Abstract
The uptake of fluoroquinolones was characterized for the fluoroquinolone-susceptible strain PG21 of Mycoplasma hominis. Accumulation of fluoroquinolones appeared to occur by passive diffusion. Addition of arginine as the energizer significantly reduced the uptake of fluoroquinolones, suggesting the presence of an energy-dependent efflux process. Reserpine and orthovanadate, two multidrug pump inhibitors, increased significantly the ciprofloxacin (CIP) uptake. In contrast, such a strong effect was not observed for moxifloxacin and pefloxacin uptakes. Two ethidium bromide (EtBr)-resistant strains, selected in vitro, showed a resistance profile compatible with a multidrug-resistant phenotype, with increased MICs for the hydrophilic fluoroquinolones, CIP and norfloxacin, EtBr, and acriflavine. Taking the EtBr-resistant strain RB1La as a model, a significant decrease of the CIP and EtBr uptakes was observed compared to the reference strain PG21. In the presence of reserpine and orthovanadate, both inhibitors of ATP-dependent efflux pumps, the CIP uptake increased significantly, reaching approximately the same level as that of the susceptible strain. Similar results were obtained with EtBr uptake and efflux experiments. Our data suggest the presence of an active efflux system, possibly an ABC-type efflux pump, implicated in the resistance to CIP and unrelated compounds like EtBr in the human mycoplasma M. hominis.
Mycoplasma hominis is a cause of urogenital tract infections and has been implicated in extragenital infections as well, especially in immunocompromised patients. This wall-less organism belongs to the class Mollicutes, characterized by a low G+C content and having arisen from a common ancestor with the gram-positive bacteria, like some clostridia (53). M. hominis is capable of metabolizing arginine as an energy source but does not ferment glucose (44). We recently reported in vitro and in vivo fluoroquinolone-resistant mutants of M. hominis associated with alterations in the topoisomerase II target genes of these antibiotics (9-10).
To date, no other mechanism of fluoroquinolone resistance has been identified in M. hominis. However, a second mechanism of resistance to fluoroquinolones, namely active efflux of the drug, might be expected, as already described for various bacteria. Indeed, energy-dependent efflux as a mechanism of quinolone resistance has been found in an increasing number of gram-negative and gram-positive bacteria (for reviews, see references 45 and 46), although the major form of quinolone resistance found in bacteria is altered DNA gyrase and topoisomerase IV (22). Furthermore, the role of an efflux mechanism in fluoroquinolone-resistant clinical isolates has been described for several bacterial species (6, 12, 16, 34, 43). This active efflux involves multidrug resistance (MDR) pumps, which transport structurally unrelated compounds, including different classes of antibiotics, antiseptics, and cationic dyes, such as ethidium bromide (EtBr) and acriflavine. These pumps are classified in two groups according to their energy source, the secondary transporters using the proton motive force (PMF) and the ATP-Binding Cassette (ABC) transporters using ATP hydrolysis (47). These pumps extrude toxic compounds, limiting their accumulation in cells. Most of the bacterial MDR pumps use the PMF, whereas only one example of an ABC-type multidrug transporter has been well identified in bacteria, LmrA in Lactococcus lactis (52).
To evaluate the possible role of efflux pump-mediated extrusion of fluoroquinolones in M. hominis, we first characterized the uptake of three compounds presenting different coefficients of hydrophobicity, the more hydrophilic ciprofloxacin (CIP), the more hydrophobic pefloxacin (PEF), and the intermediate moxifloxacin (MXF), in the susceptible strain M. hominis PG21. Then, strains resistant to the multidrug transporter substrate EtBr were selected in vitro. EtBr was chosen as a selecting agent since transporter-mediating efflux is the only mechanism known to confer resistance to this compound. Two EtBr-resistant strains, which presented a resistance profile compatible with an MDR phenotype similar to those conferred by the gram-positive Staphylococcus aureus NorA (28), Bacillus subtilis Bmr (36), and Streptococcus pneumoniae PmrA pumps (19), were studied.
MATERIALS AND METHODS
Antibiotics and chemicals.
Norfloxacin (NOR) was obtained from Merck Sharp & Dohme (Rome, Italy); PEF and sparfloxacin (SPX) were obtained from Rhône-Poulenc-Rorer (Vitry-sur-Seine, France); ofloxacin (OFX) and chloramphenicol were obtained from Hoechst Marion Roussel (Romainville, France); CIP and MXF were obtained from Bayer-Pharma (Puteaux, France); and doxycycline was obtained from Pfizer (Orsay, France). The partition coefficients between the aqueous (50 mM sodium phosphate buffer [pH 7]) and organic (n-octanol) phases previously described (4, 40) for CIP, MXF, and PEF were 0.031, 0.089, and 1.65, respectively. Silicone oil was obtained from Fluka, and EtBr, acriflavine, cetrimide, benzalkonium chloride, carbonyl cyanide m-chlorophenylhydrazone (CCCP), 2,4-dinitrophenol (DNP), and sodium orthovanadate were from Sigma.
Bacterial strains and selection of EtBr-resistant strains.
The reference strain PG21 (ATCC 23114) of M. hominis was used to select the EtBr-resistant strains. Stepwise selection of the EtBr-resistant mutants of M. hominis was performed by plating approximately 2 × 107 color-changing units (CCU) of M. hominis PG21 onto Hayflick modified agar medium containing various concentrations of EtBr. After 48 h of incubation at 37°C, resistant colonies were grown in liquid medium without EtBr and used for the next round of selection. One strain, named RB1, was obtained on 50 μg of EtBr/ml after three selection steps. The two other strains selected for the accumulation studies, named RB1La and RB1Lb, were obtained and cloned after a passage of strain RB1 in liquid medium containing 100 μg of EtBr/ml. Mycoplasmas were grown at 37°C in Hayflick modified agar or broth medium supplemented with arginine. S. aureus strains, a fluoroquinolone-susceptible isolate, SA-1199, and its efflux-resistant derived mutant, SA-1199B (28), were grown at 37°C in Luria-Bertani liquid medium or on Mueller-Hinton agar plates supplemented with 5% horse blood (bioMérieux).
MIC determinations.
MICs of antimicrobials and of the efflux substrates were determined by the agar dilution method on Hayflick modified medium for M. hominis (8) and on Mueller-Hinton medium for S. aureus. Susceptibilities to NOR, CIP, EtBr and acriflavine in the presence of 20 μg of reserpine/ml were determined as previously described (12, 16, 30). For MIC testing in the presence and absence of reserpine, results were expressed as the geometric means of at least three determinations. The method was validated with both S. aureus strains, SA-1199 and SA-1199B (28).
PCR experiments and DNA sequencing of QRDRs.
PCRs were carried out with 2 μl of a broth culture for EtBr-resistant mutants as described elsewhere (9). Primer sets MH3 and 4, MH6 and 7, MH11 and 13, and MH27 and 28 were used to amplify and sequence the gyrA, gyrB, parC, and parE quinolone resistance-determining regions (QRDRs), respectively (9).
Accumulation studies. (i) Accumulation of fluoroquinolones.
Fluoroquinolone uptake was performed as previously described (13, 33) using the silicone oil method with the following modifications. Cells were grown at 37°C in Hayflick modified broth to the mid-exponential phase of growth, harvested by centrifugation, washed once in 50 mM sodium phosphate buffer (pH 7.3), and concentrated 20-fold in this medium to approximately 2 × 109 CCU/ml. The cell suspension was supplemented with 5 g of arginine/liter (24 mM) as an energizer and preincubated for 5 min at 37°C (except for the experiments in which the absence of arginine or a different temperature was specified). After addition of fluoroquinolones at 10-μg/ml concentrations, 0.5-ml samples were removed at different time intervals and placed on 0.5-ml aliquots of silicone oil (density 1.03; Fluka). The tubes were immediately centrifuged, frozen, cut in the middle of the silicone layer, and inverted to eliminate the residual oil. Then, the cell pellets were washed, resuspended in glycine-HCl buffer (0.1 M; pH 3.0), and lysed by incubation for 10 min at 100°C. The fluorescence of the supernatant was measured with a spectrofluorimeter HITACHI F4500 at excitation and emission wavelengths depending on the fluoroquinolone tested. The natural fluorescence of cells was subtracted, and the fluorescence intensity was expressed as nanograms of product per 108 or 109 CCU. To test for the possible saturation of transport the accumulation of CIP and PEF over a concentration range of 0 to 250 μg/ml was studied with M. hominis PG21. The cells were incubated at 37°C for 20 min at each fluoroquinolone concentration.
In some experiments, efflux pumps inhibitors, reserpine (20 μg/ml; 33 μM), CCCP (100 μM), or DNP (2 mM) were added 5 min after addition of the fluoroquinolone. For studies with sodium orthovanadate (2 mM), sodium phosphate was replaced by potassium-HEPES at equimolar concentrations. Orthovanadate was added 20 min before fluoroquinolone addition at 37°C. The results were expressed as nanograms of fluoroquinolones per 109 CCU, with inoculum numbering being estimated for each uptake experiment. Each assay was performed at least three times, and the results are the means ± standard deviations for at least three separate experiments. Errors fall within 0 to 20%, except for the experiment shown in Fig. 3C with strain RB1, for which the error ranges up to 36%.
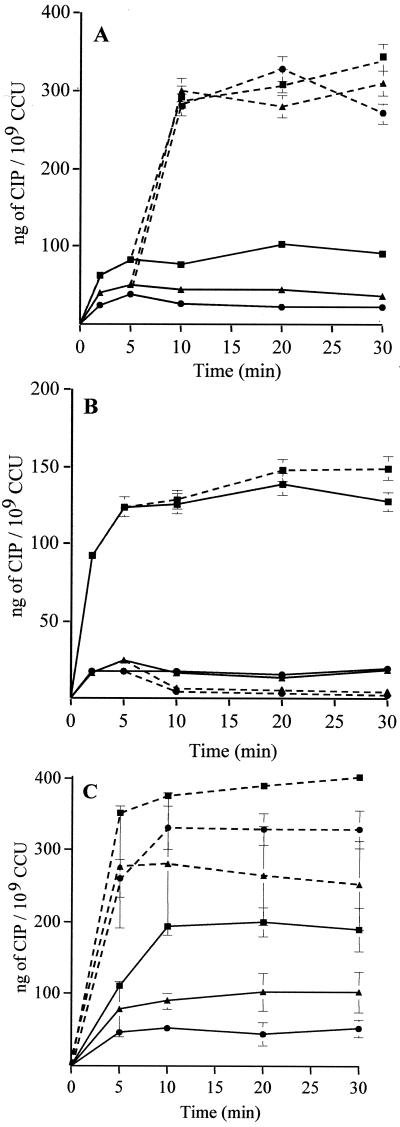
FIG. 3.
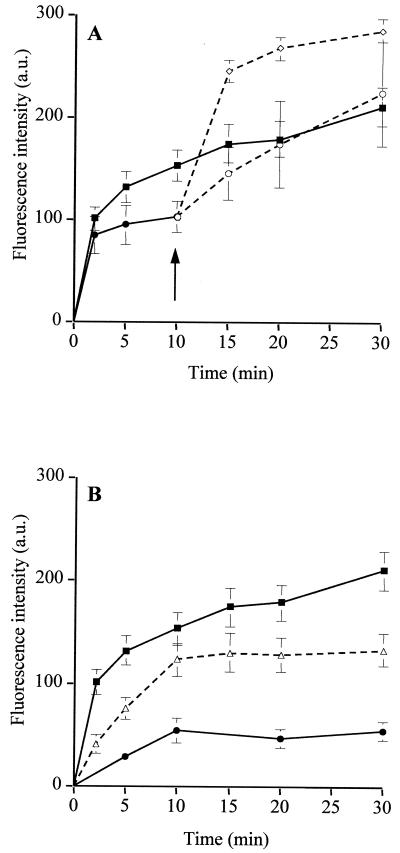
Uptake of CIP in the absence (—) and presence (---) of 20 μg of reserpine/ml (A), 100 μM CCCP (B), or 2 mM sodium orthovanadate (C) by M. hominis PG21 (▪) and the EtBr-resistant strains, RB1 (▴) and RB1La (•). Cells were energized with 5 g of arginine/liter. Reserpine and CCCP were added at 5 min, and orthovanadate was added 20 min prior the addition of the antibiotic. Error bars show standard deviations.
(ii) Accumulation and efflux of EtBr.
EtBr uptake and efflux experiments were performed as previously described (11, 26, 36) with some modifications for mycoplasmas. Bacteria were grown in Hayflick modified broth, centrifuged, washed, and resuspended in 50 mM sodium phosphate buffer (pH 7.3). For EtBr uptake the suspension was warmed to 37°C, and arginine (24 mM) and EtBr (10 μg/ml) were added. Fluorescence was used as a measure of the amount of EtBr incorporated by the cells and was recorded at frequent intervals at excitation and emission wavelengths of 530 and 600 nm, respectively. The natural fluorescence of cells was subtracted, and the fluorescence intensity was expressed in arbitrary units. CCCP (100 μM) and reserpine (20 μg/ml) were added 10 min after addition of EtBr. For studies with sodium orthovanadate, sodium phosphate was replaced by 50 mM potassium-HEPES buffer (pH 7.0). Cells were preenergized with arginine (24 mM) and preincubated for 20 min with orthovanadate (2 mM). The uptake experiment was started upon the addition of EtBr (10 μg/ml).
For EtBr efflux, 1 ml of suspension was placed at 37°C for 30 min with EtBr (10 μg/ml) and with or without either 20 μg of reserpine/ml, 100 μM CCCP, or 2 mM orthovanadate in the adapted uptake buffer. Cells were pelleted and resuspended in the same buffer without EtBr. For experiments with CCCP, cells were resuspended in the same buffer containing either no inhibitor or 100 μM CCCP (26). Arginine (24 mM) was added to energize the cells, and the fluorescence of the suspension was monitored continuously and expressed as a percentage of that at the first time point (100%). All accumulation and efflux experiments were performed at least three times.
RESULTS
Selection of the EtBr-resistant strains and their susceptibilities to antimicrobials and unrelated compounds.
In order to obtain a strain resistant to fluoroquinolones by an active efflux mechanism, EtBr-resistant strains were isolated by stepwise selection on agar with increasing concentrations of EtBr. Strain RB1 was obtained on 50-μg/ml EtBr after three steps. A passage in broth medium containing 100 μg of EtBr/ml was performed with RB1, leading to the selection of two cloned strains, RB1La and RB1Lb.
The susceptibilities of the three EtBr-selected strains to fluoroquinolones and other efflux substrates are summarized in Table 1. For the RB1 strain, MICs of the hydrophilic fluoroquinolones weakly increased (2-fold for CIP and 1.6-fold for NOR), whereas MICs of the more hydrophobic compounds like OFX, MXF, PEF, and SPX were unchanged, compared to those for the reference strain, PG21. For strains RB1La and RB1Lb, MICs of hydrophilic fluoroquinolones were higher. Compared to those for the susceptible parent strain, PG21, the MICs of NOR and CIP were two- and four-fold increased. In comparison, the previously described resistant mutant IIS1 (9), selected on SPX and mutated in gyrA and parC, did not show such a dissociated resistance profile (Table 1). A concomitant increase of MICs of EtBr and acriflavine was observed for the three EtBr-resistant strains. MICs of EtBr increased 5.6- and 8-fold for RB1 and RB1La and RB1Lb, respectively, while 1.6- and 3.2-fold increases of the acriflavine MICs were found, respectively, in comparison to M. hominis PG21. MICs of the quaternary ammoniums, cetrimide and benzalkonium chloride, were unchanged for the three EtBr-resistant strains. With regard to the other antibiotic families active against mycoplasmas, the MICs of doxycycline (0.12 μg/ml), josamycin (1 μg/ml), and chloramphenicol (8 μg/ml) were not significantly modified for the EtBr-resistant strains.
TABLE 1.
Susceptibilities of different strains of M. hominis to fluoroquinolones, cationic dyes, and antisepticsa
| M. hominis strain | MIC (μg/ml) of:
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NOR | NOR + RES | CIP | CIP + RES | OFX | MXF | PEF | SPX | EtBr | EtBr + RES | ACR | ACR + RES | CET | BZC | |
| PG21 | 16 | 12 | 2 | 1.5 | 1 | 0.12 | 4 | 0.06 | 12.5 | 12.5 | 25 | 17.5 | 25 | 30 |
| EtBr-resistant strains | ||||||||||||||
| RB1 | 25 | 8 | 4 | 1.5 | 1 | 0.12 | 4 | 0.06 | 70 | 35 | 40 | 15 | 20 | 25 |
| RB1La | 32 | 8 | 8 | 2 | 1 | 0.12 | 4 | 0.06 | 100 | 40 | 40 | 15 | 30 | 30 |
| RB1Lb | 32 | 8 | 8 | 2 | 1 | 0.12 | 4 | 0.06 | 100 | 40 | 40 | 15 | 20 | 30 |
| SPX-selected mutantb | ||||||||||||||
| IIS1 | 128 | ND | 32 | ND | 32 | 4 | 32 | 16 | ND | ND | ND | ND | ND | ND |
ACR, acriflavine; CET, cetrimide; BZC, benzalkonium chloride; RES, reserpine at 20 μg/ml.
From reference 9; it harbored amino acid changes at positions 83 and 84 in GyrA and ParC, respectively (E. coli numbering).
To detect the presence of an efflux phenotype, the susceptibilities of the strains to the hydrophilic fluoroquinolones and the other efflux substrates were determined in the presence of reserpine by the phenotypic method previously described for S. pneumoniae (12, 30). For the hydrophilic NOR and CIP MICs, reserpine caused a two- to three-fold decrease for the RB1 strain but a four-fold decrease for the RB1La and RB1Lb strains, considered as significant according to Ferrandiz et al. (16). For these three strains the EtBr and acriflavine MICs decreased 2- to 2.6-fold in the presence of reserpine, while no difference in the absence or presence of reserpine was found for M. hominis PG21. It should be noticed that while the use of reserpine decreased the CIP and NOR MICs for the EtBr mutants to the level of the M. hominis PG21 one, their MICs of EtBr in presence of reserpine remained three-fold higher than that of the reference strain (40 versus 12.5 μg/ml).
These data strongly suggested that the serial passages on EtBr selected antimycoplasmal agent-resistant strains with an MDR phenotype, similar to the efflux-mediated resistance in S. aureus (23), B. subtilis (35), and S. pneumoniae (5, 54). Indeed, the EtBr-selected strains acquired a reserpine-sensitive resistance mechanism, not only to the selective agent but also to unrelated compounds like hydrophilic fluoroquinolones and acriflavine.
For all the strains, PCR amplification and sequencing of the QRDRs from the four target genes of quinolones (gyrA, gyrB, parC, and parE) did not show any mutation in comparison to the reference strain.
Fluoroquinolone uptake by the reference strain M. hominis PG21. (i) Mechanisms of uptake and influence of energizing agents.
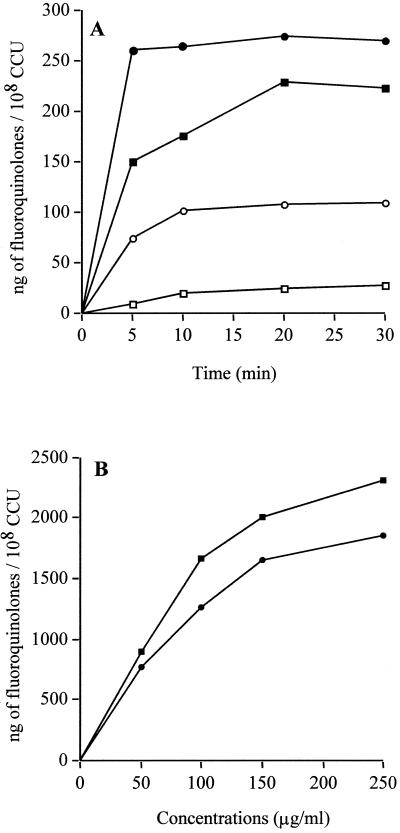
At 37°C the CIP accumulation was rapid; 65% of the maximal concentration of CIP was accumulated within the 5 min of contact with cells. The uptake level of PEF, a more hydrophobic compound, increased more rapidly, 95% of the maximal concentration of PEF being accumulated within the first 5 min of drug exposure (Fig. 1A). Uptakes of CIP and PEF were also studied at 4°C. At 37°C, the CIP uptake level increased from 150 to 223 ng/108 CCU between 5 and 30 min, whereas at 4°C, it only increased from 10 to 28 ng/108 CCU. In the same way, at 37°C, the PEF uptake level increased to 269 ng/108 CCU at 30 min but only to 110 ng/108 CCU at 4°C (Fig. 1A). Thus the absolute amount of drug that can be retained seems larger at 37°C than at 4°C in the absence of an energizing agent, suggesting that the rise in temperature could allow the unmasking of binding sites and that the fluoroquinolone uptake could be influenced by the membrane fluidity. These data have been previously described for several other bacteria (15, 42, 48). To determine the saturability of accumulation into M. hominis, the effect of exogenous concentrations of CIP and PEF was studied over a concentration range of 0 to 250 μg/ml. The concentration of accumulated CIP and PEF increased in a linear fashion up to the external fluoroquinolone concentration of 150 μg/ml. Above this value, the CIP and PEF accumulations were apparently saturable (Fig. 1B). Similar data were obtained for some fluoroquinolones in Pseudomonas aeruginosa (42) and Bacteroides fragilis (48). Overall, these results suggest that fluoroquinolone uptake is by simple passive diffusion in M. hominis.
FIG. 1.
(A) Uptake of CIP (squares) and PEF (circles) at 10 μg/ml by M. hominis PG21 in the absence of arginine at 37°C (closed symbols) and 4°C (open symbols). (B) Effect of exogenous concentrations on the CIP and PEF accumulations by M. hominis. Cells were incubated at 37°C for 20 min at each tested concentration.
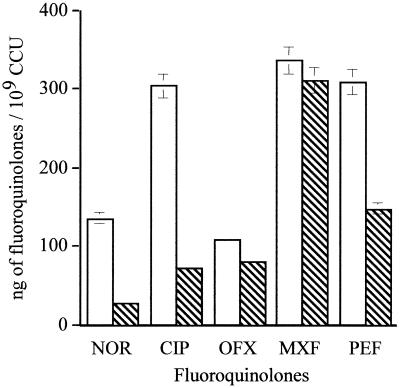
In order to reveal the activity of a putative efflux pump in M. hominis, cells were energized for accumulation studies. Addition of 20 mM lactate or 11 mM glucose did not reduce the CIP uptake level (data not shown). In the presence of 5-g/liter arginine, the metabolic energy source of M. hominis, all the accumulation rates decreased significantly except for that of MXF (Fig. 2). At 20 min, the steady state concentration (SSC) of NOR and CIP importantly decreased from 135.7 to 27.5 ng/109 CCU (79.7% decrease) and from 303.5 to 72.5 ng/109 CCU (76.1%). The PEF SSC decreased 52% while the accumulation rates of OFX and MXF, fluoroquinolones with a similar partitioning coefficient, were distinctly less reduced (26.1 and 7.74% decreases, respectively). Addition of arginine seemed to decrease the uptake levels of fluoroquinolones, as previously described with glucose for S. pneumoniae (54). The next accumulation studies were performed with arginine at 5 g/liter for CIP, MXF, and PEF.
FIG. 2.
Accumulation levels of fluoroquinolones with (hatched columns) and without (open columns) 5 g of arginine/liter by M. hominis PG21. Samples were taken at 20 min. Error bars show standard deviations.
(ii) Influence of different inhibitors of efflux pumps.
To determine if CIP, MXF, and PEF were actively transported from M. hominis PG21, the influence of several inhibitors of MDR pumps on accumulation of these fluoroquinolones was studied. The CIP data will be described first, and then the results obtained with MXF and PEF.
Reserpine, a plant alkaloïd inhibiting several MDR pumps (5, 20, 36, 52), was added 5 min after the addition of the fluoroquinolone. The concentration of CIP accumulated after 20 min importantly increased from 102.5 to 312.5 ng/109 CCU, that is to say a three-fold increase (Fig. 3A). The majority of the bacterial MDR pumps are secondary transporters, sensitive to decoupling agents or protonophores, such as CCCP and DNP, which dissipate the PMF across the cytoplasmic membrane. Fig. 3B shows the effect of 100 μM CCCP, added 5 min after the addition of CIP to the culture. An SSC from 138.5 to 147.5 ng of CIP/109 CCU was obtained with the addition of CCCP, leading to an accumulation increase of 6.5%. Similar results were obtained with CCCP added 20 min before the addition of quinolone or with 2 mM DNP added 5 min after (data not shown), suggesting that these agents did not significantly affect the accumulation of CIP. Considering the previous results, we studied the effect of orthovanadate, an inhibitor of ABC transporters, the second class of MDR pumps. Two millimolar sodium orthovanadate was added 20 min prior to the addition of the fluoroquinolone. In the presence of orthovanadate at 20 min, the CIP SSC dramatically increased, from 200 to 390 ng/109 CCU (twofold) (Fig. 3C).
The MXF and PEF accumulation rates were not significantly affected by the addition either of reserpine (20.3 and 20.9% increase) or of CCCP (12.9 and 22% decrease). Addition of orthovanadate increased the MXF SCC (59.3% increase) but did not affect the PEF SCC (16.3% decrease) (data not shown).
These data suggested the existence of an active process, affecting much more the CIP than the MXF and PEF uptakes and sensitive to both inhibitors of MDR efflux pumps, reserpine and orthovanadate, but not to CCCP or DNP.
Evidence of an active efflux in EtBr-resistant strains of M. hominis. (i) Fluoroquinolone uptake.
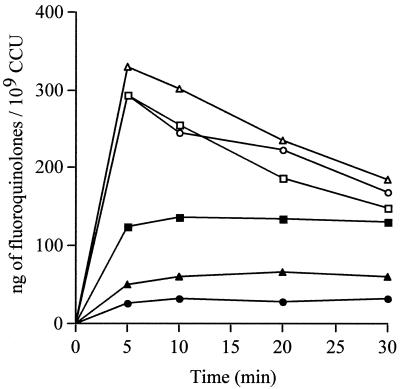
The CIP, MXF, and PEF uptake studies were realized with two out of the three EtBr-resistant strains, RB1 and RB1La. Compared to the susceptible strain PG21, at steady state, the energized accumulation of CIP was 51.3% decreased for strain RB1 but 79.8% decreased for RB1La (Fig. 4), showing that the strain most resistant to CIP also had the lower CIP SSC. Thus, both EtBr-resistant strains accumulated about two- to fivefold less CIP than the parent strain, PG21. At the opposite at 20 min, the uptake of PEF in strains RB1 and RB1La was not significantly modified compared to that in M. hominis PG21 (Fig. 4). Similar results were obtained for MXF (data not shown). It should be noticed that after 5 min, in contrast to CIP and MXF, the amount of PEF decreased in both susceptible and resistant strains. Such a decrease in the uptake level of a fluoroquinolone in a susceptible strain was previously described for P. aeruginosa (29) and in S. pneumoniae (54). The reasons for this PEF decrease are not clear at present.
FIG. 4.
Uptake of CIP and PEF by M. hominis PG21 (▪ and □) and the EtBr-resistant strains RB1 (▴ and ▵) and RB1La (• and ○). Cells were energized with 5 g of arginine/liter.
As with the PG21 susceptible strain, the effect of the three same inhibitors on the CIP, MXF, and PEF accumulations was studied for RB1 and RB1La. Here again the CIP accumulation data are presented first. With reserpine, as shown in Fig. 3A, at 20 min a large increase in the CIP SSC was observed with both strains, from 44.5 to 280 ng/109 CCU (6.3-fold increase) for strain RB1 and from 23 to 328.5 ng/109 CCU (14.3-fold increase) for strain RB1La. For both resistant strains at 20 min, after addition of reserpine, the CIP uptake level was nearly identical (around 300 ng/109 CCU) and reached the CIP level of the susceptible strain. However, the accumulation increase with reserpine was more important for strains RB1La (14.3-fold) and RB1 (6.3-fold) than for strain PG21 (3-fold). These data suggested that an active efflux process reduces the intracellular accumulation of CIP by cells in a more efficient way in the EtBr-resistant strains than in the parent strain.
Surprisingly, addition of CCCP (100 μM) strongly decreased, from 57.1 to 74.2%, the CIP SSC by the two EtBr-resistant strains (from 14 to 6 ng/109 CCU for RB1 and from 15.5 to 4 ng/109 CCU for RB1La at 20 min) (Fig. 3B). These results did not disagree with those obtained with the PG21 parent strain, for which no obvious effect of protonophores was detected (see above).
Preincubation of cells with 2 mM sodium orthovanadate increased the CIP SSC by strain RB1 from 103 to 264 ng/109 CCU (2.5-fold increase) and by strain RB1La from 44 to 329 ng/109 CCU (7.5-fold increase) (Fig. 3C, at 20 min). Thus, orthovanadate caused a dramatic increase in the CIP accumulation level only, reaching approximately the same level as that for the susceptible strain. Effects of the efflux pumps inhibitors, such as reserpine and orthovanadate, on the CIP accumulation by the RB1 and RB1La strains reinforce the hypothesis that an energy-dependent mechanism could exist in M. hominis, possibly dependent on ATP hydrolysis.
In contrast to the results obtained with CIP, after addition of reserpine, the MXF and PEF SSCs of the EtBr-resistant strains, RB1 and RB1LA, were not significantly modified, with decreases ranging from 1 to 25% at 20 min. With orthovanadate, the increase in the MXF SSC was 1.5-fold for both strains RB1 and RB1La, a factor similar to that obtained for the susceptible strain PG21. The PEF SSCs of RB1 and RB1La were not significantly modified, with 25% increases at 20 min (data not shown). The effect of CCCP on both MXF and PEF accumulations was not as pronounced as for the CIP uptake, with uptake decreases lower than 30% (data not shown).
(ii) EtBr uptake and efflux.
To understand the mechanism of EtBr resistance, fluorimetric EtBr transport assays were carried out with M. hominis PG21 and the strain most resistant to EtBr, RB1La (MIC of EtBr, 100 μg/ml). As Fig. 5A and B show, before the addition of any inhibitor, the accumulation of EtBr in RB1La was significantly decreased from that of the reference strain, PG21. Similar results were obtained for the mutant RB1, for which the EtBr MIC was 70 μg/ml (data not shown). Addition of reserpine at 10 min importantly increased the ethidium uptake level by RB1La, from 95 to 223 arbitrary units (a.u.) of fluorescence, reaching that of PG21 after 20 min. Addition of 100 μM CCCP induced also a 2.7-fold increase of fluorescence at 30 min, indicating that EtBr efflux was inhibited (Fig. 5A).
FIG. 5.
(A) Accumulation of EtBr (10 μg/ml) by M. hominis PG21 without any inhibitor (▪) and the EtBr-resistant strain RB1La in the absence of any additive (•) or in the presence of 20 μg of reserpine/ml (○) or 100 μM CCCP (◊). Reserpine and CCCP were added at 10 min as indicated by the arrow. (B) Accumulation of EtBr (10μg/ml) by M. hominis PG21 without any inhibitor (▪) and the EtBr-resistant strain RB1La in the absence (•) or in the presence (▵) of 2 mM sodium orthovanadate, added 20 min prior the addition of EtBr. Cells were energized with 5 g of arginine/liter. Uptake experiments were realized in sodium phosphate buffer for reserpine and CCCP but in 50 mM potassium-HEPES for orthovanadate. Fluorescence is expressed in arbitrary units (a.u.). Error bars show standard deviations.
When RB1La cells were preincubated with 2 mM sodium orthovanadate (Fig. 5B), the fluorescence level increased from 53 to 123 a.u. at 10 min (2.2-fold increase), compared to the level without inhibitor. Thus, orthovanadate caused a significant increase in the level of EtBr accumulation. These results confirm that an energy-dependent system was able to limit the incorporation of a toxic compound structurally unrelated to fluoroquinolones.
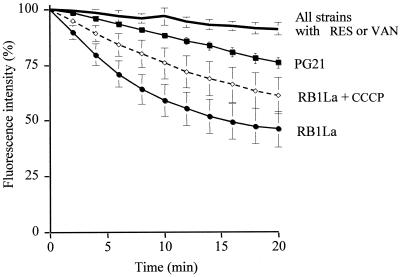
For ethidium efflux experiments the fluorescence intensity, after addition of EtBr and before addition of the energy source, was normalized at 100%. The fluorescence intensity that was measured indirectly reflected the efflux rate of EtBr. As shown in Fig. 6, the energized PG21 strain presented a moderate decrease of fluorescence intensity, from 100 to 76% (24% decrease), while the energized RB1La strain presented a distinctly greater decrease, from 100 to 46% fluorescence intensity (54% decrease). Thus, RB1La seemed to have an increased EtBr efflux, with an efflux rate approximately 4.5-fold higher than that of the wild-type strain, PG21, determined by taking the slope (tangent) of the line fitting the initial fluorescence decrease. Reserpine and sodium orthovanadate completely reverted the EtBr efflux by strain RB1La, with a fluorescence decrease from 100 to 91% only, after 20 min. Furthermore the EtBr efflux level by the inhibited strain RB1La reached that of the PG21 strain inhibited by reserpine or orthovanadate (Fig. 6). Similar results were obtained with strain RB1 (data not shown). Otherwise, after addition of 100 μM CCCP, the fluorescence decreased significantly more, from 100 to 61% (39% decrease), suggesting that CCCP had only a minor effect on EtBr efflux by RB1La (Fig. 6), in contrast to reserpine and orthovanadate. Using the technical variant described by Kaatz (26) (see Material and Methods) with CCCP did not change the results obtained with RB1La. Furthermore, CCCP did not affect the EtBr efflux in the reference strain PG21, with a fluorescence decrease from 100 to 80% (data not shown). In contrast, reserpine and orthovanadate showed a distinct effect on the EtBr efflux by M. hominis PG21, with a smaller fluorescence decrease, from 100 to 91% (Fig. 6). Finally, the particular phenotype of the resistant strains RB1 and RB1La (resistance to hydrophilic fluoroquinolones and dyes) associated with a decrease in CIP and EtBr accumulations, reversed by MDR inhibitors (reserpine and orthovanadate), is consistent with an MDR active efflux system in M. hominis.
FIG. 6.
Efflux of EtBr (10 μg/ml) by M. hominis PG21 and the EtBr-resistant strain RB1La, in the absence or presence of the three inhibitors, 20 μg of reserpine (RES)/ml, 100 μM CCCP, or 2 mM sodium orthovanadate (VAN). Cells were energized with 5 g of arginine/liter. Fluorescence is expressed as a percentage of that at the first time point (100%) for each strain. Error bars show standard deviations.
DISCUSSION
Our previous studies have shown that genetic changes in the target genes of fluoroquinolones were associated with resistance to these antimicrobials in M. hominis (9-10). We therefore investigated the possible involvement of an active efflux mechanism in resistance to fluoroquinolones in this wall-less organism.
The mode of penetration of fluoroquinolones in M. hominis being unknown, the first step of this study consisted in characterizing the uptake into a susceptible strain (M. hominis PG21). On the basis of our data, fluoroquinolones apparently enter in M. hominis by passive diffusion across the cytoplasmic membrane, as previously described for several bacterial species (18, 37, 42, 54). Fluoroquinolone accumulation by mycoplasmas showed kinetics similar to those reported for other bacteria, such as Escherichia coli, S. aureus (4, 7, 31, 33, 42), and S. pneumoniae (54).
Our studies with the susceptible strain M. hominis PG21 suggest the existence of an active process implicated in the uptake of fluoroquinolones, as shown by the differences in accumulation between arginine-energized and -nonenergized cells for all fluoroquinolones tested. Similar results were described for S. pneumoniae glucose-energized cells (54). Furthermore, the concentration of CIP but not PEF or MXF accumulated by M. hominis PG21 was much more increased with the pump blocker reserpine and the ABC-type transporter inhibitor orthovanadate. It has been suggested that hydrophilic quinolones are removed more efficiently than hydrophobic compounds, but the exact reasons of this preference are still unclear and controversial (27, 41, 46, 51). The variance between both fluoroquinolones could reflect different affinities of a putative efflux pump for them, CIP being a much better substrate than PEF and MXF. This variability in the effects upon fluoroquinolone efflux by inhibitors has been reported, notably for S. aureus (31, 41, 51).
The absence of a significant effect of the protonophore CCCP on the accumulation of CIP and PEF by M. hominis PG21 could be related to the chemical properties of fluoroquinolones rather than a direct effect of CCCP on the efflux function. As previously described (18, 37, 50), the passive distribution of fluoroquinolones which have two proton binding sites, implying four protonation species (cationic, anionic, zwitterionic, and neutral), is determined by the pH gradient of the cytoplasmic membrane. The addition of CCCP abolishes the pH gradient, modifying their distribution. Thus, the proportion of charged species of quinolones increasing extracellularly leads to a decrease of penetration across the membrane by the uncharged and zwitterionic species, masking an eventual direct effect of CCCP on a transport system. Such a lack of CCCP effect has been previously described for other secondary transporters expressed in the gram-negative E. coli (49), P. aeruginosa (3), Burkholderia pseudomallei (32), the gram-positive L. lactis (39), and mycobacteria (2, 14).
To summarize, the characteristics of the energy coupling of CIP accumulation suggest the involvement rather of an ATP-dependent than of a PMF-dependent efflux system in CIP extrusion by M. hominis, contributing to the intrinsic susceptibility of M. hominis to CIP. It is tempting to speculate that this putative efflux pump present in the wild-type strain confers a baseline low level of intrinsic resistance to CIP and the structurally unrelated EtBr, as previously described for NorA in S. aureus (27).
In order to isolate a mycoplasmal strain with an MDR phenotype compatible with an efflux mechanism, we first tried to select such a strain with hydrophilic fluoroquinolones, CIP and NOR. We did not expect to obtain easily efflux-resistant mutants using quinolones as selecting agents, but previous results obtained in vitro with S. pneumoniae (12) led us to try this selection. The phenotypic screening method with reserpine did not detect any strain with a significant difference in the CIP MIC among the resistant strains obtained (data not shown). Then, three resistant strains, RB1, RB1La, and RB1Lb, were isolated by selecting for resistance to EtBr and showed a similar pattern of cross-resistance to hydrophilic fluoroquinolones and unrelated compounds, such as EtBr and acriflavine, which were typical substrates for the bacterial MDR pumps, S. aureus NorA (24, 25, 28, 34), B. subtilis Bmr (1, 35), and S. pneumoniae PmrA (12, 54). Furthermore, fluoroquinolone and EtBr resistance could be reversed by reserpine, suggesting the existence of a reserpine-sensitive mechanism of resistance in these strains, as has been described for several MDR systems (5, 11, 12, 20, 36, 52). No cross-resistance to other families of efflux substrate antibiotics active against mycoplasmas was observed with regard to the MICs of tetracyclines, macrolides, or chloramphenicol.
The results of accumulation of CIP, MXF, and PEF by the EtBr-resistant strains RB1 and RB1La, for which the MICs of hydrophilic fluoroquinolones increased more than those of the hydrophobic ones, are consistent with an active efflux system having CIP as a preferential substrate. Indeed, a very large difference in the amount of cell-associated CIP, but not MXF and PEF, was observed between the parent strain PG21 and the two derived resistant mutants. The MXF and PEF uptake data confirmed those obtained with the reference strain, PG21. In this study, both reserpine and orthovanadate inhibited the efflux of CIP and EtBr, suggesting the involvement of an ATP-dependent efflux system. Such a hypothesis is reinforced by the sequence analysis data of the whole genome of two human mycoplasmas, Mycoplasma genitalium (17) and M. pneumoniae (21). Indeed, 11 ABC transporters but only one PMF-dependent transporter have been identified in these two microorganisms by sequence comparison (38). However, our data are insufficient to eliminate a PMF-dependent system. The lack of an effect of CCCP on the fluoroquinolone uptake by the EtBr-resistant strains could be related more to the annihilation of the ΔpH, modifying the entry of fluoroquinolones, than to a specific effect of CCCP on the efflux system (see above).
Considering the EtBr transport assays, the CCCP-induced increase of the EtBr uptake by strain RB1La (Fig. 5A) could be interpreted either as the result of a direct inhibition on the efflux system or as the effect of CCCP on the passive distribution of the cationic EtBr across the cytoplasmic membrane. The lack of a significant effect of CCCP on ethidium efflux in the RB1La strain (Fig. 6) is more consistent with the second hypothesis. It should be noticed that a CCCP-insensitive EtBr efflux system has been described very recently for the gram-positive bacterium Streptococcus pyogenes, in a strain selected on EtBr harboring an MDR phenotype with increased MICs of NOR, EtBr and acriflavine (H. E. Jones, N. P. Brenwald, K. A. Staaf, and M. J. Gill, abstract from the Seventh International Symposium on New Quinolones, J. Antimicrob. Chemother. 47(Suppl.):32-33, 2001).
In conclusion, the present study demonstrates for the first time the biological presence of an active efflux system implicated in the resistance to CIP and unrelated compounds in a wall-less organism, M. hominis. To dissect this putative drug efflux activity and to settle the question of its energy source between PFM or ATP, a genetic approach is currently being pursued.
Acknowledgments
We are grateful to Nicole Moreau for helpful comments and critical reading of the manuscript. We thank Glenn Kaatz for providing the S. aureus strains SA-1199 and SA-1199B.
This study was supported in part by a grant from Bayer-Pharma.
REFERENCES
- 1.Ahmed, M., L. Lyass, P. N. Markham, S. S. Taylor, N. Vazquez-Laslop, and A. A. Neyfakh. 1995. Two highly similar multidrug transporters of Bacillus subtilis whose expression is differentially regulated. J. Bacteriol. 177:3904-3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ainsa, J. A., M. C. Blokpoel, I. Otal, D. B. Young, K. A. De Smet, and C. Martin. 1998. Molecular cloning and characterization of Tap, a putative multidrug efflux pump present in Mycobacterium fortuitum and Mycobacterium tuberculosis. J. Bacteriol. 180:5836-5843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aires, J. R., T. Kohler, H. Nikaido, and P. Plesiat. 1999. Involvement of an active efflux system in the natural resistance of Pseudomonas aeruginosa to aminoglycosides. Antimicrob. Agents Chemother. 43:2624-2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asuquo, A. E., and L. J. Piddock. 1993. Accumulation and killing kinetics of fifteen quinolones for Escherichia coli, Staphylococcus aureus and Pseudomonas aeruginosa. J. Antimicrob. Chemother. 31:865-880. [DOI] [PubMed] [Google Scholar]
- 5.Baranova, N. N., and A. A. Neyfakh. 1997. Apparent involvement of a multidrug transporter in the fluoroquinolone resistance of Streptococcus pneumoniae. Antimicrob. Agents Chemother. 41:1396-1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bast, D. J., D. E. Low, C. L. Duncan, L. Kilburn, L. A. Mandell, R. J. Davidson, and J. C. S. Deazavedo. 2000. Fluoroquinolone resistance in clinical isolates of Streptococcus pneumoniae: contributions of type II topoisomerase mutations and efflux to levels of resistance. Antimicrob. Agents Chemother. 44:3049-3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bazile, S., N. Moreau, D. Bouzard, and M. Essiz. 1992. Relationships among antibacterial activity, inhibition of DNA gyrase, and intracellular accumulation of 11 fluoroquinolones. Antimicrob. Agents Chemother. 36:2622-2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bébéar, C., and J. Robertson. 1996. Determination of minimal inhibitory concentration, p. 189-199. In J. G. Tully and S. Razin (ed.), Molecular and diagnostic procedures in mycoplasmology, vol. II. Academic Press, San Diego, Calif. [Google Scholar]
- 9.Bébéar, C. M., H. Renaudin, A. Charron, J. M. Bové, C. Bébéar, and J. Renaudin. 1998. Alterations in topoisomerase IV and DNA gyrase in quinolone-resistant mutants of Mycoplasma hominis obtained in vitro. Antimicrob. Agents Chemother. 42:2304-2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bébéar, C. M., J. Renaudin, A. Charron, H. Renaudin, B. de Barbeyrac, T. Schaeverbeke, and C. Bébéar. 1999. Mutations in the gyrA, parC, and parE genes associated with fluoroquinolone resistance in clinical isolates of Mycoplasma hominis. Antimicrob. Agents Chemother. 43:954-956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bolhuis, H., D. Molenaar, G. Poelarends, H. W. van Veen, B. Poolman, A. J. Driessen, and W. N. Konings. 1994. Proton motive force-driven and ATP-dependent drug extrusion systems in multidrug-resistant Lactococcus lactis. J. Bacteriol. 176:6957-6964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brenwald, N. P., M. J. Gill, and R. Wise. 1998. Prevalence of a putative efflux mechanism among fluoroquinolone-resistant clinical isolates of Streptococcus pneumoniae. Antimicrob. Agents Chemother. 42:2032-2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Denis, A., and N. J. Moreau. 1993. Mechanisms of quinolone resistance in clinical isolates: accumulation of sparfloxacin and of fluoroquinolones of various hydrophobicity, and analysis of membrane composition. J. Antimicrob. Chemother. 32:379-392. [DOI] [PubMed] [Google Scholar]
- 14.De Rossi, E., M. Branzoni, R. Cantoni, A. Milano, G. Riccardi, and O. Ciferri. 1998. mmr, a Mycobacterium tuberculosis gene conferring resistance to small cationic dyes and inhibitors. J. Bacteriol. 180:6068-6071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diver, J. M., L. J. Piddock, and R. Wise. 1990. The accumulation of five quinolone antibacterial agents by Escherichia coli. J. Antimicrob. Chemother. 25:319-333. [DOI] [PubMed] [Google Scholar]
- 16.Ferrandiz, M. J., J. Oteo, B. Aracil, J. L. Gomez-Garces, and A. G. de la Campa. 1999. Drug efflux and parC mutations are involved in fluoroquinolone resistance in viridans group streptococci. Antimicrob. Agents Chemother. 43:2520-2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fraser, C. M., J. D. Gocayne, O. White, M. D. Adams, R. A. Clayton, R. D. Fleischmann, C. J. Bult, A. R. Kerlavage, G. Sutton, J. M. Kelley, et al. 1995. The minimal gene complement of Mycoplasma genitalium. Science 270:397-403. [DOI] [PubMed] [Google Scholar]
- 18.Furet, Y. X., J. Deshusses, and J. C. Pechere. 1992. Transport of pefloxacin across the bacterial cytoplasmic membrane in quinolone-susceptible Staphylococcus aureus. Antimicrob. Agents Chemother. 36:2506-2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gill, M. J., N. P. Brenwald, and R. Wise. 1999. Identification of an efflux pump gene, pmrA, associated with fluoroquinolone resistance in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 43:187-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gottesman, M. M., and I. Pastan. 1993. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu. Rev. Biochem. 62:385-427. [DOI] [PubMed] [Google Scholar]
- 21.Himmelreich, R., H. Hilbert, H. Plagens, E. Pirkl, B. C. Li, and R. Herrmann. 1996. Complete sequence analysis of the genome of the bacterium Mycoplasma pneumoniae. Nucleic Acids Res. 24:4420-4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hooper, D. C. 1998. Bacterial topoisomerases, anti-topoisomerases, and anti-topoisomerase resistance. Clin. Infect. Dis. 27:S54-S63. [DOI] [PubMed] [Google Scholar]
- 23.Kaatz, G. W., and S. M. Seo. 1995. Inducible NorA-mediated multidrug resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 39:2650-2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaatz, G. W., and S. M. Seo. 1997. Mechanisms of fluoroquinolone resistance in genetically related strains of Staphylococcus aureus. Antimicrob. Agents Chemother. 41:2733-2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaatz, G. W., S. M. Seo, and T. J. Foster. 1999. Introduction of a norA promoter region mutation into the chromosome of a fluoroquinolone-susceptible strain of Staphylococcus aureus using plasmid integration. Antimicrob. Agents Chemother. 43:2222-2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaatz, G. W., S. M. Seo, L. Obrien, M. Wahiduzzaman, and T. J. Foster. 2000. Evidence for the existence of a multidrug efflux transporter distinct from NorA in Staphylococcus aureus. Antimicrob. Agents Chemother. 44:1404-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaatz, G. W., S. M. Seo, and C. A. Ruble. 1993. Efflux-mediated fluoroquinolone resistance in Staphylococcus aureus. Antimicrob. Agents Chemother. 37:1086-1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaatz, G. W., S. M. Seo, and C. A. Ruble. 1991. Mechanisms of fluoroquinolone resistance in Staphylococcus aureus. J. Infect. Dis. 163:1080-1086. [DOI] [PubMed] [Google Scholar]
- 29.Li, X.-Z., D. Livermore, and H. Nikaido. 1994. Role of efflux pumps(s) in intrinsic resistance of Pseudomonas aeruginosa: resistance to tetracycline, chloramphenicol, and norfloxacin. Antimicrob. Agents Chemother. 38:1732-1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Markham, P. N. 1999. Inhibition of the emergence of ciprofloxacin resistance in Streptococcus pneumoniae by the multidrug efflux inhibitor reserpine. Antimicrob. Agents Chemother. 43:988-989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCaffrey, C., A. Bertasso, J. Pace, and N. H. Georgopapadakou 1992. Quinolone accumulation in Escherichia coli, Pseudomonas aeruginosa, and Staphylococcus aureus. Antimicrob. Agents Chemother. 36:1601-1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moore, R. A., D. DeShazer, S. Reckseidler, A. Weissman, and D. E. Woods. 1999. Efflux-mediated aminoglycoside and macrolide resistance in Burkholderia pseudomallei. Antimicrob. Agents Chemother. 43:465-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mortimer, P. G. S., and L. J. V. Piddock. 1991. A comparison of methods used for measuring the accumulation of quinolones by Enterobacteriaceae, Pseudomonas aeruginosa and Staphylococcus aureus. J. Antimicrob. Chemother. 28:639-653. [DOI] [PubMed] [Google Scholar]
- 34.Munoz-Bellido, J. L., M. Alonzo Manzanares, J. A. Martinez Andres, M. N. Guttierrez Zufiaurre, G. Ortiz, M. Segovia Hernandez, and J. A. Garcia-Rodriguez. 1999. Efflux pump-mediated quinolone resistance in Staphylococcus aureus strains wild type for gyrA, gyrB, grlA, and norA. Antimicrob. Agents Chemother. 43:354-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neyfakh, A. A. 1992. The multidrug efflux transporter of Bacillus subtilis is a structural and functional homolog of the Staphylococcus NorA protein. Antimicrob. Agents Chemother. 36:484-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neyfakh, A. A., V. E. Bidnenko, and L. B. Chen. 1991. Efflux-mediated multidrug resistance in Bacillus subtilis: similarities and dissimilarities with the mammalian system. Proc. Natl. Acad. Sci. USA 88:4781-4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nikaido, H., and D. G. Thanassi 1993. Penetration of lipophilic agents with multiple protonation sites into bacterial cells: tetracyclines and fluoroquinolones as examples. Antimicrob. Agents Chemother. 37:1393-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paulsen, I. T., M. K. Sliwinski, and M. H. Saier, Jr. 1998. Microbial genome analyses: global comparisons of transport capabilities based on phylogenies, bioenergetics and substrate specificities. J. Mol. Biol. 277:573-592. [DOI] [PubMed] [Google Scholar]
- 39.Perreten, V., F. V. Schwarz, M. Teuber, and S. B. Levy. 2001. Mdt(A), a new efflux protein conferring multiple antibiotic resistance in Lactococcus lactis and Escherichia coli. Antimicrob. Agents Chemother. 45:1109-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Piddock, L. J., and Y. F. Jin. 2001. Antimicrobial activity and accumulation of moxifloxacin in quinolone-susceptible bacteria. J. Antimicrob. Chemother. 42(Suppl. B):39-42. [DOI] [PubMed] [Google Scholar]
- 41.Piddock, L. J., Y. F. Jin, and D. J. Griggs. 2001. Effect of hydrophobicity and molecular mass on the accumulation of fluoroquinolones by Staphylococcus aureus. J. Antimicrob. Chemother. 47:261-270. [DOI] [PubMed] [Google Scholar]
- 42.Piddock, L. J., Y. F. Jin, V. Ricci, and A. E. Asuquo. 1999. Quinolone accumulation by Pseudomonas aeruginosa, Staphylococcus aureus and Escherichia coli. J. Antimicrob. Chemother. 43:61-70. [DOI] [PubMed] [Google Scholar]
- 43.Piddock, L. J. V., D. G. White, K. Gensberg, L. Pumbwe, and D. J. Griggs. 2000. Evidence for an efflux pump mediating multiple antibiotic resistance in Salmonella enterica serovar Typhimurium. Antimicrob. Agents Chemother. 44:3118-3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pollack, J. D. 1992. Carbohydrate metabolism and energy conservation, p. 181-200. In J. Maniloff, R. N. McElhaney, L. R. Finch, and J. B. Baseman (ed.), Mycoplasmas: molecular biology and pathogenesis. American Society for Microbiology, Washington, D.C.
- 45.Poole, K. 2000. Efflux-mediated resistance to fluoroquinolones in gram-negative bacteria. Antimicrob. Agents Chemother. 44:2233-2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Poole, K. 2000. Efflux-mediated resistance to fluoroquinolones in gram-positive bacteria and the mycobacteria. Antimicrob. Agents Chemother. 44:2595-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Putman, M., H. W. van Veen, and W. N. Konings. 2000. Molecular properties of bacterial multidrug transporters. Microbiol. Mol. Biol. Rev. 64:672-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ricci, V., and L. J. V. Piddock. 2000. Accumulation of norfloxacin by Bacteroides fragilis. Antimicrob. Agents Chemother. 44:2361-2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rosenberg, E. Y., D. Ma, and H. Nikaido. 2000. AcrD of Escherichia coli is an aminoglycoside efflux pump. J. Bacteriol. 182:1754-1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ross, D. L., S. K. Elkington, and C. M. Riley. 1992. Physicochemical properties of the fluoroquinolone antimicrobials. III. 1-Octanol/water partition coefficients and their relationships to structure. Int. J. Pharm. 88:379-389. [Google Scholar]
- 51.Takenouchi, T., F. Tabata, Y. Iwata, H. Hanzawa, M. Sugawara, and S. Ohya. 1996. Hydrophilicity of quinolones is not an exclusive factor for decreased activity in efflux-mediated resistant mutants of Staphylococcus aureus. Antimicrob. Agents Chemother. 40:1835-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Veen, H. W., K. Venema, H. Bolhuis, I. Oussenko, J. Kok, B. Poolman, A. J. Driessen, and W. N. Konings. 1996. Multidrug resistance mediated by a bacterial homolog of the human multidrug transporter MDR1. Proc. Natl. Acad. Sci. USA 93:10668-10672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Woese, C. R. 1987. Bacterial evolution. Microbiol. Rev. 51:221-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zeller, V., C. Janoir, M. D. Kitzis, L. Gutmann, and N. J. Moreau. 1997. Active efflux as a mechanism of resistance to ciprofloxacin in Streptococcus pneumoniae. Antimicrob. Agents Chemother. 41:1973-1978. [DOI] [PMC free article] [PubMed] [Google Scholar]