Abstract
Through broad screening of the compound library at Pharmacia, a naphthalene carboxamide was identified as a nonnucleoside inhibitor of human cytomegalovirus (HCMV) polymerase. Structure-activity relationship studies demonstrated that a quinoline ring could be substituted for naphthalene, resulting in the discovery of a 4-hydroxyquinoline-3-carboxamide (4-HQC) class of antiviral agents with unique biological properties. In vitro assays with the 4-HQCs have demonstrated potent inhibition of HCMV, herpes simplex virus type 1 (HSV-1), and varicella-zoster virus (VZV) polymerases but no inhibition of human α, δ, and γ polymerases. Antiviral cell culture assays have further confirmed that these compounds are active against HCMV, HSV-1, HSV-2, VZV, and many animal herpesviruses. However, these compounds were not active against several nonherpesviruses representing different DNA and RNA virus families. A strong correlation between the viral DNA polymerase and antiviral activity for this class of compounds supports inhibition of the viral polymerase as the mechanism of antiviral activity. Northern blot analysis of immediate-early and late viral transcripts also pointed to a block in the viral life cycle consistent with inhibition of viral DNA replication. In vitro HCMV polymerase assays indicate that the 4-HQCs are competitive inhibitors of nucleoside binding. However, no cross-resistance could be detected with ganciclovir-resistant HCMV or acyclovir-resistant HSV-1 mutants. The unique, broad-spectrum activities of the 4-HQCs may offer new opportunities for treating many of the diseases caused by herpesviruses.
While the treatment of herpes simplex virus type 2 (HSV-2) infections with acyclovir (ACV) and similar nucleoside analogs was one of the first success stories in antiviral chemotherapy, substantial unmet medical needs remain for diseases caused by herpesviruses. The increasing immunocompromised population, particularly AIDS patients and patients with transplants, has driven the need for improved antiviral agents to treat diseases caused by herpesviruses (6, 12). Although human cytomegalovirus (HCMV) is generally benign in the immunocompetent host, reactivation of HCMV is associated with significant morbidity and mortality in immunocompromised individuals. Active HCMV infection is associated with clinical syndromes such as pneumonia, retinitis, hepatitis, gastrointestinal disease, and congenital birth defects (2, 5, 7, 10, 12). The other human herpesviruses such as HSV-1, HSV-2, and varicella-zoster virus (VZV) also occur at a greater incidence and severity in the immunocompromised population (6, 17). Resistance to the currently available nucleoside analog antivirals does occur in this population, compounding the difficulty in treating these viral infections (11, 18). Ganciclovir (GCV), foscarnet, cidofovir, and formivirsen, the only drugs approved for treatment of HCMV infections, are less than ideal agents due to their significant toxicity, modest efficacy, and poor oral bioavailability (3, 17). Clearly, less-toxic, orally available alternatives are needed for treating herpesvirus infections in immunocompromised patients. In order to address this unmet medical need, we initiated a program to identify novel nonnucleoside inhibitors of herpesvirus DNA polymerases through broad screening of our compound collection.
Polymerases are classified according to their sequences and functional homologies. All herpesvirus polymerases belong to the family B DNA polymerases (1). Several eukaryotic polymerases, including human α and δ polymerases, also belong to this family of DNA polymerases with human δ polymerase sharing the highest degree of homology with the herpesvirus polymerases (1). These family B DNA polymerases share six to seven highly conserved domains labeled I through VII, in decreasing order of conservation (8, 9). In addition to these family B conserved domains, herpesvirus DNA polymerases share an additional conserved domain referred to as the A or δ domain (4). Hence, broad inhibition of herpesvirus polymerases may be possible if compounds target conserved domains shared among the herpesvirus polymerases but not shared among other eukaryotic polymerases such as human α and δ DNA polymerases. Approximately 80,000 compounds were screened for inhibition of the HCMV polymerase. Since the goal was to identify DNA polymerase inhibitors that demonstrate broad activities against the herpesviruses with no activity against host cell DNA polymerases, active compounds were further evaluated for inhibition of the HSV-1 and VZV polymerases and human polymerases α, δ, and γ.
One of the most promising leads from high-throughput screening for inhibitors of HCMV polymerase was a naphthalene carboxamide designated PNU-26370 (24). Structure-activity relationship (SAR) studies demonstrated that a quinoline ring could be substituted for naphthalene, resulting in the discovery of a 4-hydroxyquinoline-3-carboxamide (4-HQC) class of antivirals with unique biological properties. In this paper, we describe the broad-spectrum antiviral activities directed specifically against herpesviruses for three compounds from the 4-HQC class.
MATERIALS AND METHODS
Standard in vitro DNA polymerase assay.
HSV-1, HCMV, VZV, and human α and δ DNA polymerases were produced using a baculovirus expression system and purified by standard procedures. Purified human γ DNA polymerase was supplied by William Copeland (National Institutes of Health). Km values were determined with respect to TTP for all polymerases (data not shown). In order to compare a compound's inhibitory activity against the various DNA polymerases, substrate concentrations in the assays were significantly below Km (∼5 to 10 times) so that the 50% inhibitory concentration (IC50) for a competitive or noncompetitive inhibitor was equivalent to its Ki. Polymerases were used at a concentration that resulted in a linear rate of nucleotide incorporation during the reaction. Incorporation of 3H-labeled nucleotide into template-primer was measured using a scintillation proximity assay (SPA; Amersham Pharmacia Biotech, Piscataway, N.J.). In this assay, 3H-labeled nucleotide is incorporated into a primer-template by polymerase. The resultant radiolabeled product is captured onto a streptavidin-coated scintillant-containing microsphere. This binding event brings the beta-emitting particle in close proximity to the scintillant, allowing energy transfer and subsequent emission of light.
All polymerases except human δ polymerase were tested under identical assay conditions. In a standard assay, 5 μl of compound diluted in 100% dimethyl sulfoxide (DMSO) was added to 95 μl of polymerase in a solution containing 6.4 mM HEPES (pH 7.5), 12 mM KCl, 25 mM NaCl, 5 mM MgCl2, 46 μg of bovine serum albumin (BSA) per ml, 2 mM 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS), 5 mM dithiothreitol, 5% glycerol, 1.2 μCi of [methyl,1",2"-3H]TTP (Amersham Pharmacia Biotech), and 6 nM biotinylated oligo(dT)16-poly(dA) (Amersham Pharmacia Biotech). The polymerase reaction was incubated in a 96-well Dynatech Microlite I plate for 12 min at 26°C and stopped by the addition of 50 μl of a solution containing 4 mg of SPA beads per ml in 0.5 M EDTA (pH 8.0). The plate was sealed and counted on a Topcount Microplate Scintillation Counter (Packard Instruments, Meriden, Conn.). Percent inhibition was determined for each drug concentration compared to the value of the uninhibited control. IC50 values were calculated using EXCEL software for linear regression.
For human δ polymerase, 5 μl of compound diluted in 100% DMSO was added to 95 μl of polymerase in a solution containing 20 mM HEPES (pH 7.5), 40 μg of BSA per ml, 2 mM MnCl2, 1 mM dithiothreitol, 5% glycerol, 5% DMSO, 0.2 μM [methyl,1",2"-3H]TTP (Amersham Pharmacia Biotech), 0.4 μM dATP (Sigma, St. Louis, Mo.), and 10 μg of poly(dA-dT) primer-template per ml. Reactions were performed at 27°C for 10 min. Incorporated TMP was precipitated with an equal volume of 10% trichloroacetic acid and collected on a GF/B Millipore multiscreen filtration plate. Wells were washed three times with 5% trichloroacetic acid and dried prior to the addition of 100 μl of Microscint 40 (Packard Instruments) per well. The plate was sealed and counted on a Topcount Microplate Scintillation Counter (Packard Instruments). Percent inhibition was determined for each drug concentration compared to the value for the uninhibited control. IC50s were calculated using EXCEL software for linear regression.
Compounds used for standards in the DNA polymerase assay included zidovudine triphosphate (AZT-TP) (Moravek Biochemicals), phosphonoformic acid (foscarnet) (Sigma), and aphidicolin (Biomol).
Competitive inhibition assay.
The Ki values of PNU-145185 for inhibition of [3H]TTP incorporation into primer-template with HCMV polymerase were determined by various substrate and inhibitor concentrations in the in vitro polymerase assay described above. Substrate (TTP) concentrations spanned the calculated Km for HCMV polymerase (1.2 μM), ranging from 0.25 to 8.0 μM. Data were fit to models for competitive, mixed, and noncompetitive, or uncompetitive, inhibition using nonlinear regression analysis by the method of Marquart (15) as provided in the GraFit software package(Erithacus Software Limited, Staines, United Kingdom).
Viruses and cell culture.
Human foreskin fibroblast (HFF) cells were derived from human foreskin tissue as previously described (20). All other cell lines used to propagate viruses and to test antiviral activity in plaque reduction assays were obtained from the American Type Culture Collection (ATCC) (Rockville, Md.). HCMV and VZV were grown on HFF cells. HSV-1, HSV-2, and pseudorabies virus (PRV) were grown on African green monkey kidney cells (Vero). Rat CMV was grown on REF cells (ATCC CCL 192). Murine CMV was grown on murine 10T1/2 embryo fibroblast cells (ATCC CCL 226). Feline herpesvirus type 1 (FHV-1) was grown on CRFK cells (ATCC CCL 94). Bovine herpesvirus type 1 (BHV-1) was grown on BT cells (ATCC CRL 1390). Cells were propagated in Dulbecco's modified Eagle medium (DMEM) containing 10% fetal bovine serum (FBS) and supplemented with antibiotics. Cultures were maintained at 37°C in a humidified atmosphere of 5% CO2.
Laboratory strains of HCMV (AD169 and Davis), rat CMV, HSV-1 (KOS, McIntyre, and F), HSV-2 (MS), and VZV (Webster) were obtained from ATCC. Murine CMV (Smith strain) was originally obtained from D. Kelsey (University of Utah). The UT88 strain of FHV-1, the Iowa strain of BHV-1, and the HR strain of PRV were obtained from D. Lowrey (Pharmacia Corp.). HSV-2 strain 35D was obtained from H. Renis (Pharmacia Corp.). HSV-1 strains Patton and 294 were obtained from D. Coen (Harvard University). The ACV-resistant strains of HSV-1 and the GCV-resistant strain of HCMV were also obtained from D. Coen. The specific point mutations in the DNA polymerase gene resulting in ACV or GCV resistance for these isolates have been described previously (4, 9, 14, 21). Low-passage-number clinical isolates of HCMV and VZV were obtained from J. Nelson (University of Oregon) and A. Arvin (Stanford University), respectively.
Plaque reduction assays.
Antiviral activities of compounds against herpesviruses was determined using plaque reduction assays. Approximately 50 PFU of virus was added to each well of a 24-well culture dish containing the appropriate cells. After a 1-h incubation at 37°C, the virus inoculum was removed and drug-containing media was added. All compounds were first dissolved in 100% DMSO and diluted in 100% DMSO as 200× stocks. Dilutions of compounds were then added to DMEM containing 10% FBS and 0.8% carboxymethyl cellulose. ACV (Sigma) or GCV (obtained from a wholesale pharmacy) were used as positive controls. The plates were incubated at 37°C until plaques formed. Plaques were counted microscopically or by staining cells with crystal violet (0.1% in 20% ethanol). Percent inhibition compared to the value for the uninhibited control was determined for each drug concentration, and IC50s were calculated using EXCEL software for linear regression.
Cell viability determinations.
The toxicity of compounds on noninfected mammalian cells was determined for HFF, Vero, 10T1/2, and REF cells seeded as subconfluent monolayers and treated with compound for 3 days. Cell viability determinations were performed using both microscopic evaluation and a quantitative neutral red dye uptake assay as previously described (13).
HCMV immediate-early and late transcript analysis.
HFF cells were pretreated for 24 h with PNU-181465 or GCV at 20, 5, and 0.5 μM. Control cells were treated with 0.5% DMSO. Cell monolayers were washed and adsorbed with HCMV (Davis) at a multiplicity of infection of 1 PFU/cell. Cells were treated with compound immediately after the 1-h adsorption. Total cellular RNA was prepared from cells at 6, 24, and 48 h postinfection using RNeasy (Qiagen, Santa Clarita, Calif.) as described by the manufacturer. RNA was quantitated at A260 using a Spectramax Plus spectrophotometer (Molecular Devices, Sunnyvale, Calif.). One microgram of total cellular RNA was loaded onto a formaldehyde-morpholinepropanesulfonic acid (MOPS) 1% agarose gel, electrophoresed at 80 V for 2.5 h, transferred to a nylon membrane (Brightstar; Ambion, Austin, Tex.), and UV cross-linked. Single-stranded riboprobe for hybridization was derived by in vitro transcription from DNA containing the IE2 gene sequence (nucleotides 169231 to 170979 from EMBL data bank accession number X17403) as described by Tenney (22). RNA blots were hybridized overnight at 68°C with 32P-labeled riboprobe and autoradiographed.
RESULTS
Lead identification by high-throughput screening.
High-throughput screening of small molecules in the Pharmacia compound collection using the in vitro SPA assay identified several compounds as novel nonnucleoside inhibitors of the HCMV DNA polymerase (23, 24). One of the more promising leads was PNU-26370, a naphthalene carboxamide (Fig. 1). SAR studies on this lead identified naphthalene carboxamides with improved activities against the HCMV polymerase and specificities versus human α polymerase (24). Further SAR studies demonstrated that a quinoline ring could be substituted for naphthalene, resulting in the discovery of PNU-145185, a 4-HQC. Substitutions at the C-6 position of the quinoline ring produced a series of 4-HQC compounds such as PNU-181128 and PNU-181465 (Fig. 1) with improved activities.
FIG. 1.
Hit to lead process. Depicted are key structures that progressed from the identification of an initial lead through high-throughput screening to the discovery of 4-HQCs with potent antiviral activities against many herpesviruses.
In vitro inhibition of polymerases.
Three herpesvirus DNA polymerases (HCMV, HSV-1, and VZV) and three human polymerases (α, δ, and γ) were expressed and purified using recombinant baculovirus vectors. (Note that while human DNA γ belongs to the family A DNA polymerases sharing little homology with the herpesvirus DNA polymerases, this DNA polymerase was included in the screen as an additional test for specificity.) Inhibition of these DNA polymerases by the 4-HQCs and positive-control compounds (foscarnet, aphidicolin, and AZT-TP) was evaluated in vitro by measuring incorporation of nucleotide into a primer-template (Table 1). The 4-HQCs inhibited all three herpesvirus DNA polymerases but showed no detectable inhibition of the three human DNA polymerases. Typically, 4-HQC inhibition of the VZV polymerase was greater than for the HCMV and HSV-1 polymerases. An approximately 10-fold improvement in activity was observed from PNU-145185 to PNU-181465 for all three herpesvirus DNA polymerases. Foscarnet was used as a positive control for inhibition of the herpesvirus polymerases. Interestingly, foscarnet also strongly inhibited human δ polymerase in these assays. As expected, aphidicolin inhibited all three herpesvirus polymerases and the human α and δ polymerases. AZT-TTP was the positive control for inhibition of human γ polymerase.
TABLE 1.
Specificities of 4-HQCs for herpesvirus polymerasesa
| Compound | Inhibition of polymerases (Ki [μM])b
|
|||||
|---|---|---|---|---|---|---|
| HCMV | HSV-1 | VZV | α | δ | γ | |
| PNU-145185 | 4.0 | 2.9 | 2.0 | >50 | >20 | >20 |
| PNU-181128 | 0.95 | 0.72 | 0.19 | >50 | >20 | >20 |
| PNU-181465 | 0.35 | 0.28 | 0.07 | >50 | >20 | >20 |
| Foscarnet | 2.5 | <0.78 | <0.78 | >20 | <0.28 | >20 |
| Aphidicolin | 0.37 | 0.45 | 0.60 | 2.6 | 2.6 | >20 |
| AZT-TTP | >20 | NDc | ND | >20 | >20 | 2.3 |
HCMV, HSV-1, VZV, and human α, δ, and γ polymerases were produced using a baculovirus expression system and purified by standard procedures. Polymerase assays were performed under conditions of first-order rate kinetics.
Inhibition of polymerase activity was detected as reduced incorporation of 3H-labeled nucleotide into a primer-template using a SPA.
ND, not determined.
Antiviral activities of 4-HQCs against herpesviruses.
The 4-HQCs were tested in cell culture plaque reduction assays for antiviral activities against laboratory and clinical isolates of HCMV, VZV, HSV-1, and HSV-2 (Tables 2, 3, and 4). As predicted by their in vitro polymerase activity, PNU-181128 and PNU-181465 exhibited greater activities against these viruses than PNU-145185. PNU-181128 and PNU-181465 were as active as GCV in antiviral assays against isolates of HCMV with average IC50s of 1.3, 0.98, and 1.1 μM, respectively (Table 2). The 4-HQCs were substantially more active against isolates of VZV than ACV (Table 3). Although the IC50s generated for ACV in these VZV assays were greater than those reported in the literature (16), the submicromolar activities observed with PNU-181128 and PNU-181465 against clinical isolates of VZV indicated substantially greater potency (Table 3). The 4-HQCs were also active in plaque reduction assays against various isolates of HSV-1 and HSV-2 (Table 4). Average IC50s for PNU-181128 and PNU-181465 against HSV isolates were slightly higher than observed with ACV. In addition, the 4-HQCs were active in antiviral cell culture assays against several animal herpesviruses with species specificity for rodents, primates, or other mammals (Table 5).
TABLE 2.
Activities of 4-HQCs against HCMV isolates
| Isolatea | IC50 (μM) by plaque reduction assay
|
|||
|---|---|---|---|---|
| PNU-145185 | PNU-181128 | PNU-181465 | GCV | |
| Davis | 6.7 | 1.7 | 1.0 | 0.80 |
| AD169 | 8.6 | 0.80 | 0.80 | 0.80 |
| GDGrP53 | 5.8 | 0.70 | 0.40 | 12 |
| Adi | 19 | 1.4 | 0.95 | 1.5 |
| Gru | 4.9 | 0.80 | 0.50 | 0.90 |
| Har | 9.2 | 0.70 | 0.60 | 0.60 |
| Lys | 12 | 1.7 | 1.3 | 1.4 |
| New | 7.2 | 1.2 | 1.3 | 1.5 |
| Pho | NDc | 1.3 | 0.70 | 1.0 |
| Sal | 18 | 1.1 | 0.70 | 1.2 |
| Sch | 14 | 1.3 | 0.70 | 1.5 |
| Wat | 11 | 1.9 | 2.2 | 1.4 |
| Avg ± SDb | 11 ± 4.7 | 1.3 ± 0.40 | 0.98 ± 0.48 | 1.1 ± 0.34 |
Davis and AD169 are laboratory-passaged strains. GDGrP53 is a GCV-resistant strain derived from AD169 containing a mutation in its polymerase gene (20). The remaining virus strains are clinical isolates kindly provided by Jay Nelson (University of Oregon).
GCV-resistant strain not included in average.
ND, not determined.
TABLE 3.
Activities of 4-HQCs against VZV isolates
| Isolatea | IC50 (μM) by plaque reduction assay
|
|||
|---|---|---|---|---|
| PNU-145185 | PNU-181128 | PNU-181465 | ACV | |
| Webster | 0.70 | 0.68 | 0.17 | 8.1 |
| Car | 1.5 | 0.20 | 0.10 | 14 |
| Coh | 2.3 | 0.20 | 0.20 | 36 |
| Kle | 0.70 | 0.20 | 0.10 | 11 |
| Gei | 1.4 | 0.20 | 0.10 | 19 |
| Nag | 0.60 | 0.10 | 0.10 | 17 |
| Die | 0.60 | 0.20 | 0.10 | 14 |
| Ste | 0.90 | 0.10 | 0.10 | 46 |
| Mag | 1.1 | 0.40 | 0.10 | 46 |
| Rai | 0.50 | 0.10 | 0.10 | 13 |
| Dye | 1.2 | 0.30 | 0.10 | 37 |
| Average ± SD | 1.0 ± 0.54 | 0.24 ± 0.17 | 0.12 ± 0.03 | 24 ± 14 |
Webster is a laboratory-passaged strain. The remaining viruses are clinical isolates kindly provided by Ann Arvin (Stanford University).
TABLE 4.
Activities of 4-HQCs against HSV isolates
| Isolatea | IC50 (μM) by plaque reduction assay
|
|||
|---|---|---|---|---|
| PNU-145185 | PNU-181128 | PNU-181465 | ACV | |
| HSV-1 KOS | 16 | 7.4 | 7.4 | 4.0 |
| HSV-1 Mac | 13 | 18 | 16 | 8.7 |
| HSV-1 F | 18 | 9.5 | 12 | 5.2 |
| HSV-1 Patton | 40 | 18 | 20 | 17 |
| HSV-1 294 | 29 | 15 | 14 | 7.0 |
| HSV-1 DJL | 15 | 11 | 18 | 3.5 |
| HSV-2 MS | 26 | 14 | 15 | 17 |
| HSV-2 35D | 13 | 20 | 11 | 6.7 |
| Average ± SD | 21 ± 9.6 | 14 ± 4.5 | 14 ± 4.0 | 8.6 ± 5.4 |
HSV-1 strain DJL was a locally isolated clinical sample. The remaining HSV strains were obtained from ATCC, D. Coen (Harvard University), or H. Renis (Pharmacia Corp.).
TABLE 5.
Activities of 4-HQCs against animal herpesviruses
| Virus | IC50 (μM) by plaque reduction assay
|
|||
|---|---|---|---|---|
| PNU-145185 | PNU-181128 | PNU-181465 | GCV | |
| Murine CMV | 6.8 | 1.3 | 1.6 | 11 |
| Rat CMV | NDa | 0.6 | 0.3 | >20 |
| Simian VZV | 4.3 | 2.5 | 3.7 | >20 |
| PRV | 30 | 4.5 | 7.4 | 7.5 |
| FHV | 5.7 | 3.9 | 5.7 | 4.3 |
| BHV-1 | 27 | 3.2 | 1.7 | >20 |
ND, not determined.
Specificities of 4-HQCs.
PNU-145185, PNU-181128, and PNU-181465 were tested for cellular cytotoxicity in four different mammalian cell lines (Table 6). These compounds did not exhibit detectable cellular cytotoxicity at the drug concentrations used in this study. Comparing IC50s and 50% cellular cytotoxicities for PNU-181128 and PNU-181465 resulted in therapeutic indices ranging from 3.5 to >800 for human herpesviruses and their respective cell lines. In addition, the antiviral activities of the 4-HQCs appeared to be specific for the Herpesviridae family of viruses. These compounds were not active (IC50 > 100 μM) against vaccinia virus, simian virus 40 (SV40), adenovirus, hepatitis B virus, influenza A virus, coxsackie B virus, or vesicular stomatitis virus (data not shown).
TABLE 6.
Cytotoxicities of 4-HQCs in mammalian cells
| Cell type | Cellular cytotoxicity (CC50 [μM])a
|
||
|---|---|---|---|
| PNU-145185 | PNU-181128 | PNU-181465 | |
| HFF | >100 | 75 | >100 |
| Vero | 90 | 49 | >100 |
| REF | >100 | 58 | >100 |
| 10T1/2 | 89 | 62 | >100 |
Cytotoxicity measured by inhibition of neutral red uptake into actively proliferating cultures of cells. CC50, 50% cellular cytotoxicity.
Mechanistic studies with 4-HQCs.
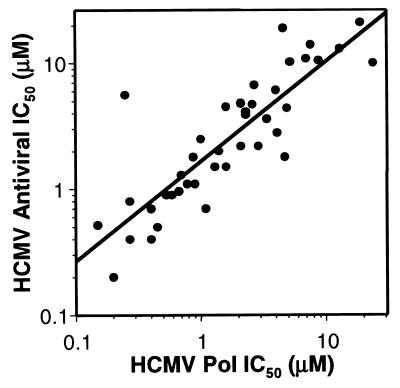
Several studies were performed to correlate the in vitro inhibition of herpesvirus polymerases by 4-HQCs with their observed antiviral activities in cell culture. A set of 41 4-HQC compounds synthesized for SAR studies were evaluated for their HCMV polymerase and antiviral activities. These compounds represented a large spectrum of potency with a 100-fold range in their IC50s for HCMV polymerase inhibition. Figure 2 plots the HCMV polymerase activities versus the HCMV antiviral activities for the 41 compounds. This plot illustrates a positive correlation (r2 = 0.73) between the relative polymerase and antiviral activities for this class of compounds and supports a linkage between in vitro polymerase inhibition and antiviral activity in cell culture.
FIG. 2.
Correlation (r2 = 0.73) between polymerase and antiviral activities. A set of 41 4-HQC compounds were assayed for inhibition of the HCMV polymerase (Pol) in vitro and for inhibition of HCMV using plaque reduction assays.
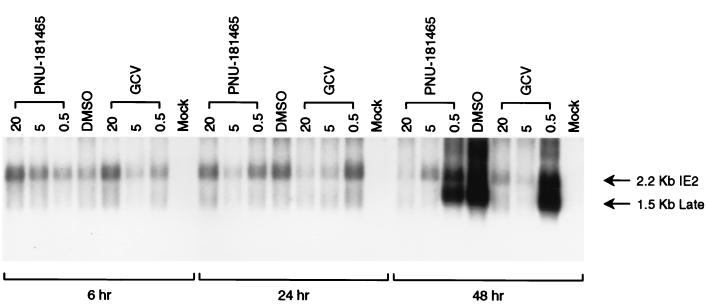
The mechanism of viral inhibition was also studied by examining inhibition of temporally regulated viral transcripts. The immediate-early 2.2-kb IE2 transcript is observed early, within 6 h postinfection, in the infection process and occurs independently of viral DNA replication (19). GCV, a known inhibitor of HCMV DNA polymerase, did not inhibit the 2.2-kb IE2 transcript which is observed 6 h postinfection (Fig. 3). Similarly, PNU-181465 did not inhibit the 2.2-kb immediate-early transcript. The late 1.5-kb transcript occurs only after viral DNA replication and is not easily detectable until 48 h postinfection (19). GCV and PNU-181465 completely inhibited this 1.5-kb late transcript at 20 and 5 μM (Fig. 3). Appearance of the late transcript was observed at 0.5 μM with both compounds. This pattern of viral mRNA inhibition by PNU-181465, similar to GCV, supports a block in the viral life cycle consistent with inhibition of viral DNA replication.
FIG. 3.
Northern blot analysis of immediate-early (2.2-kb IE2 transcript) and late (1.5-kb IE2 transcript) HCMV transcripts from cells treated with PNU-181465 or GCV from 24 h prior to infection until harvest at 6, 24, or 48 h postinfection. Mock-infected cells served as the negative control, and cells mock treated with 0.5% DMSO served as the positive control for hybridization of viral transcripts.
Competitive inhibition of 4-HQCs and nucleotide for binding to the viral polymerase.
Inhibition of HCMV polymerase activity by PNU-145185 was evaluated by measuring initial velocity in the presence of various concentrations of inhibitor and substrate. Figure 4 shows a Lineweaver-Burk plot for the kinetic analysis of PNU-145185. The intersection of the substrate and inhibitor lines was slightly to the left of the y axis. A statistical analysis (independent sum of squares) comparing the fit of this data to different models for inhibition indicated that PNU-145185 may act as a mixed inhibitor with respect to nucleotide substrate. Mixed inhibitors can inhibit by competitively binding to the enzyme alone (Kic) and also by binding to the enzyme-substrate complex (Kiu). However, comparison of the Kic (4.7 μM) and Kiu (23.9 μM) values indicates that PNU-145185 is predominantly a competitive inhibitor of TTP.
FIG. 4.
Inhibition of HCMV polymerase by PNU-145185. A Lineweaver-Burk plot was constructed to demonstrate the effects of various concentrations (Conc.) of the inhibitor, PNU-145185, on HCMV DNA polymerase. Substrate concentrations bracketed the calculated Km for the polymerase (1.2 μM), ranging from 0.25 to 8 μM TTP. The mixed inhibition scheme depicted to the right of the plot was fit to the data with the best-fit values (i.e., Kic and Kiu) shown underneath the scheme. The straight lines shown in the figure were calculated using these best-fit values.
Lack of cross-resistance between 4-HQCs and nucleotide analogs.
Although the 4-HQCs appear to be competitive inhibitors of substrate binding to the viral polymerase, these compounds do not appear to bind the identical site(s) to which nucleotide analogs such as ACV-TP or GCV-TP bind. A series of ACV-resistant HSV-1 isolates containing point mutations in different conserved domains of the viral polymerase remained sensitive to PNU-145185 (Table 7). Most of these mutants exhibited greater sensitivity to PNU-145185 than the wild-type parent strain of HSV-1 used to generate the mutants. In addition, an HCMV isolate (GDGrp53) containing a polymerase mutation resulting in ganciclovir resistance also remained sensitive to the 4-HQCs (Table 2).
TABLE 7.
Activities of 4-HQCs against ACV-resistant HSV
| HSV-1 isolatea | Pol mutation (Pol domain)b | IC50 (μM) by plaque reduction assay
|
|
|---|---|---|---|
| PNU-145185 | ACV | ||
| KOS | None (WT) | 15 | 2.7 |
| F891C | F891C (I) | 3.3 | 52 |
| AraAr13 | V813M (III) | 1.5 | 36 |
| PAAr5 | R841S (III) | 4.6 | 36 |
| AraAr9 | N961K (V) | 18 | 8.1 |
| PFAr2 | T566A, A605V (δ) | 1.5 | 34 |
| TsD9 | E597 (δ) | 7.6 | 39 |
Mutants were derived in the laboratory from the KOS wild-type strain and have been described previously (4, 9, 14).
The polymerase (Pol) mutations are shown as follows: the single-letter symbol for the wild type (WT) amino acid precedes its numbered position within the polymerase, and the symbol for the new amino acid resulting from the point mutation follows the numbered position. The conserved domains within polymerase (Pol) containing the mutation causing ACV resistance are shown in parentheses.
DISCUSSION
The 4-HQCs represent a novel class of nonnucleoside herpesvirus DNA polymerase inhibitors. This class of compounds was discovered through a combination of high-throughput screening for small-molecule inhibitors of herpesvirus DNA polymerases and SAR studies to improve the characteristics of the initial lead templates. These studies help to illustrate the power of a molecular-target-based screening approach for the discovery of new drugs to treat infectious diseases.
The 4-HQCs appear to be specific inhibitors of herpesvirus DNA polymerases. These compounds did not inhibit human DNA polymerases using in vitro polymerase activity assays. The compounds also did not inhibit SV40 DNA replication in cell culture (data not shown). Since SV40 does not encode its own viral polymerase and must rely upon eukaryotic polymerases to replicate its DNA, the lack of viral inhibition provides further evidence for the specificity of the 4-HQCs. In addition, the 4-HQCs did not inhibit a panel of nonherpesviruses representing several different families of DNA and RNA viruses. However, within the Herpesviridae, the 4-HQCs demonstrated broad antiviral activities, inhibiting isolates of HCMV, HSV-1, HSV-2, VZV, and several animal herpesviruses. This spectrum of activity is presumably related to specific interactions with the herpesvirus polymerases. While herpesvirus polymerases contain the conserved domains characteristic of all family B DNA polymerases, the degree of homology within the herpesvirus family is higher than that for unrelated family B polymerases (1). The 4-HQCs may bind to regions of the DNA polymerase specifically conserved within the herpesviruses. Mutational analysis of these polymerases will be critical in understanding the spectrum of antiviral activities for the 4-HQCs.
Enzyme kinetic studies indicated that the 4-HQCs were primarily competitive inhibitors of substrate binding to the polymerase. These studies used TTP as the substrate, so relative degrees of competitive behavior may vary for other nucleotide substrates. These studies also indicated that the 4-HQCs may have a small uncompetitive component. Under these circumstances, the 4-HQCs may have a high affinity for enzyme alone, but a low-level affinity for the enzyme-substrate complex. Alternatively, the 3"→5" exonuclease activity found with herpesvirus DNA polymerases may have introduced experimental error into these enzyme kinetic studies, since the level of exonuclease activity would change with various substrate concentrations. We are currently preparing an exonuclease-deficient herpesvirus polymerase to examine this possibility.
One of the critical correlates for molecular-target-based antiviral drug discovery is demonstrating that in vivo antiviral activity results directly from inhibition of the viral target identified in vitro. The positive correlation between in vitro HCMV DNA polymerase activity and HCMV antiviral cell culture activity for the 4-HQC class of compounds provides strong evidence that the in vivo mechanism of action is inhibition of the viral DNA polymerase. The approximately 1:1 ratio for polymerase and antiviral IC50s was maintained through a 100-fold range of potency. Additionally, analysis of temporally regulated viral transcripts indicated that the 4-HQCs blocked the herpesvirus life cycle during the early phase of viral replication, after the immediate-early phase and prior to the synthesis of late transcripts, which is compatible with inhibition of viral DNA replication. While these studies clearly implicate inhibition of the herpesvirus DNA polymerase, conclusive proof of the in vivo mechanism of action must await the generation and mapping of resistant mutants.
The 4-HQC class of compounds offers an exciting opportunity for the development of new antivirals to treat many of the diseases caused by herpesviruses that affect humans. These compounds also offer a unique tool to study herpesvirus DNA polymerases and their conserved functions. Future studies will focus on mapping the binding site(s) for these compounds on the viral DNA polymerase.
REFERENCES
- 1.Braithwaite, D. K., and J. Ito. 1993. Compilation, alignment, and phylogenetic relationships of DNA polymerases. Nucleic Acids Res. 21:787-802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cisneros, J. M., P. Munoz, J. Torre-Cisneros, M. Gurgui, M. J. Rodriguez-Hernandez, J. M. Aguado, A. Echaniz, and the Spanish Transplantation Infection Study Group. 1998. Pneumonia after heart transplantation: a multiinstitutional study. Clin. Infect. Dis. 27:324-331. [DOI] [PubMed] [Google Scholar]
- 3.deJong, M. D., G. J. Galaso, B. Gazzard, P. D. Griffiths, D. A. Jabs, E. A. Kern, and S. A. Spector. 1998. Summary of the Second International Symposium on Cytomegalovirus. Antivir. Res. 37:141-146. [DOI] [PubMed] [Google Scholar]
- 4.Gibbs, J. S., H. C. Chiou, K. F. Bastow, Y. C. Cheng, and D. M. Coen. 1988. Identification of amino acids in herpes simplex virus DNA polymerase involved in substrate and drug recognition. Proc. Natl. Acad. Sci. USA 85:6672-6676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goodgame, R. W. 1993. Gastrointestinal cytomegalovirus disease. Ann. Intern. Med. 119:924-935. [DOI] [PubMed] [Google Scholar]
- 6.Griffiths, P. A. 1996. Herpesviruses and AIDS. Scand. J. Infect. Dis. Suppl. 100:3-7. [PubMed] [Google Scholar]
- 7.Halwachs-Baumann, G., B. Genser, M. Danda, H. Engele, H. Rosegger, B. Folsch, U. Maurer, H. Lackner, and M. Truschnig-Wilders. 2000. Screening and diagnosis of congenital cytomegalovirus infection: a 5-year study. Scand. J. Infect. Dis. 32:137-142. [DOI] [PubMed] [Google Scholar]
- 8.Hindges, R., and U. Hubscher. 1997. DNA polymerase δ, an essential enzyme for DNA transactions. Biol. Chem. 378:345-362. [DOI] [PubMed] [Google Scholar]
- 9.Hwang, C. B. C., K. L. Ruffner, and D. M. Coen. 1992. A point mutation within a distinct conserved region of the herpes simplex virus DNA polymerase gene confers drug resistance. J. Virol. 66:1774-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ives, D. V. 1997. Cytomegalovirus disease in AIDS. AIDS 11:1791-1797. [DOI] [PubMed] [Google Scholar]
- 11.Jabs, D. A., C. Enger, J. P. Dunn, and M. Forman. 1998. Cytomegalovirus retinitis and viral resistance: ganciclovir resistance. J. Infect. Dis. 177:770-773. [DOI] [PubMed] [Google Scholar]
- 12.Ljungman, P. 1996. Cytomegalovirus infections in transplant patients. Scand. J. Infect. Dis. Suppl. 100:59-63. [PubMed] [Google Scholar]
- 13.Lowik, C. W. G. M., M. J. Alblas, M. van de Ruit, S. E. Papapoulos, and G. van der Pluijm. 1993. Quantitation of adherent and nonadherent cells cultured in 96 well plates using the supravital stain neutral red. Anal. Biochem. 213:426-433. [DOI] [PubMed] [Google Scholar]
- 14.Marcy, A. I., C. B. C. Hwang, K. L. Ruffner, and D. M. Coen. 1990. Engineered herpes simplex virus DNA polymerase point mutations: the most highly conserved region shared among α-like DNA polymerases is involved in substrate recognition. J. Virol. 64:5883-5890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marquardt, D. W. 1993. An algorithm for least-squares estimation of nonlinear parameters. J. Soc. Appl. Math. 11:431-444. [Google Scholar]
- 16.O'Brien, J. J., and D. M. Campoli-Richards. 1989. Acyclovir. An updated review of its antiviral activity, pharmacokinetic properties and therapeutic efficacy. Drugs 37:233-309. [DOI] [PubMed] [Google Scholar]
- 17.Pillay, D., D. Mutimer, S. Singhal, A. Turner, K. Ward, and M. Wood. 2000. Management of herpes virus infections following transplantation. J. Antimicrob. Chemother. 45:729-748. [DOI] [PubMed] [Google Scholar]
- 18.Reusser, P., C. Cordonnier, H. Einsele, D. Engelhard, H. Link, A. Locasciulli, and P. Ljungman. 1996. European survey of herpesvirus resistance to antiviral drugs in bone marrow transplant recipients. Bone Marrow Transplant. 17:813-817. [PubMed] [Google Scholar]
- 19.Stenberg, R. M., A. S. Depto, J. Fortney, and J. A. Nelson. 1989. Regulated expression of early and late RNAs and proteins from the human cytomegalovirus immediate-early gene region. J. Virol. 63:2699-2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stinski, J. F. 1978. Sequence of protein synthesis in cells infected by human cytomegalovirus: early and late virus-induced polypeptides. J. Virol. 26:686-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sullivan, V., K. A. Biron, C. Talarico, S. C. Stanat, M. Davis, L. M. Pozzi, and D. M. Coen. 1993. A point mutation in the human cytomegalovirus DNA polymerase gene confers resistance to ganciclovir and phosphonylmethoxyalkyl derivatives. Antimicrob. Agents Chemother. 37:19-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tenney, D. J., and A. M. Colberg-Poley. 1991. Human cytomegalovirus UL36-38 and US3 immediate-early genes: temporally regulated expression of nuclear, cytoplasmic, and polysome-associated transcripts during infection. J. Virol. 65:6724-6734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tucker, J. A., T. L. Clayton, C. G. Chidester, M. W. Schulz, L. E. Harrington, S. J. Conrad, Y. Yagi, N. L. Oien, D. Yurek, and M. S. Kuo. 2000. Structure-activity relationships of acyloxyamidine cytomegalovirus DNA polymerase inhibitors. Bioorg. Med. Chem. 8:601-615. [DOI] [PubMed] [Google Scholar]
- 24.Vaillancourt, V. A., M. M. Cudahy, S. A. Staley, R. J. Brideau, S. J. Conrad, M. L. Knechtel, N. L. Oien, J. L. Wieber, Y. Yagi, and M. W. Wathen. 2000. Naphthalene carboxamides as inhibitors of human cytomegalovirus DNA polymerase. Bioorg. Med. Chem. Lett. 10:2079-2081. [DOI] [PubMed] [Google Scholar]