0.06 | 0.03 | 0.06 | |||||
| Levofloxacin | 0.12-1 | 0.25 | 0.5 | |||||
| Ciprofloxacin | 0.25-2 | 0.5 | 1 | |||||
| Sparfloxacin | 0.03-0.12 | 0.06 | 0.12 | |||||
| Tosufloxacin | 0.015-0.12 | 0.06 | 0.06 | |||||
| Gatifloxacin | 0.06-0.25 | 0.12 | 0.25 | |||||
| Moxifloxacin | 0.03-0.25 | 0.12 | 0.12 | |||||
| T-3811ME | 0.008-0.06 | 0.03 | 0.03 | |||||
| Gemifloxacin | 0.015-0.06 | 0.06 | 0.06 | |||||
| Imipenem | 0.5-32 | 4 | 32 | |||||
| Arbekacin | 4->128 | 16 | 64 | |||||
| Minocycline | 0.12-8 | 0.12 | 8 | |||||
| Vancomycin | 1-2 | 1 | 2 | |||||
| Teicoplanin | 0.5-2 | 0.5 | 2 | |||||
| Linezolid | 1-2 | 2 | 2 | |||||
| Oxacillin | 16->128 | 64 | >128 | |||||
| Ofloxacin-resistant (25) | ||||||||
| DQ-113 | 0.03-2 | 0.06 | 0.25 | |||||
| Sitafloxacin | 0.25-32 | 1 | 4 | |||||
| Levofloxacin | 4->128 | 16 | 64 | |||||
| Ciprofloxacin | 16->128 | 128 | >128 | |||||
| Sparfloxacin | 2->128 | 8 | 32 | |||||
| Tosufloxacin | 1-64 | 32 | 32 | |||||
| Gatifloxacin | 2->128 | 8 | 16 | |||||
| Moxifloxacin | 1-64 | 4 | 8 | |||||
| T-3811ME | 0.25-64 | 2 | 8 | |||||
| Imipenem | 4-128 | 64 | 128 | |||||
| Arbekacin | 4-64 | 8 | 32 | |||||
| Minocycline | 0.12-32 | 8 | 32 | |||||
| Vancomycin | 0.5-2 | 1 | 2 | |||||
| Teicoplanin | 0.5-4 | 1 | 4 | |||||
| Linezolid | 1-2 | 2 | 2 | |||||
| Oxacillin | 64->128 | >128 | >128 | |||||
| MSCNS (27) | ||||||||
| DQ-113 | ≤0.004-0.06 | 0.008 | 0.03 | |||||
| Sitafloxacin | 0.008-0.25 | 0.03 | 0.25 | |||||
| Levofloxacin | 0.12-8 | 0.25 | 4 | |||||
| Ciprofloxacin | 0.06-64 | 0.25 | 8 | |||||
| Sparfloxacin | 0.03-8 | 0.12 | 4 | |||||
| Tosufloxacin | 0.015-16 | 0.06 | 4 | |||||
| Gatifloxacin | 0.06-4 | 0.25 | 2 | |||||
| Moxifloxacin | 0.03-4 | 0.12 | 1 | |||||
| T-3811ME | 0.015-4 | 0.06 | 1 | |||||
| Gemifloxacin | 0.008-2 | 0.06 | 0.25 | |||||
| Imipenem | 0.015-0.03 | 0.03 | 0.03 | |||||
| Arbekacin | 1-64 | 4 | 16 | |||||
| Minocycline | 0.06-16 | 0.12 | 0.5 | |||||
| Vancomycin | 0.5-4 | 2 | 2 | |||||
| Teicoplanin | 0.25-16 | 1 | 2 | |||||
| Linezolid | 1-2 | 1 | 2 | |||||
| Oxacillin | 0.06-0.25 | 0.25 | 0.25 | |||||
| MRCNS (36) | ||||||||
| DQ-113 | ≤0.004-0.12 | 0.03 | 0.06 | |||||
| Sitafloxacin | 0.008-0.5 | 0.12 | 0.5 | |||||
| Levofloxacin | 0.12-32 | 4 | 16 | |||||
| Ciprofloxacin | 0.12-128 | 8 | 64 | |||||
| Sparfloxacin | 0.06-16 | 4 | 8 | |||||
| Tosufloxacin | 0.03-16 | 4 | 16 | |||||
| Gatifloxacin | 0.12-4 | 2 | 4 | |||||
| Moxifloxacin | 0.06-8 | 1 | 4 | |||||
| T-3811ME | 0.03-4 | 1 | 4 | |||||
| Gemifloxacin | 0.015-4 | 0.5 | 2 | |||||
| Imipenem | 0.12->128 | 64 | 128 | |||||
| Arbekacin | 0.25-128 | 8 | 32 | |||||
| Minocycline | 0.12-16 | 0.5 | 4 | |||||
| Vancomycin | 1-4 | 1 | 2 | |||||
| Teicoplanin | 0.25-32 | 1 | 8 | |||||
| Linezolid | 1-2 | 2 | 2 | |||||
| Oxacillin | 1->128 | 128 | >128 | |||||
| PSSP (25) | ||||||||
| DQ-113 | 0.008-0.06 | 0.03 | 0.03 | |||||
| Sitafloxacin | 0.03-0.12 | 0.12 | 0.12 | |||||
| Levofloxacin | 1-4 | 2 | 2 | |||||
| Ciprofloxacin | 1-8 | 2 | 4 | |||||
| Sparfloxacin | 0.25-1 | 0.5 | 1 | |||||
| Tosufloxacin | 0.25-0.5 | 0.25 | 0.5 | |||||
| Gatifloxacin | 0.25-1 | 0.5 | 0.5 | |||||
| Moxifloxacin | 0.12-0.5 | 0.25 | 0.5 | |||||
| T-3811ME | 0.03-0.12 | 0.12 | 0.12 | |||||
| Gemifloxacin | 0.03-0.12 | 0.12 | 0.12 | |||||
| Cefotaxime | 0.008-0.25 | 0.12 | 0.25 | |||||
| Imipenem | 0.008-0.03 | 0.015 | 0.015 | |||||
| Vancomycin | 0.25-0.5 | 0.5 | 0.5 | |||||
| Teicoplanin | 0.12-0.5 | 0.5 | 0.5 | |||||
| Linezolid | 0.5-1 | 1 | 1 | |||||
| Ampicillin | 0.015-0.12 | 0.06 | 0.12 | |||||
| Benzylpenicillin | 0.015-0.06 | 0.06 | 0.06 | |||||
| PISP and PRSP (50) | ||||||||
| DQ-113 | ≤0.008-0.015 | ≤0.008 | 0.015 | |||||
| Sitafloxacin | 0.015-0.06 | 0.03 | 0.06 | |||||
| Levofloxacin | 0.25-1 | 1 | 1 | |||||
| Ciprofloxacin | 0.25-4 | 1 | 2 | |||||
| Sparfloxacin | 0.12-0.5 | 0.25 | 0.5 | |||||
| Tosufloxacin | 0.06-0.25 | 0.12 | 0.25 | |||||
| Gatifloxacin | 0.06-0.5 | 0.25 | 0.25 | |||||
| Moxifloxacin | 0.06-0.25 | 0.12 | 0.25 | |||||
| T-3811ME | 0.015-0.06 | 0.03 | 0.06 | |||||
| Gemifloxacin | 0.015-0.06 | 0.03 | 0.06 | |||||
| Imipenem | 0.12-0.5 | 0.25 | 0.5 | |||||
| Vancomycin | 0.25-0.5 | 0.5 | 0.5 | |||||
| Teicoplanin | 0.12-1 | 0.25 | 0.5 | |||||
| Linezolid | 0.25-0.5 | 0.5 | 0.5 | |||||
| Benzylpenicillin | 1-4 | 2 | 2 | |||||
| S. pyogenes (25) | ||||||||
| DQ-113 | ≤0.004-0.015 | 0.008 | 0.015 | |||||
| Sitafloxacin | 0.015-0.06 | 0.03 | 0.06 | |||||
| Levofloxacin | 0.06-1 | 0.25 | 0.5 | |||||
| Ciprofloxacin | 0.12-1 | 0.25 | 1/PICK>{tt} | |||||
| Sparfloxacin | 0.12-1 | 0.5 | 1 | |||||
| Tosufloxacin | 0.06-1 | 0.12 | 0.5 | |||||
| Gatifloxacin | 0.12-0.5 | 0.25 | 0.5 | |||||
| Moxifloxacin | 0.12-0.5 | 0.12 | 0.5 | |||||
| T-3811ME | 0.03-0.25 | 0.06 | 0.25 | |||||
| Ceftazidime | 0.12-0.5 | 0.12 | 0.25 | |||||
| Cefotaxime | 0.008-0.03 | 0.015 | 0.015 | |||||
| Imipenem | ≤0.004-0.015 | ≤0.004 | ≤0.004 | |||||
| Vancomycin | 0.06-0.5 | 0.25 | 0.25 | |||||
| Teicoplanin | 0.12-1 | 0.25 | 0.5 | |||||
| Linezolid | 1-2 | 1 | 2 | |||||
| Ampicillin | 0.015-0.12 | 0.015 | 0.03 | |||||
| E. faecalis (25) | ||||||||
| DQ-113 | 0.015-0.25 | 0.03 | 0.25 | |||||
| Sitafloxacin | 0.06-2 | 0.12 | 2 | |||||
| Levofloxacin | 0.5-64 | 1 | 32 | |||||
| Ciprofloxacin | 0.25-64 | 1 | 32 | |||||
| Sparfloxacin | 0.25-32 | 0.5 | 16 | |||||
| Tosufloxacin | 0.25-16 | 0.25 | 16 | |||||
| Gatifloxacin | 0.25-16 | 0.5 | 16 | |||||
| Moxifloxacin | 0.25-16 | 0.25 | 8 | |||||
| T-3811ME | 0.12-4 | 0.25 | 2 | |||||
| Cefotaxime | 0.25->128 | >128 | >128 | |||||
| Imipenem | 0.06-8 | 2 | 4 | |||||
| Vancomycin | 0.5-4 | 1 | 2 | |||||
| Teicoplanin | 0.12-0.5 | 0.25 | 0.5 | |||||
| Linezolid | 2 | 2 | 2 | |||||
| Ampicillin | 0.5-4 | 1 | 2 | |||||
| E. faecium (23) | ||||||||
| DQ-113 | 0.06-4 | 0.5 | 2 | |||||
| Sitafloxacin | 0.12-8 | 1 | 8 | |||||
| Levofloxacin | 2->128 | 32 | 128 | |||||
| Ciprofloxacin | 1->128 | 32 | 64 | |||||
| Sparfloxacin | 0.5-64 | 16 | 32 | |||||
| Tosufloxacin | 0.5-16 | 8 | 16 | |||||
| Gatifloxacin | 1-64 | 8 | 32 | |||||
| Moxifloxacin | 0.5-64 | 4 | 16 | |||||
| T-3811ME | 0.25-64 | 4 | 16 | |||||
| Cefotaxime | >128 | >128 | >128 | |||||
| Imipenem | 8->128 | >128 | >128 | |||||
| Vancomycin | 0.5-2 | 1 | 2 | |||||
| Teicoplanin | 0.25-1 | 0.5 | 1 | |||||
| Linezolid | 1-2 | 2 | 2 | |||||
| Ampicillin | 2-128 | 128 | 128 | |||||
| VRE (26) | ||||||||
| DQ-113 | 0.25-2 | 0.5 | 2 | |||||
| Sitafloxacin | 1-8 | 2 | 8 | |||||
| Levofloxacin | 16-64 | 32 | 64 | |||||
| Ciprofloxacin | 32->128 | 64 | >128 | |||||
| Sparfloxacin | 8-64 | 32 | 64 | |||||
| Tosufloxacin | 16-32 | 32 | 32 | |||||
| Gatifloxacin | 4-64 | 16 | 32 | |||||
| Moxifloxacin | 2-32 | 16 | 32 | |||||
| T-3811ME | 1-32 | 4 | 8 | |||||
| Gemifloxacin | 1-32 | 4 | 32 | |||||
| Imipenem | 2->128 | 128 | >128 | |||||
| Arbekacin | 2->128 | 8 | 64 | |||||
| Minocycline | 0.06-16 | 0.12 | 16 | |||||
| Vancomycin | >128 | >128 | >128 | |||||
| Teicoplanin | 0.5->128 | 32 | 128 | |||||
| Linezolid | 2 | 2 | 2 | |||||
Abbreviations and resistance criteria are as follows: MSSA, oxacillin MIC ≤ 2 μg/ml; MRSA, oxacillin MIC ≥ 4 μg/ml; MSCNS, oxacillin MIC ≤ 0.25 μg/ml; MRCNS, oxacillin MIC ≥ 0.5 μg/ml; PSSP, penicillin-susceptible S. pneumoniae (penicillin MIC ≤ 0.06μg/ml); PISP and PRSP, penicillin-intermediate and -resistant S. pneumoniae, respectively (penicillin MIC ≥ 0.12 μg/ml) VRE, vancomycin MIC ≥ 32μg/ml; ofloxacin-susceptible, ofloxacin MIC ≤ 2 μg/ml; ofloxacin-resistant, ofloxacin MIC ≥ 8 μg/ml.
TABLE 2.
Antibacterial activities of DQ-113 and reference drugs against gram-negative bacteriaa
| Organism (no. of strains) and compound | MIC (μg/ml)
|
|
Organism (no of strains) and compound | MIC (μg/ml)
|
||||
|---|---|---|---|---|---|---|---|---|
| Range | MIC50 | MIC90 | Range | MIC50 | MIC90 | |||
| E. coli (22) | ||||||||
| DQ-113 | ≤0.004-2 | 0.03 | 0.25 | |||||
| Sitafloxacin | ≤0.004-0.5 | 0.008 | 0.06 | |||||
| Levofloxacin | ≤0.004-2 | 0.015 | 0.5 | |||||
| Ciprofloxacin | ≤0.004-2 | 0.008 | 0.5 | |||||
| Sparfloxacin | ≤0.004-16 | 0.015 | 0.5 | |||||
| Tosufloxacin | ≤0.004-16 | 0.015 | 0.5 | |||||
| Gatifloxacin | ≤0.004-2 | 0.03 | 0.5 | |||||
| Moxifloxacin | ≤0.004-8 | 0.06 | 1 | |||||
| T-3811ME | ≤0.004-16 | 0.06 | 1 | |||||
| K. pneumoniae (25) | ||||||||
| DQ-113 | 0.03-2 | 0.06 | 0.25 | |||||
| Sitafloxacin | 0.015-1 | 0.03 | 0.25 | |||||
| Levofloxacin | 0.06-8 | 0.06 | 1 | |||||
| Ciprofloxacin | 0.015-4 | 0.03 | 0.5 | |||||
| Sparfloxacin | 0.03-4 | 0.06 | 0.5 | |||||
| Tosufloxacin | 0.03-2 | 0.03 | 0.5 | |||||
| Gatifloxacin | 0.03-4 | 0.06 | 1 | |||||
| Moxifloxacin | 0.12-4 | 0.25 | 1 | |||||
| T-3811ME | 0.06-4 | 0.12 | 2 | |||||
| Gemifloxacin | 0.03-4 | 0.06 | 1 | |||||
| S. marcescens (22) | ||||||||
| DQ-113 | ≤0.25-4 | 0.5 | 1 | |||||
| Sitafloxacin | 0.06-1 | 0.12 | 0.25 | |||||
| Levofloxacin | 0.12-8 | 0.25 | 2 | |||||
| Ciprofloxacin | 0.06-8 | 0.12 | 1 | |||||
| Sparfloxacin | 0.25-8 | 0.5 | 2 | |||||
| Tosufloxacin | 0.12-8 | 0.25 | 1 | |||||
| Gatifloxacin | 0.25-4 | 0.5 | 2 | |||||
| Moxifloxacin | 0.25-8 | 1 | 2 | |||||
| T-3811ME | 1-16 | 2 | 8 | |||||
| Gemifloxacin | 0.12-8 | 0.5 | 2 | |||||
| Enterobacter spp. (23) | ||||||||
| DQ-113 | 0.03-4 | 0.06 | 1 | |||||
| Sitafloxacin | 0.008-1 | 0.03 | 0.25 | |||||
| Levofloxacin | 0.03-8 | 0.06 | 2 | |||||
| Ciprofloxacin | 0.008-16 | 0.03 | 2 | |||||
| Sparfloxacin | 0.015-4 | 0.06 | 2 | |||||
| Tosufloxacin | ≤0.004-4 | 0.06 | 1 | |||||
| Gatifloxacin | 0.03-8 | 0.12 | 1 | |||||
| Moxifloxacin | 0.06-4 | 0.25 | 2 | |||||
| T-3811ME | 0.06-16 | 0.25 | 2 | |||||
| Gemifloxacin | 0.03-8 | 0.06 | 1 | |||||
| P. mirabilis (25) | ||||||||
| DQ-113 | 0.06-2 | 0.12 | 2 | |||||
| Sitafloxacin | 0.015-0.5 | 0.03 | 0.25 | |||||
| Levofloxacin | 0.03-4 | 0.06 | 1 | |||||
| Ciprofloxacin | 0.015-16 | 0.03 | 0.5 | |||||
| Sparfloxacin | 0.12-16 | 0.25 | 8 | |||||
| Tosufloxacin | 0.06-16 | 0.12 | 4 | |||||
| Gatifloxacin | 0.12-8 | 0.12 | 2 | |||||
| Moxifloxacin | 0.12-16 | 0.25 | 2 | |||||
| T-3811ME | 0.25-32 | 0.5 | 16 | |||||
| Gemifloxacin | 0.06-16 | 0.12 | 4 | |||||
| Indole-positive Proteus (25) | ||||||||
| DQ-113 | 0.06-2 | 0.12 | 1 | |||||
| Sitafloxacin | 0.008-0.5 | 0.03 | 0.25 | |||||
| Levofloxacin | 0.03-2 | 0.06 | 1 | |||||
| Ciprofloxacin | 0.008-4 | 0.03 | 1 | |||||
| Sparfloxacin | 0.06-4 | 0.25 | 4 | |||||
| Tosufloxacin | 0.03-4 | 0.12 | 2 | |||||
| Gatifloxacin | 0.03-2 | 0.12 | 2 | |||||
| Moxifloxacin | 0.12-4 | 0.25 | 4 | |||||
| T-3811ME | 0.25-8 | 0.5 | 4 | |||||
| Gemifloxacin | 0.03-8 | 0.12 | 4 | |||||
| P. aeruginosa, ofloxacin-susceptible (24) | ||||||||
| DQ-113 | 0.25-2 | 0.5 | 2 | |||||
| Sitafloxacin | 0.03-0.5 | 0.12 | 0.25 | |||||
| Levofloxacin | 0.25-2 | 1 | 2 | |||||
| Ciprofloxacin | 0.03-0.5 | 0.12 | 0.25 | |||||
| Sparfloxacin | 0.12-2 | 1 | 2 | |||||
| Tosufloxacin | 0.06-0.5 | 0.25 | 0.5 | |||||
| Gatifloxacin | 0.25-4 | 1 | 2 | |||||
| Moxifloxacin | 0.5-8 | 2 | 4 | |||||
| T-3811ME | 0.5-4 | 1 | 4 | |||||
| Acinetobacter spp. (24) | ||||||||
| DQ-113 | 0.015-0.5 | 0.03 | 0.25 | |||||
| Sitafloxacin | 0.015-1 | 0.03 | 0.25 | |||||
| Levofloxacin | 0.12-4 | 0.25 | 1 | |||||
| Ciprofloxacin | 0.12-4 | 0.25 | 2 | |||||
| Sparfloxacin | 0.008-1 | 0.03 | 0.5 | |||||
| Tosufloxacin | 0.015-1 | 0.06 | 0.5 | |||||
| Gatifloxacin | 0.03-2 | 0.12 | 0.5 | |||||
| Moxifloxacin | 0.06-4 | 0.12 | 1 | |||||
| T-3811ME | 0.015-4 | 0.06 | 1 | |||||
| Gemifloxacin | 0.06-2 | 0.12 | 0.5 | |||||
| H. influenzae | ||||||||
| Ampicillin-susceptible (38) | ||||||||
| DQ-113 | ≤0.004-0.03 | 0.008 | 0.015 | |||||
| Sitafloxacin | ≤0.004-0.008 | ≤0.004 | ≤0.004 | |||||
| Levofloxacin | ≤0.004-0.03 | 0.015 | 0.015 | |||||
| Ciprofloxacin | ≤0.004-0.06 | 0.008 | 0.015 | |||||
| Sparfloxacin | ≤0.004-0.03 | 0.008 | 0.008 | |||||
| Tosufloxacin | ≤0.004-0.015 | ≤0.004 | 0.008 | |||||
| Gatifloxacin | ≤0.004-0.03 | 0.008 | 0.008 | |||||
| Moxifloxacin | 0.008-0.06 | 0.03 | 0.03 | |||||
| T-3811MEE | ≤0.004-0.015 | ≤0.004 | 0.008 | |||||
| Gemifloxacin | ≤0.004-0.03 | ≤0.004 | 0.008 | |||||
| Cefaclor | 1-64 | 2 | 32 | |||||
| Ampicillin | 0.12-1 | 0.25 | 1 | |||||
| β-Lactamase-negative, ampicillin-resistant (14) | ||||||||
| DQ-113 | ≤0.004-0.015 | 0.015 | 0.015 | |||||
| Sitafloxacin | ≤0.004 | ≤0.004 | ≤0.004 | |||||
| Levofloxacin | 0.008-0.015 | 0.015 | 0.015 | |||||
| Ciprofloxacin | ≤0.004-0.015 | 0.008 | 0.015 | |||||
| Sparfloxacin | ≤0.004-0.015 | 0.008 | 0.015 | |||||
| Tosufloxacin | ≤0.004-0.008 | ≤0.004 | 0.008 | |||||
| Gatifloxacin | ≤0.004-0.008 | 0.008 | 0.008 | |||||
| Moxifloxacin | 0.008-0.03 | 0.015 | 0.03 | |||||
| T-3811ME | ≤0.004-0.015 | 0.008 | 0.008 | |||||
| Gemifloxacin | ≤0.004-0.008 | ≤0.004 | 0.008 | |||||
| Cefaclor | 32-128 | 64 | 128 | |||||
| Ampicillin | 2-8 | 2 | 4 | |||||
| β-Lactamase-positive, ampicillin-resistant (21) | ||||||||
| DQ-113 | ≤0.004-0.03 | 0.008 | 0.015 | |||||
| Sitafloxacin | ≤0.004 | ≤0.004 | ≤0.004 | |||||
| Levofloxacin | 0.008-0.015 | 0.008 | 0.015 | |||||
| Ciprofloxacin | ≤0.004-0.015 | 0.008 | 0.008 | |||||
| Sparfloxacin | ≤0.004-0.015 | 0.008 | 0.008/PICK>{tt} | |||||
| Tosufloxacin | ≤0.004-0.008 | ≤0.004 | 0.008 | |||||
| Gatifloxacin | ≤0.004-0.015 | 0.008 | 0.008 | |||||
| Moxifloxacin | 0.015-0.06 | 0.015 | 0.03 | |||||
| T-3811ME | ≤0.004-0.03 | ≤0.004 | 0.008 | |||||
| Gemifloxacin | ≤0.004-0.015 | ≤0.004 | 0.008 | |||||
| Cefaclor | 1-64 | 8 | 32 | |||||
| Ampicillin | 4->128 | 16 | 128 | |||||
| M. catarrhalis (25) | ||||||||
| DQ-113 | ≤0.004-0.03 | 0.015 | 0.03 | |||||
| Sitafloxacin | ≤0.004-0.03 | 0.015 | 0.015 | |||||
| Levofloxacin | 0.03-0.06 | 0.06 | 0.06 | |||||
| Ciprofloxacin | 0.015-0.06 | 0.06 | 0.06 | |||||
| Sparfloxacin | ≤0.004-0.03 | 0.015 | 0.03 | |||||
| Tosufloxacin | ≤0.004-0.03 | 0.015 | 0.03 | |||||
| Gatifloxacin | 0.015-0.06 | 0.03 | 0.06 | |||||
| Moxifloxacin | 0.03-0.12 | 0.12 | 0.12 | |||||
| T-3811ME | 0.004-0.06 | 0.03 | 0.03 | |||||
| Gemifloxacin | ≤0.004-0.06 | 0.03 | 0.03 | |||||
| N. gonorrhoeae (35) | ||||||||
| DQ-113 | ≤0.004-2 | 0.25 | 1 | |||||
| Sitafloxacin | ≤0.004-0.5 | 0.12 | 0.25 | |||||
| Levofloxacin | 0.015-16 | 4 | 16 | |||||
| Ciprofloxacin | 0.008-32 | 4 | 16 | |||||
| Sparfloxacin | ≤0.004-16 | 2 | 8 | |||||
| Tosufloxacin | 0.008-32 | 2 | 16 | |||||
| Gatifloxacin | 0.008-4 | 1 | 4 | |||||
| Moxifloxacin | ≤0.004-8 | 2 | 8 | |||||
| T-3811ME | ≤0.004-8 | 0.5 | 4 | |||||
| Gemifloxacin | ≤0.004-16 | 1 | 8 | |||||
Resistance criteria are as follows: ampicillin-susceptible, ampicillin MIC ≤ 1 μg/ml; ampicillin-resistant, ampicillin MIC ≥ 4 μg/ml.
MIC90s for penicillin-susceptible and -resistant S. pneumoniae and Streptococcus pyogenes were 0.03, 0.015, and 0.015 μg/ml, respectively, which were at least fourfold lower than those of the quinolones tested. Against Enterococcus faecalis and Enterococcus faecium, DQ-113 showed the highest activity among the compounds tested, with MIC90s of 0.25 and 2 μg/ml, respectively. Against VRE, DQ-113 also showed the highest activity among quinolones tested. The MIC ranged from 0.06 to 2 μg/ml.
H. influenzae, including ampicillin-resistant strains, and M. catarrhalis were susceptible to DQ-113, with MIC90s for these strains being 0.015 and 0.03 μg/ml, respectively. Against these strains, DQ-113 activity was roughly comparable to those of the reference quinolones. Against various members of the Enterobacteriaceae, DQ-113 activity was up to four times that of levofloxacin at MIC90 levels. DQ-113 inhibited 90% of isolates of Escherichia coli, Klebsiella pneumoniae, Serratia marcescens, Enterobacter spp., Proteus mirabilis, and indole-positive Proteus spp. at 0.25, 0.25, 1, 1, 2, and 1 μg/ml, respectively. At MIC90 levels, DQ-113 showed activity comparable to those of levofloxacin, sparfloxacin, and gatifloxacin; was four to eight times less active than sitafloxacin, ciprofloxacin, and tosufloxacin; was two times more potent than moxifloxacin and T-3811ME; and was eight times more active than imipenem against ofloxacin-susceptible P. aeruginosa. Against Acinetobacter spp., DQ-113 activity was comparable to that of sitafloxacin and was at least twofold higher than those of the other reference quinolones, with a MIC90 of 0.25 μg/ml. Against N. gonorrhoeae, including ofloxacin-resistant strains, the MIC90 of DQ-113 was 1 μg/ml. The interpretive MICs of the compounds tested against the reference strains for quality control were reproducible throughout the study.
This study showed that DQ-113, a recently synthesized quinolone, possesses the most potent antibacterial activity against staphylococci, streptococci, and enterococci among the reference compounds, such as sitafloxacin, levofloxacin, ciprofloxacin, sparfloxacin, tosufloxacin, gatifloxacin, moxifloxacin, T-3811ME, gemifloxacin, vancomycin, teicoplanin, and linezolid. Moreover, DQ-113 showed good antibacterial activity against strains of Enterobacteriaceae, Pseudomonas aeruginosa, H. influenzae, M. catarrhalis, and N. gonorrhoeae, known clinically as significant pathogens.
DQ-113 showed a potent antibacterial activity against gram-positive bacteria as well as favorable safety and pharmacokinetic profiles (H. Takahashi, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1505, 2000). The relative potency of DQ-113 will be better understood when the human pharmacokinetics are available. Further studies of DQ-113 are warranted based upon the available data.
REFERENCES
- 1.Cunha, B. A. 1998. Antibiotic resistance. Control strategies. Crit. Care Clin. 14:309-327. [DOI] [PubMed] [Google Scholar]
- 2.Dalhoff, A. 1994. Quinolone resistance in Pseudomonas aeruginosa and Staphylococcus aureus. Development during therapy and clinical significance. Infection 22(Suppl.):S111-S121. [DOI] [PubMed] [Google Scholar]
- 3.Endtz, H. P., N. van den Braak, H. A. Verbrugh, and A. van Belkum. 1999. Vancomycin resistance: status quo and quo vadis. Eur. J. Clin. Microbiol. Infect. Dis. 18:683-690. [DOI] [PubMed] [Google Scholar]
- 4.File, T. M., Jr. 1999. Overview of resistance in the 1990s. Chest 115(Suppl.):3S-8S. [DOI] [PubMed] [Google Scholar]
- 5.Hooper, D. C., and J. S. Wolfson. 1985. The fluoroquinolones: pharmacology, clinical uses, and toxicities in humans. Antimicrob. Agents Chemother. 28:716-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Committee for Clinical Laboratory Standards. 2000. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 5th ed. Approved standard M7-A5. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 7.Piercy, E. A., D. Barbaro, J. P. Luby, and P. A. Mackowiak. 1989. Ciprofloxacin for methicillin-resistant Staphylococcus aureus infections. Antimicrob. Agents Chemother. 33:128-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rubinstein, E., P. Prokocimer, and G. H. Talbot. 1999. Safety and tolerability of quinupristin/dalfopristin: administration guidelines. J. Antimicrob. Chemother. 44(Suppl. A):37-46. [DOI] [PubMed] [Google Scholar]
- 9.Witte, W. 1999. Antibiotic resistance in Gram-positive bacteria: epidemiological aspects. J. Antimicrob. Chemother. 44(Suppl. A):1-9. [DOI] [PubMed] [Google Scholar]
- 10.Wolfson, J. S., and D. C. Hooper. 1989. Bacterial resistance to quinolones: mechanisms and clinical importance. Rev. Infect. Dis. 11:S960-S968. [DOI] [PubMed] [Google Scholar]
- 11.Yamaguchi, K., S. Miyazaki, F. Kashitani, M. Iwata, and Levofloxacin Surveillance Group. 2000. Activities of antimicrobial agents against 5,180 clinical isolates obtained from 26 medical institutions during 1998 in Japan. Jpn. J. Antibiot. 53:387-408. [PubMed] [Google Scholar]