Abstract
Drug-associated dysfunction of mitochondria is believed to play a role in the etiology of the various adverse symptoms that occur in human immunodeficiency virus (HIV)-infected patients treated with the nucleoside reverse transcriptase inhibitors (NRTIs). Tenofovir, a nucleotide analog recently approved for use in the treatment of HIV infection, was evaluated in vitro for its potential to cause mitochondrial toxicity and was compared to currently used NRTIs. Treatment with tenofovir (3 to 300 μM) for up to 3 weeks produced no significant changes in mitochondrial DNA (mtDNA) levels in human hepatoblastoma (HepG2) cells, skeletal muscle cells (SkMCs), or renal proximal tubule epithelial cells. The potencies of inhibition of mtDNA synthesis by the NRTIs tested were zalcitabine (ddC) > didanosine (ddI) > stavudine > zidovudine (ZDV) > lamivudine = abacavir = tenofovir, with comparable relative effects in the three cell types. Unlike ddC and ddI, tenofovir did not affect cellular expression of COX II and COX IV, two components of the mitochondrial cytochrome c oxidase complex. Lactate production was elevated by less than 20% in HepG2 cells or SkMCs following treatment with 300 μM tenofovir. In contrast, lactate synthesis increased by >200% in the presence of 300 μM ZDV. Thus, treatment of various human cell types with tenofovir at concentrations that greatly exceed those required for it both to have in vitro anti-HIV type 1 activity in peripheral blood mononuclear cells (50% effective concentration, 0.2 μM) and to achieve therapeutically relevant levels in plasma (maximum concentrations in plasma, 0.8 to 1.3 μM) is not associated with mitochondrial toxicity.
A variety of clinical symptoms such as myopathy, polyneuropathy, lactic acidosis, liver steatosis, pancreatitis, and lipodystrophy have been identified in human immunodeficiency virus (HIV)-infected patients treated with antiretroviral therapy containing one or more nucleoside reverse transcriptase inhibitors (NRTIs) (6, 34). Some of these adverse effects, which usually occur after prolonged treatment, are linked to mitochondrial toxicity, as demonstrated in a number of in vitro and in vivo studies with various NRTIs. Zidovudine (ZDV) is known to induce mitochondrial toxicity in rat heart muscle, skeletal muscles, and other tissues (24, 27) as well as cause an increase in the oxidative damage of mitochondrial DNA (mtDNA) in mouse skeletal muscle and liver tissues (18, 19). More importantly, morphological changes in mitochondria, cytochrome c oxidase deficiency, and reductions in mtDNA levels have been detected in muscle tissue from HIV-infected patients with ZDV-induced myopathy (2, 17, 46). Zalcitabine (ddC) has been implicated in the induction of neuropathy in HIV-infected patients (20) and simian immunodeficiency virus-infected macaques (44). It has been shown that ddC can cause mitochondrial alterations in Schwann cells in a rabbit model of ddC-induced neuropathy (1). Didanosine (ddI) and stavudine (d4T) therapy can induce adverse effects such as hepatic steatosis and lactic acidosis, which are presumably also a consequence of drug-associated mitochondrial toxicity (5, 32). In contrast, the etiology of abacavir-associated adverse effects such as hypersensitivity does not seem to involve mitochondrial toxicity (21, 22). Lamivudine (3TC) appears to produce fewer side effects than the other NRTIs (6, 38).
Clinical toxicities due to the mitochondrial dysfunction induced by NRTIs may limit certain treatment regimens and may even produce fatal complications, as documented for some cases of severe lactic acidosis (43). Therefore, it is important to evaluate new drugs from the NRTI class for their potential to cause mitochondrial dysfunction. NRTI-associated mitochondrial toxicity can be assessed in vitro by measuring specific markers such as mtDNA synthesis or production of lactic acid in drug-treated cell cultures (4, 36). Active phosphorylated forms of some NRTIs are potent inhibitors of DNA polymerase γ (DNA pol γ), an enzyme solely responsible for mtDNA replication, causing inhibition of de novo mtDNA synthesis during the process of mitochondrial division (28). In addition, drug-related deficiencies in the mitochondrial oxidative phosphorylation system may result in a blockage of pyruvate oxidation, leading to an elevated level of production of lactic acid (6).
Tenofovir (Fig. 1) is a novel nucleotide analog with potent anti-HIV activity and a favorable resistance profile. Its oral prodrug, tenofovir disoproxil [bis(isopropyloxymethylcarbonyl)-9-R-(2-phosphonomethoxypropyl)adenine] (Fig. 1), has recently been approved for use in the treatment of HIV-infected patients (3; R. Schooley, P. Ruane, R. Myers, G. Beall, H. Lampiris, et al., Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. I-1929, 2001). In this study, tenofovir was characterized and compared with the currently used NRTIs for its potential to cause mitochondrial toxicity in various types of cells of human origin. The results indicate that the potential for tenofovir to interfere with mitochondrial functions is low.
FIG. 1.
Structures of tenofovir and its oral prodrug tenofovir disoproxil.
MATERIALS AND METHODS
Drugs.
ZDV, d4T, ddC, and ddI were purchased from Sigma (St. Louis, Mo.), 3TC was supplied by Moravek Biochemicals (Brea, Calif.), tenofovir was prepared by Gilead Sciences (Foster City, Calif.), and abacavir was provided by GlaxoWellcome (Research Triangle Park, N.C.).
Cells.
A human hepatoblastoma cell line (HepG2 cells; American Type Culture Collection, Manassas, Va.) was maintained in minimal essential medium supplemented with 10% fetal bovine serum, 1 mM pyruvate, and antibiotics. Primary normal human skeletal muscle cells (SkMCs) were purchased from Clonetics (San Diego, Calif.) and were grown for a maximum of four passages in a serum-free medium supplied by the vendor. Primary human renal proximal tubule epithelial cells (RPTECs) were provided by Kenneth McMartin (Louisiana State University, Shreveport) and were maintained on plastic dishes coated with Vitrogen-100 (Collagen Biomaterials, Palo Alto, Calif.). RPTECs were grown for a maximum of three passages in Dulbecco's minimal essential medium-F-12 medium (1:1) supplemented with 5 ng of selenium per ml, 5 μg of insulin per ml, 5 μg of transferrin per ml, 40 ng of hydrocortisone per ml, 10 ng of epidermal growth factor (Collaborative Research, Bedford, Mass.) per ml, and 4 pg of triiodothyronine per ml (33).
Determination of mtDNA content.
DNA probes were generated by PCR from total DNA isolated from HepG2 cells with a QIAmp tissue kit (Qiagen, Valencia, Calif.). A mitochondrial DNA probe specific for cytochrome b was amplified with primers 5"-TGACCCCAATACGCAAAATTAACC-3" and 5"-CATTTGAGTATTTTGTTTTCAATTAGG-3" and encompassed nucleotides 14172 to 15306 of the mitochondrial genome (GenBank accession no. X93334). A chromosomal DNA-specific β-actin probe (nucleotides 2039 to 3065 of the DNA fragment comprising the β-actin gene; GenBank accession no. E01094) was amplified by PCR with primers 5"-AGACCTTCAACACCCCAGCCATGTACG-3" and 5"-TCTTGTTTTCTGCGCAAGTTAGGTTTTGTC-3". Both probes were purified by gel electrophoresis and labeled with [33P]dCTP with the Prime-It II labeling kit (Stratagene, La Jolla, Calif.). The specificity of each probe was determined by hybridization with samples of DNA from nuclear and mitochondrial fractions isolated from RPTECs.
HepG2 cells and SkMCs were plated into 24-well plates (3,000 cells/cm2). At 24 h, fresh medium containing test drugs at 10-fold serial dilutions was added. The cells were maintained in the presence of the drugs for 9 or 18 days, with replacement of the drug-containing medium every 4 days. In the experiment with quiescent SkMCs, the cells were seeded into Vitrogen-100-coated 24-well plates (10,000 cells/cm2). After the cells reached confluence, fresh medium containing the test drugs was added and the cells were maintained for 21 days with a medium change every 5 days. RPTECs were seeded into Vitrogen-100-coated 12-mm Millicell-CM inserts (Millipore, Bedford, Mass.) at a density of 150,000 cells/insert, and the inserts were placed into 24-well plates. After formation of tight junctions, which was assessed by measuring the transepithelial resistance (33), the cells were fed fresh medium containing the test drug in both the apical and the basolateral compartments and incubated for 12 or 21 days with medium changes every 3 days. At the end of incubation, HepG2 cells and SkMCs were trypsinized, pelleted by centrifugation, and lysed in 0.5 M NaOH-12.5 mM EDTA. The inserts containing RPTECs were washed in phosphate-buffered saline (PBS), and the membranes with cells were removed from the inserts with a scalpel and placed in 0.5 ml of the lysis solution described above. All lysates were heated at 100°C for 10 min, centrifuged, and slot blotted onto a Zeta-Probe membrane (Bio-Rad, Hercules, Calif.) according to the vendor's protocol. DNA was cross-linked to the membrane with UV light, and the membrane was prehybridized and sequentially hybridized with the β-actin- and cytochrome b-specific probes (107 dpm/ml) under the conditions recommended by the manufacturer. Following each hybridization, the signals were quantified with a PhosphorImager (Molecular Dynamics, Sunnyvale, Calif.). The relative amount of mtDNA was calculated as the ratio of the cytochrome b signal to the β-actin signal and was expressed as a percentage relative to that for the untreated control.
Immunoblot analysis.
Cells were seeded into 75-cm2 flasks at a density of 3,000 cells/cm2. Test compounds were added 24 h later, and the cells were incubated for 9 days with one change of the medium. After a wash of the cells with PBS and a 10-min incubation in the presence of 2 ml of lysis buffer (150 mM NaCl, 50 mM Tris-HCl [pH 7.5], 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate [SDS], 1% Nonidet P-40, 5 mM EDTA, mammalian protease inhibitor cocktail purchased from Roche Molecular Biochemicals), the cells were scraped and disintegrated with a Dounce homogenizer. The lysate was clarified by centrifugation, and proteins were concentrated with a Centricon YM-3 apparatus (Millipore). Following separation by SDS-polyacrylamide gel electrophoresis (PAGE; 10 μg of protein/sample), the proteins were electroblotted onto a polyvinylidene difluoride membrane (Millipore). The membrane was blocked for 2 h in PBS-0.1% Tween 20 (PBS-Tween) containing 5% nonfat dry milk, washed with PBS-Tween, and incubated with a mixture of mouse monoclonal antibodies, anti-COX II (2.2 μg/ml; Molecular Probes, Eugene, Oreg.), anti-COX IV (5.5 μg/ml; Molecular Probes), and anti-G3PD (5 μg/ml; Advanced ImmunoChemical, Long Beach, Calif.), in PBS-Tween containing 1% bovine serum albumin. After extensive washing with PBS-Tween, the membrane was incubated in the presence of 2.5 μCi of anti-mouse [35S]immunoglobulin G (Amersham Pharmacia Biotech, Piscataway, N.J.) per ml. The washed membrane was analyzed with the PhosphorImager, and the signal corresponding to each protein was quantified.
Measurement of lactate production.
Twenty-four hours after plating of HepG2 cells or SkMCs into 24-well plates (3,000 cells/cm2), fresh medium containing the test drugs at concentrations of 30 and 300 μM was added. Medium was collected 3 or 6 days later and was extracted with 2 volumes of ice-cold 10% trichloroacetic acid. The lactic acid concentration in the deproteinized medium was measured with a diagnostic kit (Sigma) by the protocol specified by the manufacturer. The treated cells were washed on plates, trypsinized, and counted. Following assay calibration, the concentration of lactate in the medium was determined and expressed as the number of milligrams per 106 cells.
RESULTS
Effects of tenofovir and other NRTIs on mtDNA content in liver and skeletal muscle cells.
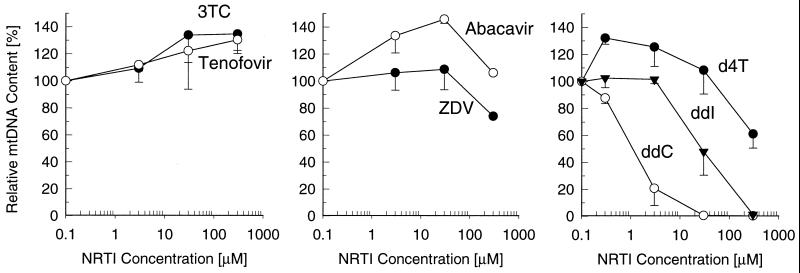
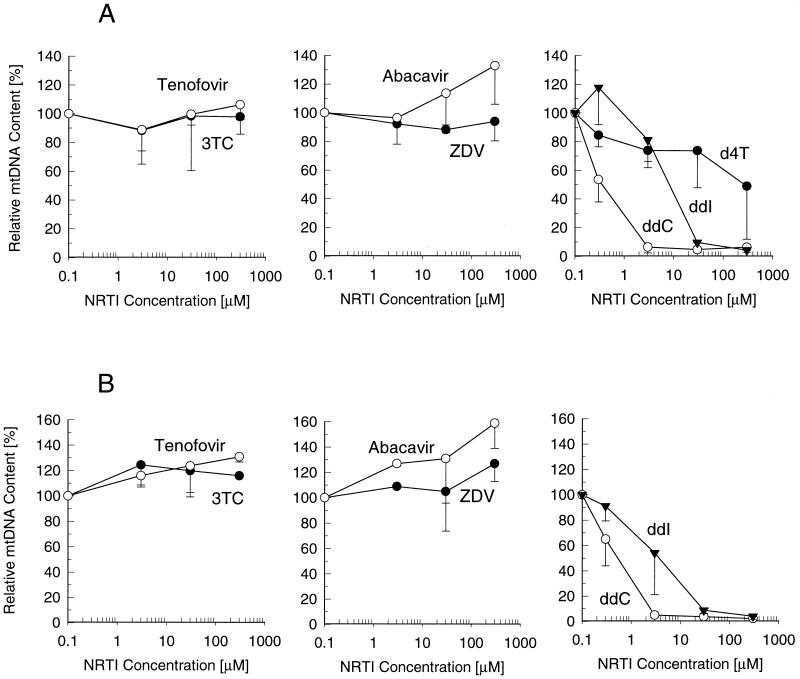
Clinical adverse effects due to NRTI-induced mitochondrial toxicity frequently occur in liver and muscle tissues (6). Therefore, the in vitro experiments performed to assess the mitochondrial toxicity of tenofovir were carried out with HepG2 cells and SkMCs. The effects of tenofovir and other NRTIs on mtDNA synthesis were determined from the ratio of mtDNA and chromosomal DNA calculated following sequential hybridization of total cellular DNA with specific probes derived from mtDNA and chromosomal DNA sequences. The analyses revealed no interference with mtDNA synthesis in HepG2 cells following a 9-day incubation with tenofovir at concentrations as high as 300 μM (Fig. 2). In contrast, ddC and ddI at concentrations of 30 and 300 μM, respectively, completely depleted the mtDNA in HepG2 cells. d4T and ZDV at concentrations up to 30 μM showed no deleterious effects, but exposure of the cells to concentrations of 300 μM reduced the mtDNA contents by approximately 40 and 25%, respectively. Similar to tenofovir, 3TC and abacavir did not significantly change the levels of mtDNA in HepG2 liver cells.
FIG. 2.
Effects of tenofovir and other NRTIs on mtDNA content in HepG2 cells. The cells were incubated with various drug concentrations for 9 days, and the relative mtDNA content was determined as the ratio of hybridization signals from mtDNA- and chromosomal DNA-specific probes. Data represent the percentages of the values for the untreated control, given as the means ± standard deviations of two independent experiments performed in duplicate.
Tenofovir disoproxil, a lipophilic oral prodrug of tenofovir, was also tested for its effects on the mtDNA content in HepG2 cells and was found not to induce any changes at concentrations as high as 3 μM. After 9 days of treatment with tenofovir disoproxil at concentrations of 0.3 and 3 μM, the mtDNA contents were 90.5% ± 20.6% and 99.4% ± 12.6% (the values are means ± standard deviations of two independent experiments performed in triplicate) compared with those in control mtDNA, respectively. As a positive control, after 9 days of treatment with ddC at concentrations of 0.3 and 3 μM, the mtDNA contents were 66.2% ± 5.6% and 8.2% ± 4.5%, respectively. The prodrug of tenofovir can enter cells by passive diffusion across the plasma membrane and delivers a greater than 1,000-fold higher concentration of the active metabolite, tenofovir diphosphate (tenofovir-DP), into the cells compared to the concentration delivered by free tenofovir (41).
Exposure of SkMCs to tenofovir and other NRTIs resulted in effects similar to those observed with HepG2 cells. Proliferating cells did not show any decrease in mtDNA levels following a 9-day exposure to concentrations of tenofovir as high as 300 μM (Fig. 3A). Likewise, 3TC, abacavir, and ZDV did not deplete mtDNA under the same experimental conditions. d4T at a concentration of 300 μM reduced mtDNA levels by approximately 50%. ddC and ddI showed even more pronounced effects in SkMCs than in HepG2 cells, causing almost complete depletion of mtDNA at 3 and 30 μM, respectively. Virtually identical effects, including no depletion of mtDNA by tenofovir, were observed for the test drugs following an 18-day treatment of proliferating SkMCs (Fig. 3B) or a 3-week treatment of quiescent nonproliferating SkMCs maintained on a collagen-coated surface (data not shown).
FIG. 3.
Effects of tenofovir and other NRTIs on mtDNA content in skeletal muscle cells. The dividing cells were incubated in the presence of drugs for 9 days (A) or 18 days (B). The relative mtDNA content was determined as described in the legend to Fig. 2, with data representing the percentages of the values for the untreated control (mean ± standard deviations) from two independent experiments performed in duplicate.
mtDNA content in renal proximal tubule cells treated with NRTIs.
Tenofovir can potentially accumulate in the kidney due to its active transport by the organic anion transporter 1 expressed in renal proximal tubules (12). Thus, in addition to liver and skeletal muscle cells, the effects of tenofovir and other NRTIs on mtDNA content were characterized in normal human RPTECs. As shown in Table 1, changes in relative mtDNA levels were minimal following treatment of differentiated RPTECs cultured on microporous membranes in the presence of 300 μM tenofovir or 200 μM ZDV for up to 21 days. While 200 μM d4T produced only a minor decrease in the mtDNA content, ddC and ddI showed potent inhibition of mtDNA synthesis in RPTECs at markedly lower concentrations.
TABLE 1.
Effects of tenofovir and other NRTIs on mtDNA contents in differentiated human RPTECs
| Drug | Drug concn (μM) | mtDNA content (% of control)a
|
|
|---|---|---|---|
| 12 days | 21 days | ||
| Tenofovir | 30 | 124 ± 10.8 | 118 ± 3.9 |
| 300 | 118 ± 5.9 | 109 ± 9.2 | |
| ZDV | 40 | 103 ± 2.9 | 111 ± 0.9 |
| 200 | 109 ± 3.0 | 104 ± 14.7 | |
| d4T | 40 | 79.4 ± 0.5 | 87.8 ± 6.0 |
| 200 | 92.3 ± 13.9 | 77.0 ± 10.9 | |
| ddC | 2 | 21.1 ± 1.8 | 5.1 ± 0.6 |
| 10 | 12.1 ± 1.1 | 6.9 ± 0.9 | |
| ddI | 40 | 47.5 ± 5.3 | 25.7 ± 0.1 |
| 200 | 30.1 ± 0.3 | 10.4 ± 0.7 | |
Relative content of mtDNA in RPTECs after 12- and 21-day drug treatments given as mean ± standard deviations of a representative experiment performed in duplicate.
Effect of NRTIs on cellular expression of cytochrome c oxidase.
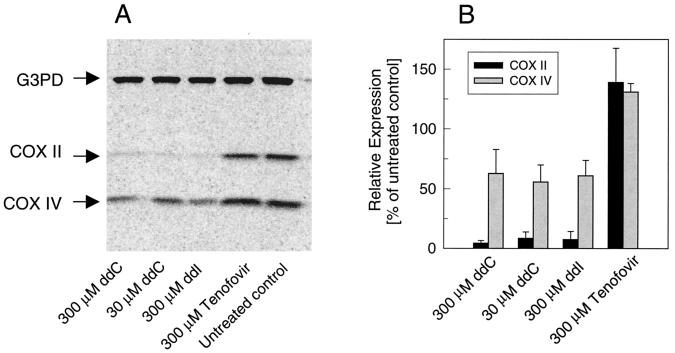
In order to determine whether the drug-induced depletion of mtDNA exerts an impact on the levels of mitochondrial proteins, we characterized the effects of ddC, ddI, and tenofovir on the expression of cytochrome c oxidase, a multiprotein mitochondrial complex comprising 13 subunits (13). Three subunits (COX I to III) constitute the catalytic core of the enzyme and are encoded by mtDNA; the remaining 10 subunits are expressed from nuclear genes. Immunoblot analysis following 9 days of drug treatment was used to characterize changes in the levels of the cellular expression of the COX II and COX IV subunits of cytochrome c oxidase encoded by mtDNA and nuclear DNA, respectively. Glyceraldehyde-3-phosphate dehydrogenase was quantified simultaneously with the mitochondrial proteins and was used as a standard to determine the relative changes in the levels of expression of COX II and COX IV. Treatment of HepG2 cells with 300 μM tenofovir did not reduce the level of cellular expression of either of the two proteins (Fig. 4). In contrast, exposure of HepG2 cells to 30 μM ddC or 300 μM ddI inhibited the level of expression of COX II by >90%. These two NRTIs also reduced cellular levels of COX IV by approximately 40 to 50%. Depletion of both COX II and COX IV was also detected in SkMCs treated with 30 μM ddC or 300 μM ddI (data not shown). However, only minimal changes in the levels of expression of COX II and COX IV (83 and 103% relative to the levels of expression by the untreated control, respectively) were detected in SkMCs cultured for 9 days in the presence of 300 μM tenofovir.
FIG. 4.
Effects of ddC, ddI, and tenofovir on the expression of COX II and COX IV in HepG2 cells. Following 9 days of treatment, cells were lysed and the level of protein expression was analyzed by using specific monoclonal antibodies in combination with [35S]immunoglobulin G. (A) Detection of proteins following SDS-PAGE and immunoblot analysis (10 μg of cellular proteins per each sample). (B) Quantification of the signal from immunoblot analysis. The relative expression levels were determined as a ratio between the specific cytochrome c oxidase signal and the glyceraldehyde-3-phosphate dehydrogenase signal, with the relative expression in control untreated cells being 100%.
Production of lactate in cells treated with NRTIs.
Lactic acidosis is one of the most serious complications induced by prolonged therapy with NRTIs. When diagnosed, it often requires intensive care (32) and can be fatal in some patients (43). Therefore, lactic acid production is considered an important marker for characterization of NRTI-induced mitochondrial dysfunction. Prior in vitro studies have demonstrated that ZDV can increase the level of extracellular production of lactate in HepG2 cells (36) and SkMCs (4). In contrast, 3TC has been shown to have no effect on the levels of lactate produced by HepG2 cells (15). However, the studies with 3TC were conducted with relatively low drug concentrations (10 μM).
Tenofovir was tested at concentrations up to 300 μM, and its effect on lactate production by HepG2 cells and SkMCs was compared with those of ZDV, ddC, and 3TC. As shown in Table 2, tenofovir increased the levels of extracellular lactate production by <20% in HepG2 cells and SkMCs after incubation for 3 and 6 days, respectively. Similarly, an insignificant (<20%) increase in the level of lactate production compared to that by the untreated control was detected after incubation of the cells with 3TC, indicating that this drug has no effect even at concentrations higher than those studied previously (36). In contrast, ZDV treatment produced a concentration-dependent elevation in the level of lactate production in both HepG2 cells and SkMCs, with two- to threefold increases at 300 μM, a result consistent with observations described previously (4, 36). Even though 30 μM ddC caused almost complete depletion of mtDNA, leading to a virtual knockout of COX II expression, it only increased the level of lactate production by 30 to 50% in SkMCs and HepG2 cells, an effect comparable to that of ZDV.
TABLE 2.
Effects of NRTIs on in vitro production of lactic acid
| Drug | Concn (μM) | Lactic acid production (mg/106 cells)a
|
|
|---|---|---|---|
| HepG2 cells | SkMCs | ||
| None | 1.61 ± 0.25 (100) | 7.53 ± 0.83 (100) | |
| Tenofovir | 30 | 1.34 ± 0.18 (83) | 7.23 ± 1.21 (96) |
| 300 | 1.62 ± 0.06 (101) | 8.79 ± 1.97 (116) | |
| ZDV | 30 | 2.26 ± 0.04 (141) | 10.39 ± 0.56 (138) |
| 300 | 3.32 ± 0.05 (207) | 21.94 ± 4.04 (291) | |
| 3TC | 30 | 1.92 ± 0.67 (119) | 7.29 ± 1.47 (97) |
| 300 | 1.94 ± 0.14 (121) | 8.13 ± 0.95 (108) | |
| ddC | 3 | 1.95 ± 0.26 (121) | 11.22 ± 3.15 (149) |
| 30 | 2.15 ± 0.42 (133) | 11.45 ± 2.09 (152) | |
The data are given as means ± standard deviations from a representative experiment performed in duplicate. HepG2 cells and SkMCs were incubated with the drugs for 3 and 6 days, respectively. Data in parentheses represent the percentages of the values for the untreated control.
DISCUSSION
Various therapy-limiting adverse effects observed in HIV-infected patients treated with NRTIs have been linked to mitochondrial toxicity (6, 34). One of the proposed mechanisms by which NRTIs interfere with mitochondrial functions is the depletion of mtDNA due to the inhibition of DNA pol γ by NRTI triphosphates (22, 23). Some NRTI triphosphates such as ddCTP inhibit DNA pol γ and HIV type 1(HIV-1) reverse transcriptase with similar potencies, resulting in a narrow therapeutic window (9). Inhibition studies with DNA pol γ are therefore important for evaluation of the potential of NRTIs to cause mitochondrial dysfunction. However, because differences in levels of mitochondrial accumulation and/or phosphorylation of NRTIs may exist in different tissues, the conclusions derived from the results of inhibition studies with DNA pol γ should be confirmed by experiments determining the effects of the test drugs on mtDNA synthesis in various cell types.
Tenofovir-DP, the active metabolite of tenofovir and a structural analog of dATP, is a weaker inhibitor of DNA pol γ than most of the other NRTI triphosphates, with a Ki of 60 μM (9). In contrast, HIV-1 reverse transcriptase is efficiently inhibited by tenofovir-DP, with Ki values of 1.55 and 0.022 μM when the values are determined with DNA and RNA templates, respectively (9). Incorporation of tenofovir into a DNA primer-template by DNA pol γ is also less efficient than that of other NRTIs (11), suggesting a weak inhibition of mtDNA synthesis in cells treated with tenofovir. In order to confirm this assumption in various cell types, the effects of tenofovir and six clinically used NRTIs on mtDNA synthesis were evaluated in HepG2 cells, normal SkMCs, and normal human RPTECs. In all three cell types tested, tenofovir showed no interference with mtDNA synthesis at concentrations as high as 300 μM. Similarly, treatment of the cells with tenofovir did not result in elevated levels of production of lactic acid. This is consistent with the low level of cytotoxicity of tenofovir observed in the three cell types (10, 12). Tenofovir disoproxil, a lipophilic oral prodrug of tenofovir which substantially increases the cellular permeation of tenofovir (41), was also evaluated at concentrations up to 3 μM and did not inhibit synthesis of mtDNA. In comparison, tenofovir and tenofovir disoproxil inhibit HIV-1 replication in peripheral blood mononuclear cells, with 50% inhibitory concentrations of 0.18 and 0.005 μM, respectively (41). In vitro stability studies and preclinical pharmacokinetic studies suggest that tenofovir disoproxil is rapidly cleaved to tenofovir upon intestinal absorption. The therapeutic dose of tenofovir disoproxil in HIV-infected patients (300 mg once daily) delivers tenofovir into the systemic circulation and produces peak concentrations in plasma of 240 to 374 ng/ml (0.8 to 1.3 μM) (3). Thus, at concentrations that greatly exceed their effective antiviral concentrations and therapeutically relevant levels in plasma, both tenofovir and its prodrug show no significant effects on mtDNA synthesis.
Similar to tenofovir, 3TC and abacavir had minimal effects on the mtDNA contents in HepG2 cells and SkMCs. This observation confirms data from prior studies with 3TC in HepG2 cells (36) and T cells (28) and extends them to other cell types. Likewise, prior results with carbovir, the active metabolite of abacavir, in T-lymphoblastoid cells indicate a low potential for abacavir to interfere with mtDNA replication (37). Carbovir triphosphate was identified as one of the weakest DNA pol γ inhibitors among a number of NRTI triphosphates, with a Ki/Km value of 100 (28), a result similar to that obtained with tenofovir-DP (9).
Consistent with our data, ZDV has been shown to have only minor effects on the mtDNA content compared with those of other NRTIs such as ddC or ddI in various cell culture models (8, 14, 36). In contrast, severely reduced mtDNA contents have been found in skeletal muscle tissue from ZDV-treated mice (24) and from patients with fully developed ZDV-induced myopathy (2). Recent in vitro studies have demonstrated that ZDV can reduce the capacity of the respiratory chain in mitochondria without depleting mtDNA (36). In addition, oxidative damage of mtDNA has been detected in muscle and liver tissues from mice treated with ZDV (18, 19), suggesting that the primary mechanism of ZDV-associated mitochondrial dysfunction may also involve some other effects besides the direct inhibition of mtDNA replication. This hypothesis is supported by a substantially weaker inhibitory effect of ZDV triphosphate on DNA pol γ compared to those of other NRTI triphosphates such as ddCTP, ddATP, and d4TTP (9, 28). Additional support for this hypothesis is provided by our observations that ZDV can substantially increase the levels of lactate production in both liver and skeletal muscle cells without substantial depletion of mtDNA.
Our study revealed that d4T has concentration-dependent effects on mtDNA levels in HepG2 cells and SKMCs. Likewise, a detectable effect of d4T was observed in RPTECs. It is apparent that the effects of d4T on the mtDNA levels are cell type dependent. While 10 μM d4T reduced the mtDNA content by 50% in different T-lymphoblastoid cell lines (28, 31), HepG2 cells and SkMCs exhibited similar decreases in mtDNA synthesis following exposure to 300 μM d4T. Since d4T triphosphate is a potent inhibitor of DNA pol γ (Ki = 0.05 μM) (28) and also undergoes efficient incorporation into DNA primer-template by this enzyme (11), differences in the levels of drug accumulation and/or phosphorylation in the mitochondria of various cell types presumably account for the cell type-dependent effects of d4T on mtDNA synthesis.
A number of prior in vitro studies have demonstrated a profound depletion of mtDNA in the presence of dideoxynucleosides. ddI and ddC inhibit mtDNA synthesis in a broad spectrum of cell types including neuronal pheochromocytoma cells (14), T-lymphoblastoid cells (8, 31), HepG2 cells (36), and a monocyte-derived cell line (42). In all cases, the effects of ddC are more severe than those produced by ddI, with significant decreases in mtDNA levels detected at submicromolar concentrations. In our study, ddC caused more severe depletions of mtDNA than ddI did in all three cell types tested. However, ddATP, the active metabolite of ddI, shows an affinity toward DNA pol γ as both a substrate (11) and an inhibitor (9) which is comparable to that of ddCTP. The differential effects of the two drugs on mtDNA might be due to differences in their intracellular phosphorylation (23).
The rank order of the effects of the NRTIs tested on mtDNA content was similar in the three human cell types studied (ddC > ddI > d4T > ZDV > 3TC = abacavir = tenofovir) and is approximately concordant with the efficiencies at which the respective NRTI triphosphates are incorporated into DNA by DNA pol γ (ddCTP = ddATP > d4TTP > 3TC triphosphate > tenofovir-DP > ZDV triphosphate) (11). This correlation can be explained, at least in part, by the fact that none of the tested NRTIs contains a 3"-like hydroxyl group in their molecules, and thus, they all act as absolute chain terminators following their incorporation into de novo synthesized DNA (11). In contrast, fialuridine (FIAU), a nucleoside analog that induces severe clinical symptoms due to its mitochondrial toxicity (30), contains a 3"-like hydroxyl and can be elongated following its incorporation into DNA by DNA pol γ (25, 26). FIAU is efficiently phosphorylated by mitochondrial thymidine kinase (45), and the affinity of FIAU triphosphate toward DNA pol γ is comparable to that of ddC triphosphate (25, 28). Yet, FIAU does not induce depletion of mtDNA as profound as that induced by ddC (16, 25; G. Birkus and T. Cihlar, unpublished data), suggesting that the internal incorporation of FIAU may result in functional damage of mtDNA in the absence of its substantial depletion.
Depletion of mtDNA caused by treatment of cells with ddC or ddI reduced the level of expression of COX II, a subunit of the cytochrome c oxidase complex encoded by mtDNA. It has been previously shown that the mtDNA depletion induced by treatment of cells with ethidium bromide ([rho0] phenotype) causes reductions in the levels of multiple mtDNA-encoded proteins (7). Thus, the reduced levels of COX II expression in ddI- or ddC-treated cells is probably representative of the situation for other mtDNA-encoded proteins as well. In addition to low levels of COX II, COX IV, a nuclear DNA-encoded component of the complex, is also present at lower levels in ddC- and ddI-treated cells. Reduced levels of nuclear DNA-encoded cytochrome c oxidase subunits in conjunction with suppressed levels of mtDNA-encoded subunits have been observed in primary cells from patients with various forms of cytochrome c oxidase deficiency including COX II deficiency (40) as well as in cell lines with the [rho0] phenotype (29). Similarly, analysis of muscle tissue from patients with ZDV-induced myopathy revealed reduced levels of COX II as well as reduced levels of COX IV (46). These concerted reductions in the levels of mitochondrial proteins expressed from mtDNA and the levels of proteins expressed from nuclear DNA may be due to a limited stability of uncomplexed nuclear DNA-encoded cytochrome c oxidase subunits since the mtDNA-encoded subunits play essential roles in the early steps of the complex assembly (35). In addition, there may be communication between the mitochondrion and the nucleus via metabolic and signal transduction pathways to control the expression of nuclear DNA-encoded mitochondrial proteins such as cytochrome c oxidase subunits (39).
In summary, this study demonstrates that the potential of the anti-HIV nucleotide analog tenofovir to induce mitochondrial toxicity in different human cell types is low. Comparison with the other NRTIs currently used for treatment of HIV infection indicates that within the class of NRTIs, the potential that tenofovir will produce adverse effects caused by drug-associated mitochondrial dysfunction is low. These data are consistent with the favorable tolerability profile of tenofovir observed in HIV-infected patients (Schooley et al., 41st ICAAC).
Acknowledgments
We thank Craig Gibbs from Gilead Sciences for critical reading of the manuscript and Kenneth McMartin from Louisiana State University, Shreveport, for RPTECs.
REFERENCES
- 1.Anderson, T. D., A. Davidovich, D. Feldman, T. J. Sprinkle, J. Arezzo, C. Brosnan, R. O. Calderon, L. H. Fossom, J. T. DeVries, and G. H. DeVries. 1994. Mitochondrial schwannopathy and peripheral myelinopathy in a rabbit model of dideoxycytidine neurotoxicity. Lab. Investig. 70:724-739. [PubMed] [Google Scholar]
- 2.Arnaudo, E., M. Dalakas, S. Shanske, C. T. Moraes, S. DiMauro, and E. A. Schon. 1991. Depletion of muscle mitochondrial DNA in AIDS patients with zidovudine-induced myopathy. Lancet 337:508-510. [DOI] [PubMed] [Google Scholar]
- 3.Barditch-Crovo, P., S. G. Deeks, A. Collier, S. Safrin, D. F. Coakley, M. Miller, B. P. Kearney, R. L. Coleman, P. D. Lamy, J. O. Kahn, I. McGowan, and P. S. Lietman. 2001. Phase I/II trial of the pharmacokinetics, safety, and antiretroviral activity of tenofovir disoproxil fumarate in human immunodeficiency virus-infected adults. Antimicrob. Agents Chemother. 45:2733-2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benbrik, E., P. Chariot, S. Bonavaud, M. Ammi-Said, E. Frisdal, C. Rey, R. Gherardi, and G. Barlovatz-Meimon. 1997. Cellular and mitochondrial toxicity of zidovudine (AZT), didanosine (ddI) and zalcitabine (ddC) on cultured human muscle cells. J. Neurol. Sci. 149:19-25. [DOI] [PubMed] [Google Scholar]
- 5.Bissuel, F., F. Bruneel, F. Habersetzer, D. Chassard, L. Cotte, M. Chevallier, J. Bernuau, J. C. Lucet, and C. Trepo. 1994. Fulminant hepatitis with severe lactate acidosis in HIV-infected patients on didanosine therapy. J. Intern. Med. 235:367-371. [DOI] [PubMed] [Google Scholar]
- 6.Brinkman, K., H. J. ter Hofstede, D. M. Burger, J. A. Smeitink, and P. P. Koopmans. 1998. Adverse effects of reverse transcriptase inhibitors: mitochondrial toxicity as common pathway. AIDS 12:1735-1744. [DOI] [PubMed] [Google Scholar]
- 7.Chandel, N. S., and P. T. Schumacker. 1999. Cells depleted of mitochondrial DNA (rho0) yield insight into physiological mechanisms. FEBS Lett. 454:173-176. [DOI] [PubMed] [Google Scholar]
- 8.Chen, C. H., M. Vazquez-Padua, and Y. C. Cheng. 1991. Effect of anti-human immunodeficiency virus nucleoside analogs on mitochondrial DNA and its implication for delayed toxicity. Mol. Pharmacol. 39:625-628. [PubMed] [Google Scholar]
- 9.Cherrington, J. M., S. J. W. Allen, N. Bischofberger, and M. S. Chen. 1995. Kinetic interaction of the diphosphates of 9-(2-phosphonylmethoxyethyl)adenine and other anti-HIV active purine congeners with HIV reverse transcriptase and human DNA polymerases alpha, beta, and gamma. Antivir. Chem. Chemother. 6:217-221. [Google Scholar]
- 10.Cihlar, T., G. Birkus, D. E. Greenwalt, and M. J. M. Hitchcock. Tenofovir exhibits low cytotoxicity in various human cell types: comparison with other nucleoside reverse transcriptase inhibitors. Antivir. Res., in press. [DOI] [PubMed]
- 11.Cihlar, T., and M. S. Chen. 1997. Incorporation of selected nucleoside phosphonates and anti-human immunodeficiency virus nucleotide analogs into DNA by human DNA polymerase alpha, beta, and gamma. Antivir. Chem. Chemother. 8:187-195. [Google Scholar]
- 12.Cihlar, T., E. S. Ho, D. C. Lin, and A. S. Mulato. 2001. Human renal organic anion transporter 1 (hOAT1) and its role in the nephrotoxicity of antiviral nucleotide analogs. Nucleosides Nucleotides Nucleic Acids 20:641-648. [DOI] [PubMed] [Google Scholar]
- 13.Cooper, C. E., P. Nicholls, and J. A. Freedman. 1991. Cytochrome c oxidase: structure, function, and membrane topology of the polypeptide subunits. Biochem. Cell. Biol. 69:586-607. [DOI] [PubMed] [Google Scholar]
- 14.Cui, L., L. Locatelli, M. Y. Xie, and J. P. Sommadossi. 1997. Effect of nucleoside analogs on neurite regeneration and mitochondrial DNA synthesis in PC-12 cells. J. Pharmacol. Exp. Ther. 280:1228-1234. [PubMed] [Google Scholar]
- 15.Cui, L., R. F. Schinazi, G. Gosselin, J. L. Imbach, C. K. Chu, R. F. Rando, G. R. Revankar, and J. P. Sommadossi. 1996. Effect of beta-enantiomeric and racemic nucleoside analogues on mitochondrial functions in HepG2 cells. Implications for predicting drug hepatotoxicity. Biochem. Pharmacol. 52:1577-1584. [DOI] [PubMed] [Google Scholar]
- 16.Cui, L., S. Yoon, R. F. Schinazi, and J. P. Sommadossi. 1995. Cellular and molecular events leading to mitochondrial toxicity of 1-(2-deoxy-2-fluoro-1-beta-d-arabinofuranosyl)-5-iodouracil in human liver cells. J. Clin. Investig. 95:555-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dalakas, M. C., I. Illa, G. H. Pezeshkpour, J. P. Laukaitis, B. Cohen, and J. L. Griffin. 1990. Mitochondrial myopathy caused by long-term zidovudine therapy. N. Engl. J. Med. 322:1098-1105. [DOI] [PubMed] [Google Scholar]
- 18.de la Asuncion, J., M. L. del Olmo, J. Sastre, F. V. Pallardo, and J. Vina. 1999. Zidovudine (AZT) causes an oxidation of mitochondrial DNA in mouse liver. Hepatology 29:985-987. [DOI] [PubMed] [Google Scholar]
- 19.de la Asuncion, J. G., M. L. del Olmo, J. Sastre, A. Millan, A. Pellin, F. V. Pallardo, and J. Vina. 1998. AZT treatment induces molecular and ultrastructural oxidative damage to muscle mitochondria. Prevention by antioxidant vitamins. J. Clin. Investig. 102:4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dubinsky, R. M., R. Yarchoan, M. Dalakas, and S. Broder. 1989. Reversible axonal neuropathy from the treatment of AIDS and related disorders with 2",3"-dideoxycytidine (ddC). Muscle Nerve 12:856-860. [DOI] [PubMed] [Google Scholar]
- 21.Hervey, P. S., and C. M. Perry. 2000. Abacavir: a review of its clinical potential in patients with HIV infection. Drugs 60:447-479. [DOI] [PubMed] [Google Scholar]
- 22.Kakuda, T. N. 2000. Pharmacology of nucleoside and nucleotide reverse transcriptase inhibitor-induced mitochondrial toxicity. Clin. Ther. 22:685-708. [DOI] [PubMed] [Google Scholar]
- 23.Lewis, W., and M. C. Dalakas. 1995. Mitochondrial toxicity of antiviral drugs. Nat. Med. 1:417-422. [DOI] [PubMed] [Google Scholar]
- 24.Lewis, W., B. Gonzalez, A. Chomyn, and T. Papoian. 1992. Zidovudine induces molecular, biochemical, and ultrastructural changes in rat skeletal muscle mitochondria. J. Clin. Investig. 89:1354-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lewis, W., E. S. Levine, B. Griniuviene, K. O. Tankersley, J. M. Colacino, J. P. Sommadossi, K. A. Watanabe, and F. W. Perrino. 1996. Fialuridine and its metabolites inhibit DNA polymerase gamma at sites of multiple adjacent analog incorporation, decrease mtDNA abundance, and cause mitochondrial structural defects in cultured hepatoblasts. Proc. Natl. Acad. Sci. USA 93:3592-3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lewis, W., R. R. Meyer, J. F. Simpson, J. M. Colacino, and F. W. Perrino. 1994. Mammalian DNA polymerases alpha, beta, gamma, delta, and epsilon incorporate fialuridine (FIAU) monophosphate into DNA and are inhibited competitively by FIAU triphosphate. Biochemistry 33:14620-14624. [DOI] [PubMed] [Google Scholar]
- 27.Lewis, W., T. Papoian, B. Gonzalez, H. Louie, D. P. Kelly, R. M. Payne, and W. W. Grody. 1991. Mitochondrial ultrastructural and molecular changes induced by zidovudine in rat hearts. Lab. Investig. 65:228-236. [PubMed] [Google Scholar]
- 28.Martin, J. L., C. E. Brown, N. Matthews-Davis, and J. E. Reardon. 1994. Effects of antiviral nucleoside analogs on human DNA polymerases and mitochondrial DNA synthesis. Antimicrob. Agents Chemother. 38:2743-2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marusich, M. F., B. H. Robinson, J. W. Taanman, S. J. Kim, R. Schillace, J. L. Smith, and R. A. Capaldi. 1997. Expression of mtDNA and nDNA encoded respiratory chain proteins in chemically and genetically derived Rho0 human fibroblasts: a comparison of subunit proteins in normal fibroblasts treated with ethidium bromide and fibroblasts from a patient with mtDNA depletion syndrome. Biochim. Biophys. Acta 1362:145-159. [DOI] [PubMed] [Google Scholar]
- 30.McKenzie, R., M. W. Fried, R. Sallie, H. Conjeevaram, A. M. Di Bisceglie, Y. Park, B. Savarese, D. Kleiner, M. Tsokos, C. Luciano, et al. 1995. Hepatic failure and lactic acidosis due to fialuridine (FIAU), an investigational nucleoside analogue for chronic hepatitis B. N. Engl. J. Med. 333:1099-1105. [DOI] [PubMed] [Google Scholar]
- 31.Medina, D. J., C. H. Tsai, G. D. Hsiung, and Y. C. Cheng. 1994. Comparison of mitochondrial morphology, mitochondrial DNA content, and cell viability in cultured cells treated with three anti-human immunodeficiency virus dideoxynucleosides. Antimicrob. Agents Chemother. 38:1824-1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller, K. D., M. Cameron, L. V. Wood, M. C. Dalakas, and J. A. Kovacs. 2000. Lactic acidosis and hepatic steatosis associated with use of stavudine: report of four cases. Ann. Intern. Med. 133:192-196. [DOI] [PubMed] [Google Scholar]
- 33.Morshed, K. M., and K. E. McMartin. 1995. Transient alterations in cellular permeability in cultured human proximal tubule cells: implications for transport studies. In Vitro Cell. Dev. Biol. Anim. 31:107-114. [DOI] [PubMed] [Google Scholar]
- 34.Moyle, G. 2000. Clinical manifestations and management of antiretroviral nucleoside analog-related mitochondrial toxicity. Clin. Ther. 22:911-936. [DOI] [PubMed] [Google Scholar]
- 35.Nijtmans, L. G., J. W. Taanman, A. O. Muijsers, D. Speijer, and C. Van den Bogert. 1998. Assembly of cytochrome-c oxidase in cultured human cells. Eur. J. Biochem. 254:389-394. [DOI] [PubMed] [Google Scholar]
- 36.Pan-Zhou, X. R., L. Cui, X. J. Zhou, J. P. Sommadossi, and V. M. Darley-Usmar. 2000. Differential effects of antiretroviral nucleoside analogs on mitochondrial function in HepG2 cells. Antimicrob. Agents Chemother. 44:496-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parker, W. B., S. C. Shaddix, R. Vince, and L. L. Bennett. 1997. Lack of mitochondrial toxicity in CEM cells treated with carbovir. Antivir. Res. 34:131-136. [DOI] [PubMed] [Google Scholar]
- 38.Perry, C. M., and D. Faulds. 1997. Lamivudine. A review of its antiviral activity, pharmacokinetic properties and therapeutic efficacy in the management of HIV infection. Drugs 53:657-680. [DOI] [PubMed] [Google Scholar]
- 39.Poyton, R. O., and J. E. McEwen. 1996. Crosstalk between nuclear and mitochondrial genomes. Annu. Rev. Biochem. 65:563-607. [DOI] [PubMed] [Google Scholar]
- 40.Rahman, S., J. W. Taanman, J. M. Cooper, I. Nelson, I. Hargreaves, B. Meunier, M. G. Hanna, J. J. Garcia, R. A. Capaldi, B. D. Lake, J. V. Leonard, and A. H. Schapira. 1999. A missense mutation of cytochrome oxidase subunit II causes defective assembly and myopathy. Am. J. Hum. Genet. 65:1030-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robbins, B. L., R. V. Srinivas, C. Kim, N. Bischofberger, and A. Fridland. 1998. Anti-human immunodeficiency virus activity and cellular metabolism of a potential prodrug of the acyclic nucleoside phosphonate 9-R-(2-phos-phonomethoxypropyl)adenine (PMPA), bis(isopropyloxymethylcarbonyl)PMPA. Antimicrob. Agents Chemother. 42:612-617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rossi, L., S. Serafini, G. F. Schiavano, A. Casabianca, G. Vallanti, L. Chiarantini, and M. Magnani. 1999. Metabolism, mitochondrial uptake and toxicity of 2",3"-dideoxycytidine. Biochem. J. 344:915-920. [PMC free article] [PubMed] [Google Scholar]
- 43.Sundar, K., M. Suarez, P. E. Banogon, and J. M. Shapiro. 1997. Zidovudine-induced fatal lactic acidosis and hepatic failure in patients with acquired immunodeficiency syndrome: report of two patients and review of the literature. Crit. Care Med. 25:1425-1430. [DOI] [PubMed] [Google Scholar]
- 44.Tsai, C. C., K. E. Follis, M. Yarnall, and G. A. Blakley. 1989. Toxicity and efficacy of 2",3"-dideoxycytidine in clinical trials of pigtailed macaques infected with simian retrovirus type 2. Antimicrob. Agents Chemother. 33:1908-1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang, J., and S. Eriksson. 1996. Phosphorylation of the anti-hepatitis B nucleoside analog 1-(2"-deoxy-2"-fluoro-1-beta-d-arabinofuranosyl)-5-iodouracil by human cytosolic and mitochondrial thymidine kinase and implications for cytotoxicity. Antimicrob. Agents Chemother. 40:1555-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yerroum, M., C. Pham-Dang, F. J. Authier, I. Monnet, R. Gherardi, and P. Chariot. 2000. Cytochrome c oxidase deficiency in the muscle of patients with zidovudine myopathy is segmental and affects both mitochondrial DNA- and nuclear DNA-encoded subunits. Acta Neuropathol. (Berlin) 100:82-86. [DOI] [PubMed] [Google Scholar]