Abstract
Free-living amoebae of the genus Acanthamoeba are causing serious chronic conditions such as destructive keratitis in contact lens wearers or granulomatous amoebic encephalitis in individuals with compromised immune systems. Both are characterized by the lack of availability of sufficiently effective and uncomplicated, manageable treatments. Hexadecylphosphocholine (miltefosine) is licensed for use as a topical antineoplastic agent, but it is also active in vitro against several protozoan parasites, and it was applied very successfully for the treatment of human visceral leishmaniasis. The aim of our study was to evaluate the efficacy of hexadecylphosphocholine and other alkylphosphocholines (APCs) against Acanthamoeba spp. The in vitro activities of eight different APCs against three Acanthamoeba strains of various pathogenicities were determined. All substances showed at least amoebostatic effects, and some of them disrupted the amoebae, as shown by the release of cytoplasmic enzyme activity. Hexadecylphosphocholine exhibited the highest degree of cytotoxicity against trophozoites, resulting in complete cell death at a concentration as low as 40 μM, and also displayed significant cysticidal activity. Hexadecylphosphocholine may be a promising new candidate for the topical treatment of Acanthamoeba keratitis and, conceivably, even for the oral treatment of granulomatous amoebic encephalitis.
Free-living amoebae of the genus Acanthamoeba are the causative agents of both a painful and often seriously progressing keratitis (11) and granulomatous amoebic encephalitis (GAE), which predominantly occurs in immunodeficient individuals. As of October 1996, more than 100 cases of Acanthamoeba GAE and more than 700 cases of Acanthamoeba keratitis had been reported worldwide (18). Moreover, acanthamoebae cause several disseminating infections such as Acanthamoeba dermatitis and Acanthamoeba pneumonitis in the immunocompromised host (18).
The genus Acanthamoeba has been classified into three morphological groups (21), with the vast majority of pathogenic strains belonging to morphological group II. However, various representatives of morphological group III have also been reported to cause disease.
To date there is no effective treatment for GAE or disseminated acanthamoebiasis. Only in a very few cases have the patients responded favorably to therapy. One example was a case of disseminated Acanthamoeba infection without central nervous system involvement in a transplant patient who was successfully treated with intravenous pentamidine isethionate and local chlorhexidine and ketoconazole (26). In a previously healthy 7-year-old girl, GAE was controlled with ketoconazole chemotherapy and total excision of the infected area (20), and recently, for the first time, a case of AIDS-associated Acanthamoeba GAE was successfully treated by chemotherapy with fluconazole and sulfadiazine, also in combination with surgical excision of the lesion (25). The immunosuppressed state of the patients, the low levels of activity of the antiamoebic agents against trophozoites and particularly cysts, and the low levels of these agents in the brain due to the blood-brain barrier have resulted in a grave prognosis for patients with Acanthamoeba GAE.
Acanthamoeba keratitis is usually treated with a combination of cationic antiseptics which inhibit membrane function, such as polyhexamethylene biguanide, and aromatic diamidines which inhibit DNA synthesis, such as propamidine isethionate (Brolene), which must be applied every hour for 3 days and subsequently up to six times a day (3, 22). However, sufficient efficacy is achieved in only half of the patients, and resistance to propamidine was observed to develop in a temporal series of Acanthamoeba isolates from one patient (7). A valid alternative to polyhexamethylene biguanide is chlorhexidine, which has also been combined with propamidine isethionate (8). More recently, chlorhexidine alone has also been applied successfully (15). All these therapies need to be applied hourly at least for several days and therefore usually require stationary treatment.
Alkylphosphocholines (APCs), a group of compounds consisting of phosphocholine esterified to various long-chain aliphatic alcohols, exhibit in vitro and in vivo antineoplastic activities (6, 28). In addition, they are active against several protozoan parasites such as Leishmania donovani (1, 17), Trypanosoma cruzi (23), and Entamoeba histolytica (24). In humans, a clinical study demonstrated that visceral leishmaniasis could be treated successfully by oral treatment with hexadecylphosphocholine (APC1) (10).
The aim of the present study was to evaluate the potential efficacies of APCs against free-living amoebae of the genus Acanthamoeba. The activities of eight different APCs against three Acanthamoeba group II and group III strains with various pathogenicities were evaluated in vitro.
MATERIALS AND METHODS
Chemicals.
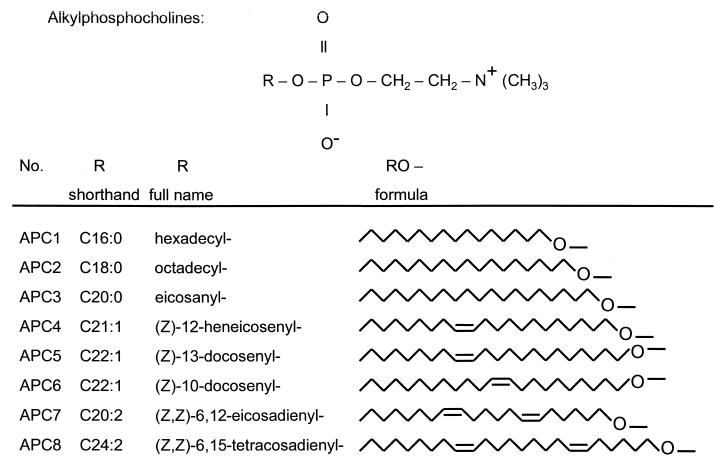
Eight different APCs were investigated for their antiamoebic activities. Their formulas are shown in Fig. 1. The compounds were hexadecylphosphocholine APC1 (miltefosine), octadecylphosphocholine (APC2), eicosanylphosphocholine (APC3), (Z)-12-heneicosenylphosphocholine (APC4), (Z)-13-docosenylphosphocholine (APC5), (Z)-10-docosenylphosphocholine (APC6), (Z,Z)-6,12-eicosadienylphosphocholine (APC7), and (Z,Z)-6,15-tetracosadienylphosphocholine (APC8). The APCs were prepared from the respective alcohols as described by Eibl (4). More detailed conditions have been reported for the synthesis of APC1 (5). For the cytotoxicity measurements, 2 mM stock solutions of each APC were prepared. All APCs except APC7 were dissolved in distilled water containing 5% (wt/vol) ethanol. APC7 was dissolved in distilled water.
FIG. 1.
APCs examined in the study. The APCs are phosphocholine (top) esters with various long-chain saturated and unsaturated alkyl groups. The shorthand designations for the alkyl chains indicate the number of C atoms of the alkyl chain, followed by a colon and the number of double bonds.
Amoeba strains.
Two Acanthamoeba strains isolated from clinical specimens (strain 1BU and strain 5SU) and one strain isolated from mouse brain (strain 72/2) were used in the study. Strains 1BU and 5SU are representatives of Acanthamoeba sp. group II, while strain 72/2 exhibits the Acanthamoeba sp. group III morphology, according to the classification of Pussard and Pons (21). Strain 1BU (Acanthamoeba castellanii) was isolated from clinical specimens of a patient with a severe keratitis in the left eye (30). This strain was highly pathogenic in tissue culture (31). Strain 5SU (Acanthamoeba polyphaga) was isolated from the contact lens case of a keratitis patient, but careful examination showed that it was nonpathogenic and not the causative agent of the patient's keratitis (31). Strain 72/2 (Acanthamoeba lenticulata) had originally been isolated from the nasal mucosa of a healthy individual (19); however, it was highly virulent in mice after intranasal instillation (2). The isolate of strain 72/2 used in the present study was reisolated from mouse brain and was thermophilic and also highly pathogenic for a human cell line (31).
Growth conditions.
The acanthamoebae were grown axenically in 150-cm2 conical tissue culture flasks (Costar; Corning, Bodenheim, Germany). Axenic cultures were obtained as described previously (30). Briefly, cysts were harvested from 7-day xenic plate cultures with sterile cotton-tipped applicators, washed three times in sterile saline, transferred into 3% HCl, and incubated at room temperature overnight in order to inactivate the bacteria. After another washing step the cysts were transferred into proteose-peptone-yeast extract-glucose medium (29) and incubated at 30°C. Actively growing trophozoites were harvested after 72 h by centrifugation at 500 × g for 7 min. For encystment of the amoebae, MgCl2 was added to the medium to a final concentration of 50 mM and the cultures were incubated at 30°C. The process of encystment including morphological changes to the cyst wall was observed daily by phase-contrast microscopy at ×1,000 magnification. In all three strains, essentially all the cysts had matured after 7 days. A pure cyst population was then harvested on day 14 by centrifugation at 500 × g for 7 min. The cells (trophozoites or cysts) were resuspended in 10 ml of sterile medium, counted in a Bürker-Türk hemocytometer, and brought to a concentration of 105 cells ml−1.
Cytotoxicity measurements.
Experiments were carried out in 24-well microtiter plates at 30 or 37°C under sterile conditions. Each well was seeded with 1 ml (105 cells) of a trophozoite or cyst suspension. In the first experiment, each APC was tested at a concentration of 80 μM against trophozoites and cysts of all three strains. The log reduction of amoeba cells was recorded after 30 min and 24, 48, and 72 h by counting the surviving cells in a Bürker-Türk hemocytometer. Viability was determined by trypan blue exclusion and phase-contrast microscopy. Statistical evaluation was done with the SPSS program package (SPSS Inc., Chicago, Ill.). One hundred percent eradication was confirmed by transferring 100 μl of the suspension to an Escherichia coli-coated agar plate and recording the amoebal growth for 14 days. In a second experiment, APC1 was tested at six concentrations (5, 10, 20, 40, 80, and 160 μM) against trophozoites and cysts of all three strains. Cell morphology was observed by phase-contrast microscopy. The APC cytotoxicity assays were performed in two independent experiments, each time in triplicate.
Cell disruption assay.
The release of the cytoplasmic enzyme hexokinase from APC1-treated acanthamoebae was used as a measure for cell disruption. A culture of 2 × 106 trophozoites was harvested by centrifugation at 500 × g for 7 min. The pellet was resuspended in 1 ml of 0.9% NaCl and divided into two aliquots of 500 μl (106 cells) each. One part was treated for 30 min with APC1 at a final concentration of 200 μM. The other part was disrupted by freezing-thawing three times to liberate cytoplasmic components. The spontaneous release was determined by measuring hexokinase activity in the culture supernatants. Untreated cells were precipitated as described above, and for the treated samples, debris was removed by centrifugation at 12,000 × g for 5 min at room temperature. Triplicate measurements were carried out. Hexokinase activity was assessed by the standard glucose-6-phosphate dehydrogenase-coupled assay, which measures in a spectrophotometer the absorption of the NADPH produced at 340 nm. The assay mixture contained 5 mM glucose, 1 mM ATP, 0.7 mM NADP+, and 1 U of glucose-6-phosphate dehydrogenase ml−1 (final concentrations). The sample was added immediately before measurement of the activity, and the change in optical density was recorded for 1 min. One unit of activity corresponds to the phosphorylation of 1 μM glucose per min. For control reasons, the effect of APC1 on hexokinase activity was also measured; no inhibition was observed at a concentration of 200 μM.
RESULTS
Cytotoxic activities of APCs.
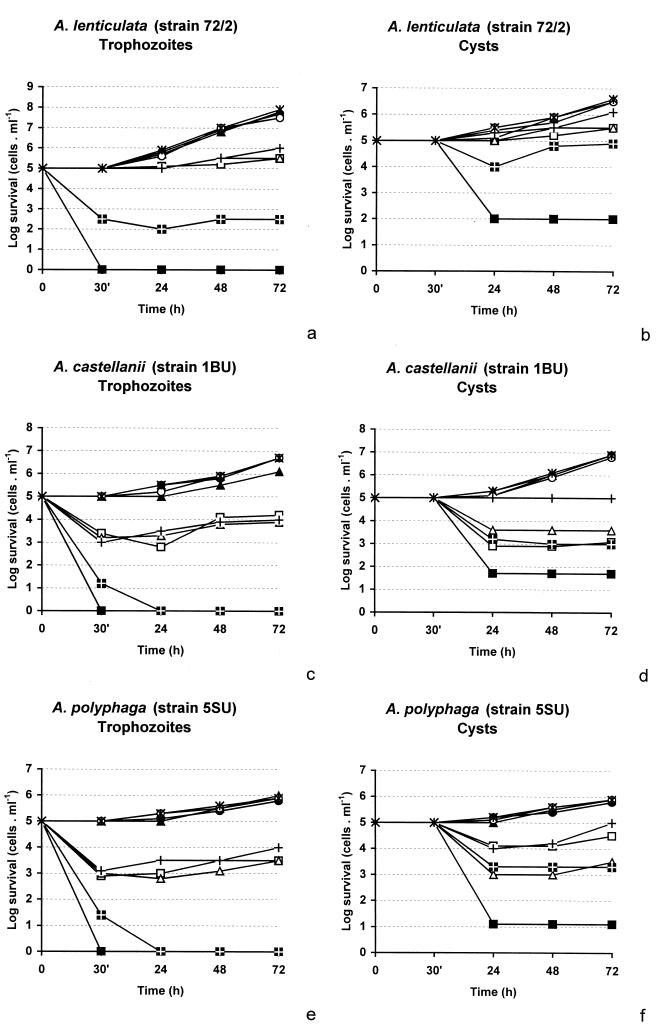
The time-dependent cytotoxicities of the eight different APCs (Fig. 1), each at a fixed concentration of 80 μM, were tested against trophozoites and cysts of three different isolates of Acanthamoeba at 30°C. Most of the APCs tested showed at least amoebostatic effects against each of the strains of Acanthamoeba (Fig. 2). APC1 had the highest level of activity against all strains. A. lenticulata 72/2 (morphological group III) was generally the most resistant, while strain 5SU (morphological group II) was the most sensitive. Substances APC1, APC2, APC4, APC7, and APC8 showed various levels of activity; however, only APC1 had a 100% amoebicidal effect against all strains, and APC7 was 100% amoebicidal for group II strains 1BU (A. castellanii) and 5SU (A. polyphaga). Essentially the same log reductions of the acanthamoebae were obtained in a series of experiments carried out at an incubation temperature of 37°C (data not shown).
FIG. 2.
Antiamoebic activities of various APCs against trophozoites (a, c, and e) and cysts (b, d, and f) of A. lenticulata (a and b), A. castellanii (c and d), and A. polyphaga (e and f). ▪, APC1; □, APC2; ▴, APC3; ▵, APC4; •, APC5; ○, APC6; , APC7; +, APC8. Each APC was used at a concentration of 80 μM; , untreated control. APC1 had a significantly higher level of cytotoxic activity than the second best substance, APC7 (P < 0.01), and much higher levels of activity than the rest of the substances (P < 0.001). Group III strain A. lenticulata was significantly less susceptible than group II strains A. castellanii and A. polyphaga (P < 0.001).
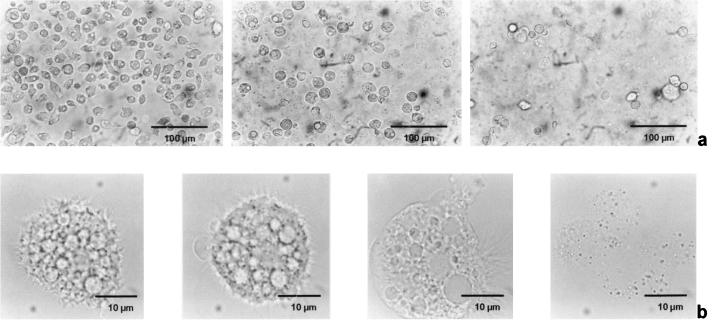
Figure 3 consists of a series of light microscopic images of A. castellanii (1BU) trophozoites treated with APC1 at a concentration of 80 μM. Within 10 min after the addition of the APCs the acanthamoebae rounded up and detached from the bottom of the tissue culture well. The visible structures within the cytoplasm disappeared. Cell death set in after about 30 min, as determined by trypan blue staining. After 1 h the amoebae underwent complete lysis, whereby only the nucleus remained visible. Acanthamoebae treated with other APCs had the same responses, but higher concentrations of the substances were needed and the rate of killing was slower.
FIG. 3.
Cytotoxic effect of APC1 on trophozoites of A. castellanii (strain 1BU). (a) Trophozoite monolayer before treatment, 30 min after treatment, and 1 h after treatment with 80 μM APC1, from left to right, respectively. (b) Representative single trophozoite before treatment, after 15 min (note the blebbing of the cell), after 30 min (disappearance of cytoplasmic structures), and after 1 h (lysis), from left to right, respectively.
In all cases the cysts were less sensitive than the trophozoites. A 100% eradication of the cysts was not achieved for any of the Acanthamoeba strains tested; however, APC1 was strongly cysticidal for all three strains, resulting in between 60% (A. lenticulata 72/2) and 80% (A. polyphaga 5SU) log reductions, i.e., killing of beween 99.9 and 99.99% of the cysts (Fig. 2). Moreover, in a separate experiment, APC1 inhibited excystment at a concentration as low as 5 μM (data not shown). APC2, APC4, and APC7 displayed significant cysticidal activities only against A. castellanii 1BU and A. polyphaga 5SU, whereas the rest of the substances were not cysticidal.
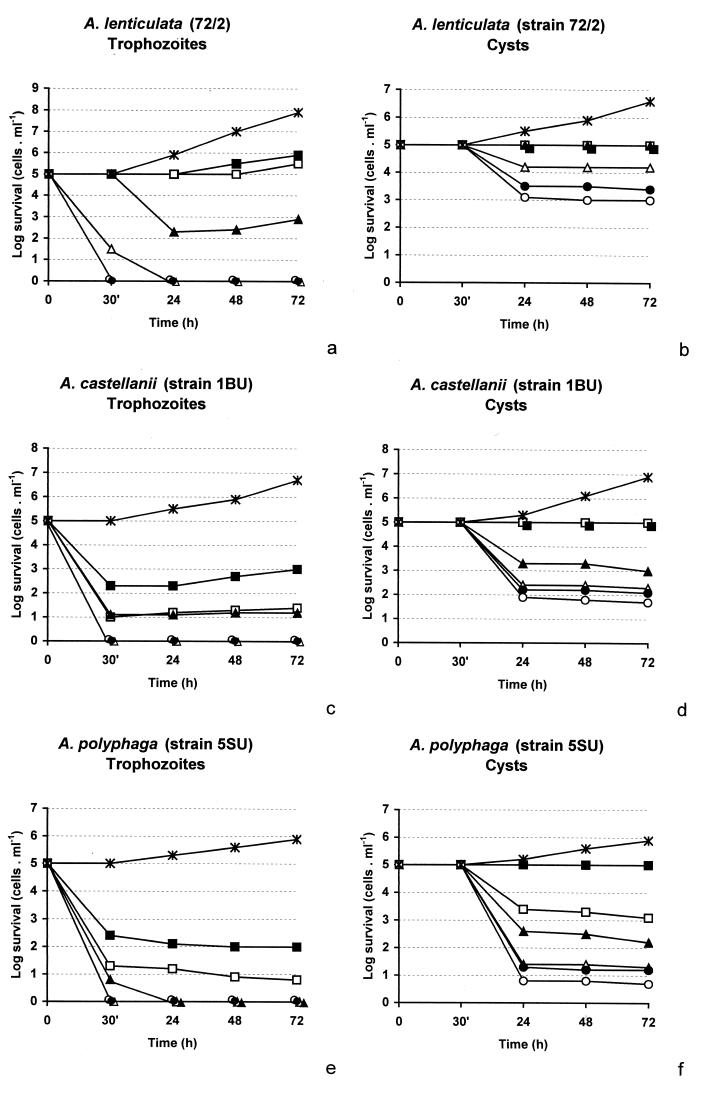
Figure 4 illustrates the cytotoxicities of APC1 at various concentrations against trophozoites and cysts of the three Acanthamoeba strains. Generally, the two morphological group II strains (A. castellanii 1BU and A. polyphaga 5SU) were somewhat more sensitive than group III strain A. lenticulata 72/2. Treatment with APC1 at a concentration of 40 μM resulted in a 100% log reduction of trophozoites of the group II acanthamoebae after just 30 min and resulted in a 100% log reduction of trophozoites of the group III strain after 24 h. A concentration of 80 μM was enough to eradicate the group III strain after 30 min.
FIG. 4.
Effects of different concentrations of APC1 against trophozoites and cysts of A. lenticulata (a and b), A. castellanii (c and d), and A. polyphaga (e and f). Concentrations of 5 μM (▪), 10 μM (□), 20 μM (▴), 40 μM (▵), 80 μM (•), and 160 μM (○) were used. ∗, control.
Release of cytoplasmic components by APC1.
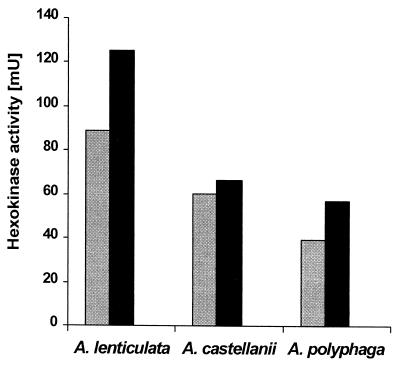
Acanthamoeba trophozoites contain the glycolytic enzyme hexokinase (EC 2.7.1.1), which could be liberated from all three Acanthamoeba strains by repeated cycles of freezing and thawing (Fig. 5). The hexokinase activity in the cell culture supernatants of untreated trophozoites was always <0.5% of the activity liberated by freezing-thawing. Treatment of the three strains with 200 μM APC1 for 30 min disrupted the cells so that significant amounts (70 to 90%) of hexokinase were released into the medium (Fig. 5).
FIG. 5.
Release of cytoplasmic enzyme hexokinase in Acanthamoeba strains 1BU, 5SU, and 72/2 by treatment with 200 μM APC1 (left bars) in comparison to the total amount liberated by freezing-thawing of the cells (right bars). The y axis shows the activity per 106 cells. The spontaneous release was less than 0.5% of the total amounts in all cases (data not shown).
DISCUSSION
Susceptibilities of trophozoites.
The amoebicidal activities of eight different APCs against three strains of Acanthamoeba of different pathogenicities were studied. Most of the APCs had at least cytostatic effects against cultures of Acanthamoeba spp. APC1 showed by far the highest level of amoebicidal activity against each of the Acanthamoeba strains, resulting in a 100% log reduction within 30 min at a concentration of 40 μM for strains 1BU and 5SU and resulting in a 100% log reduction at a concentration of 80 μM for strain 72/2. In comparison, Kuhlencord et al. (17) found that APC1 at concentrations between 2.2 and 5.5 μM had a 50% growth inhibitory effect for Leishmania strains. Santa-Rita et al. (23) reported that 30 μM APC1 resulted in total lysis of T. cruzi.
The diverse protozoan species appear to react to the APCs differently, depending on the lengths of the alkyl chains. Whereas APC1 was the most active substance against acanthamoebae, APC2 and other APCs are reported to be more effective against entamoebae (24). APC1 was also less active than longer-chain APCs against L. donovani (H. Eibl et al., unpublished data). Similarly, APCs with long alkyl chains appear to be more cytotoxic than those with short chains against leukemia cells (14).
Treatment with APC1 resulted in vacuolization, rounding up of cells, blebbing, and finally, complete lysis of the cells as soon as 1 h after the addition of the substance. Similar effects were observed in the treatment of T. cruzi in vitro (23): extensive vacuolization in the cytoplasm, blebbing of the flagellar membrane, and finally, cell lysis. At high concentrations, the membrane architecture appears to be a primary target of the APCs in both species. This is supported by the hexokinase liberation experiment, in which treatment with 200 μM APC1 for only 30 min resulted in the release of between 70 and 90% of the total hexokinase activity from the cytoplasm of the acanthamoebae. This effect was much more pronounced in acanthamoebae than in entamoebae, although both species are eventually lysed (K. Seifert, unpublished data).
Susceptibilities of cysts.
One difficulty in the treatment of Acanthamoeba infections is the high degree of resistance of the cysts. In Acanthamoeba keratitis as well as in GAE, some cysts may survive in the tissue, resulting in reinfection. Cysts are resistant to diamidines and polyhexamethylene biguanide at high concentrations (27). Cysts are sensitive to hydrogen peroxide (2.1% [vol/vol]) or 3 M HCl, which is useful only for disinfection. In the present study we demonstrated that APC1 is cysticidal, resulting in a log reduction of up to 90% at the highest concentration tested (160 μM); in addition, at a concentration of as low as 5 μM APC1 inhibited excystment of all strains tested.
Cysts of strain 72/2 (morphological group III) were significantly less sensitive to APC1 than cysts of the two group II strains. Recently, we showed that Acanthamoeba cysts could be killed by microwave treatment. In the present study the cysts of strain 72/2 were also far more resistant than those of the group II strains tested (9). Acanthamoebae of morphological group III have a very thick cyst wall (21), which may provide protection against physical as well as chemical influences.
Perspectives for treatment.
In rats, APC1 is able to cross the blood-brain barrier, and levels in brain as high as 170 nmol g−1 were obtained in a tissue distribution study (16). This would be comparable to 170 μM in solution, a lethal concentration for the Acanthamoeba trophozoites. In addition, APC1 might exert significant cysticidal activity, killing more than 99% of the cysts and preventing excystation of the remaining ones. If similar levels could be reached in the central nervous systems of GAE patients, the elimination of the microorganism could be conceivable. The success of the chemotherapy would, however, also depend both on how the patients tolerate the substance and on the inflammatory effect caused by the death of large numbers of the parasites.
Much of the possible success will depend on the method of application. Pure APC1 cannot be given intravenously, and oral treatment is accompanied by significant gastrointestinal side effects. To increase the tolerability of the APCs, liposomal preparations of APCs containing cholesterol have been developed (12, 13). These preparations are suitable for parenteral application as well as oral or topical application, and they may potentially be useful for the treatment of GAE or Acanthamoeba keratitis. As the list of target organisms grows, it will become more likely that the APCs will be developed into drugs available for use as antiprotozoal agents in humans.
Acknowledgments
We thank Rolf Michel from the Ernst-Rodenwaldt-Institute, Koblenz, Germany, for providing A. lenticulata strain 72/2.
K.S. was supported in part by grant P12295-MED from the Austrian Science Fund and by a grant from the Hygiene Fund from the Clinical Institute for Hygiene at the University of Vienna.
REFERENCES
- 1.Croft, S. L., R. A. Neal, W. Pendergast, and J. H. Chan. 1987. The activity of alkyl phosphorylcholines and related derivatives against Leishmania donovani. Biochem. Pharmacol. 36:2633-2636. [DOI] [PubMed] [Google Scholar]
- 2.De Jonckheere, J. F., and R. Michel. 1988. Species identification and virulence of Acanthamoeba strains from human nasal mucosa. Parasitol. Res. 74:314-316. [DOI] [PubMed] [Google Scholar]
- 3.Duguid, I. G., J. K. Dart, N. Morlet, B. D. Allan, M. Matheson, L. Ficker, and S. Tuft. 1997. Outcome of Acanthamoeba keratitis treated with polyhexamethyl biguanide and propamidine. Ophthalmology 104:1587-1592. [DOI] [PubMed] [Google Scholar]
- 4.Eibl, H. 1978. Phospholipid synthesis. Oxazaphospholanes and dioxaphospholanes as intermediates. Proc. Natl. Acad. Sci. USA 75:4074-4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eibl, H., and J. Engel. 1992. Synthesis of hexadecylphosphocholine (miltefosine), p. 1-5. In H. Eibl, P. Hilgard, and C. Unger (ed.), New drugs in cancer therapy, Karger, Basel, Switzerland.
- 6.Eibl, H., and C. Unger. 1990. Hexadecylphosphocholine: a new and selective antitumor drug. Cancer Treat. Rev. 17:233-242. [DOI] [PubMed] [Google Scholar]
- 7.Ficker, L., D. Seal, D. Warhurst, and P. Wright. 1990. Acanthamoeba keratitis—resistance to medical therapy. Eye 4:835-838. [DOI] [PubMed] [Google Scholar]
- 8.Hay, J., C. M. Kirkness, D. V. Seal, and P. Wright. 1994. Drug resistance and Acanthamoeba keratitis: the quest for alternative antiprotozoal chemotherapy. Eye 8:555-563. [DOI] [PubMed] [Google Scholar]
- 9.Hiti, K., J. Walochnik, C. Faschinger, E. M. Haller-Schober, and H. Aspöck. 2001. Microwave treatment of contact lens cases contaminated with Acanthamoeba. Cornea 20:467-470. [DOI] [PubMed] [Google Scholar]
- 10.Jha, T. K., S. Sundar, C. P. Thakur, P. Bachmann, J. Karbwang, C. Fischer, A. Voss, and J. Berman. 1999. Miltefosine, an oral agent, for the treatment of Indian visceral leishmaniasis. N. Engl. J. Med. 341:1795-1800. [DOI] [PubMed] [Google Scholar]
- 11.Jones, D. B. 1986. Acanthamoeba—the ultimate opportunist? Am. J. Ophthalmol. 102:527.. [DOI] [PubMed] [Google Scholar]
- 12.Kaufmann-Kolle, P., M. R. Berger, C. Unger, and H. Eibl. 1996. Systemic administration of APCs, erucylphosphocholine and liposomal hexadecylphosphocholine. Adv. Exp. Med. Biol. 416:165-168. [PubMed] [Google Scholar]
- 13.Kaufmann-Kolle, P., J. Drevs, M. R. Berger, J. Kotting, N. Marschner, C. Unger, and H. Eibl. 1994. Pharmacokinetic behaviour and antineoplastic activity of liposomal hexadecylphosphocholine. Cancer Chemother. Pharmacol. 34:393-398. [DOI] [PubMed] [Google Scholar]
- 14.Konstantinov, S. M., H. Eibl, and M. R. Berger. 1998. APCs induce apoptosis in HL-60 and U-937 leukemic cells. Cancer Chemother. Pharmacol. 41:210-216. [DOI] [PubMed] [Google Scholar]
- 15.Kosrirukvongs, P., D. Wanachiwanawin, and G. S. Visvesvara. 1999. Treatment of Acanthamoeba keratitis with chlorhexidine. Ophthalmology 106:798-802. [DOI] [PubMed] [Google Scholar]
- 16.Kotting, J., M. R. Berger, C. Unger, and H. Eibl. 1992. Alkylphosphocholines: influence of structural variation on biodistribution at antineoplastically active concentrations. Cancer Chemother. Pharmacol. 30:105-112. [DOI] [PubMed] [Google Scholar]
- 17.Kuhlencord, A., T. Maniera, H. Eibl, and C. Unger. 1992. Hexadecylphosphocholine: oral treatment of visceral leishmaniasis in mice. Antimicrob. Agents Chemother. 36:1630-1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinez, A. J., and G. S. Visvesvara. 1997. Free-living, amphizoic and opportunistic amebas. Brain Pathol. 7:583-598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michel, R., R. Röhl, and H. Schneider. 1982. Isolation of free-living amoebae from nasal mucosa of healthy individuals. Zentbl. Bakteriol. Mikrobiol. Hyg. B 176:155-159. [PubMed] [Google Scholar]
- 20.Ofori-Kwakye, S. K., D. G. Sidebottom, J. Herbert, E. G. Fischer, and G. S. Visvesvara. 1986. Granulomatous brain tumor caused by Acanthamoeba. Case report. J. Neurosurg. 64:505-509. [DOI] [PubMed] [Google Scholar]
- 21.Pussard, M., and R. Pons. 1977. Morphologie de la paroi kystique et taxonomie du genre Acanthamoeba (Protozoa, Amoebida). Protistologica 8:557-598. [Google Scholar]
- 22.Radford, C. F., O. J. Lehmann, and J. K. G. Dart. 1998. Acanthamoeba keratitis: multicentre survey in England 1992-6. Br. J. Ophthalmol. 82:1387-1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Santa-Rita, R. M., H. Santos Barbosa, M. N. Meirelles, and S. L. de Castro. 2000. Effect of the alkyl-lysophospholipids on the proliferation and differentiation of Trypanosoma cruzi. Acta Trop. 75:219-228. [DOI] [PubMed] [Google Scholar]
- 24.Seifert, K., M. Duchêne, W. H. Wernsdorfer, H. Kollaritsch, O. Scheiner, G. Wiedermann, T. Hottkowitz, and H. Eibl. 2001. Effects of miltefosine and other alkylphosphocholines on human intestinal parasite Entamoeba histolytica. Antimicrob. Agents Chemother. 45:1505-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seijo-Martinez, M., G. Gonzalez-Mediero, P. Santiago, A. Rodriguez de Lope, J. Diz, C. Conde, and G. S. Visvesvara. 2000. Granulomatous amebic encephalitis in a patient with AIDS: isolation of Acanthamoeba sp. group II from brain tissue and successful treatment with sulfadiazine and fluconazole. J. Clin. Microbiol. 38:3892-3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Slater, C. A., J. Z. Sickel, G. S. Visvesvara, R. C. Pabico, and A. A. Gaspari. 1994. Successful treatment of disseminated Acanthamoeba infection in an immunocompromised patient. N. Engl. J. Med. 331:85-88. [DOI] [PubMed] [Google Scholar]
- 27.Turner, N. A., A. D. Russel, J. R. Furr, and D. Lloyd. 2000. Emergence of resistance to biocides during differentiation of Acanthamoeba castellanii. J. Antimicrob. Chemother. 46:27-34. [DOI] [PubMed] [Google Scholar]
- 28.Unger, C., W. Damenz, E. A. Fleer, D. J. Kim, A. Breiser, P. Hilgard, J. Engel, G. Nagel, and H. Eibl. 1989. Hexadecylphosphocholine, a new ether lipid analogue. Studies on the antineoplastic activity in vitro and in vivo. Acta Oncol. 28:213-217. [DOI] [PubMed] [Google Scholar]
- 29.Visvesvara, G. S., and W. Balamuth. 1975. Comparative studies on related free-living and pathogenic amebae with special reference to Acanthamoeba. J. Protozool. 22:245-256. [DOI] [PubMed] [Google Scholar]
- 30.Walochnik, J., E. Haller-Schober, H. Kolli, O. Picher, A. Obwaller, and H. Aspöck. 2000. Discrimination between clinically relevant and nonrelevant Acanthamoeba strains isolated from contact lens-wearing keratitis patients in Austria. J. Clin. Microbiol. 38:3932-3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walochnik, J., A. Obwaller, and H. Aspöck. 2000. Correlations between morphological, molecular biological, and physiological characters in clinical and nonclinical isolates of Acanthamoeba spp. Appl. Environ. Microbiol. 66:4408-4413. [DOI] [PMC free article] [PubMed] [Google Scholar]