Abstract
Polymyxin B (PXB) and the cecropin A-melittin hybrid CA(1-8)M(1-18) (KWKLFKKIGIGAVLKVLTTGLPALIS-NH2) were compared for antibiotic activity on reference and multiresistant Acinetobacter baumannii strains. Significant differences for both peptides were observed on their inner membrane interaction and inhibition by environmental factors, supporting the use of CA(1-8)M(1-18) as a potential alternative to PXB against Acinetobacter.
Fatty acid-acylated polymyxins are among the most active antibiotics against gram-negative bacteria (5). Although not completely unveiled, the mechanism of action of these cyclic cationic peptides is based on self-promoted uptake through interaction with lipopolysaccharide (LPS), disorganization of the outer membrane, and further binding to the inner membrane, whose permeation is not required. (4).
Polymyxin B (PXB) was the only drug universally active against nosocomial multiresistant strains from the opportunistic pathogen Acinetobacter baumannii (2). A recent description of a PXB-resistant isolate raised clinical concern (C. Urban, N. Mariano, J. J. Rahal, E. Tay, C. Ponio, T. Koprivnjak, and J. Weiss, Letter, Antimicrob. Agents Chemother. 45:994-995, 2001). Nevertheless, this strain was susceptible to peptides such as cecropin P1 and rBPI21, supporting eukaryotic antibiotic peptides as alternative drugs for use against Acinetobacter. In tune with this, we have compared the antibiotic activity of PXB with that of the cecropin A-melittin hybrid CA(1-8)M(1-18) (KWKLFKKIGIGAVLKVLTTGLPALIS-NH2), for which strong bactericidal (17) and antiendotoxic activities (8), but no animal cytotoxicity (6, 8), have been reported. CA(1-8)M(1-18) is active against other members of the family Enterobacteriaceae in vitro (8, 9, 19) and has been tested successfully on a set of A. baumannii nosocomial isolates with different antibiotic resistance patterns (1).
Because it had a broad multiresistance pattern, the nosocomial Acinetobacter baumannii isolate Ac157 was chosen from a panel of 17 nosocomial isolates from the Microbiology Department (Hospital de la Princesa, Madrid, Spain) and tested against ticarcillin, cefotaxime, imipenem, tobramycin, amikacin, ofloxacin, and doxycycline (1). This strain was resistant to the complete panel, except for showing intermediate resistance to tobramycin as defined in reference 12. CA(1-8)M(1-18) was synthesized by solid-phase methods (3), purified by reverse-phase chromatography, and satisfactorily characterized by high-performance liquid chromatography, amino acid analysis, and matrix-assisted laser desorption ionization-time of flight mass spectrometry. Reagents were purchased from Sigma (St. Louis, Mo.), and fluorescence dyes were obtained from Molecular Probes (Leiden, Holland).
MICs were assayed at 5 × 105 CFU/ml on Mueller-Hinton (MH) broth at 37°C on polypropylene microwell plates by twofold serial dilution of the peptides (8.0 to 0.012 μM). MICs for the reference strain were 2.0 and 1.0 μM for CA(1-8)M(1-18) and PXB, respectively, whereas those for Ac157 were 2.0 to 4.0 and 1.0 to 2.0 μM. These MICs were in the same range as those determined with the previously assayed (1) panel of A. baumannii nosocomial multiresistant (to one to six drugs) strains. All strains were susceptible to colistin and to CA(1-8)M(1-18)—in this case, with MICs between 2 and 4 μM— except for one strain for which the MIC was 8 μM (1). No correlation was found among MICs and the class or number of drugs to which they were resistant (1), in agreement with the results obtained for the same peptide on other bacterial species with multiresistance to standard antibiotics. Unless otherwise stated, only data for strain Ac157 are shown, because differences from the reference strain were not significant (P > 0.1).
Determinations of parameters involving peptide-pathogen interaction were made at 107 CFU/ml, the same used for the membrane perturbation assays. Bacteria were resuspended in Hanks medium plus 20 mM glucose (Hanks+Glc) and incubated with the peptide alone or with the corresponding reagent for 30 min at 37°C. Afterwards 10-μl aliquots were transferred into a 96-well microplate containing 100 μl of MH broth, and MICs were determined by the checkerboard method by twofold dilution. At this concentration, killing curves showed a fast killing kinetics, with a reduction of 4 log orders for CA(1-8)M(1-18) and PXB after 5 or 15 min, respectively.
Although CA(1-8)M(1-18) and PXB showed identical increases in their MICs in the presence of divalent cations (or, conversely, enhanced activity in the presence of EDTA), PXB was more susceptible to changes in salinity (Table 1). In contrast, the activity of CA(1-8)M(1-18) was much more susceptible than that of PXB to inhibition by polyanions. A possible explanation is that a flexible, linear peptide such as CA(1-8)M(1-18) has a broader repertoire of potential polyanion-binding conformations than the more rigid PXB. Interestingly, CA(1-8)M(1-18) retains more activity than PXB on depolarized and/or metabolically quiescent Acinetobacter cells, like those in the stationary phase or biofilms (6).
TABLE 1.
MICs of CA(1-8)M(1-18) and PXB for A. baumannii at 107 CFU/ml under different experimental conditions
| Agenta | MIC (μM)
|
|
|---|---|---|
| CA(1-8)M(1-18) | PXB | |
| Standard conditions (Hanks+Glc) | 2 | 1 |
| NaCl (mM) | ||
| 27 | 1 | 0.25 |
| 81 | 2 | 0.5 |
| 136 | 2 | 1 |
| Divalent cations | ||
| Ca2+ (mM) | ||
| 0.05 | 2 | 1 |
| 1.0 | 8 | 2 |
| 5.0 | 16 | 4 |
| Mg2+ (mM) | ||
| 0.05 | 2 | 1 |
| 1.0 | 8 | 2 |
| 5.0 | 16 | 4 |
| EDTA (mM) | ||
| 0.25 | 1 | 0.5 |
| 1.0 | 0.5 | 0.25 |
| 5.0 | 0.25 | 0.12 |
| Polyanionsb | ||
| Heparin (mg/ml) | ||
| 0.01 | 2 | 1 |
| 0.1 | 8 | 2 |
| 1.0 | 16 | 2 |
| Alginate (mg/ml) | ||
| 0.01 | 4 | 1 |
| 0.1 | 16 | 2 |
| 1.0 | >16 | 2 |
| Uncoupler CCCP (μM)b | ||
| 12.5 | 2 | 4 |
| 50 | 4 | 4 |
| 100 | 4 | 8 |
Unless otherwise stated, bacteria were preincubated with the corresponding agent for 15 min prior to peptide addition.
Peptide and the corresponding agent were preincubated for 15 min before being added to the bacterial suspension. CCCP, carbonyl cyanide m-chlorophenylhydrazone.
Outer membrane sensitization to detergents (16) by both peptides was demonstrated by the fact that all MICs were decreased to 0.25 μM in the presence of 0.05% Triton X-100. Interaction with LPS was confirmed by peptide displacement of dansyl polymyxin (DPXB) bound to purified A. baumannii LPS (18) or to isolated cells (10, 14). The 50% maximal displacements (I50) of DPXB by CA(1-8)M(1-18) were 1.6 and 4.1 μM on LPS and bacterial cells, respectively, and 1.3 and 5.5 μM for PXB. The values are in the same range as those reported for Pseudomonas aeruginosa (13).
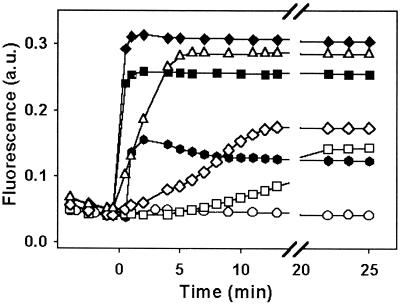
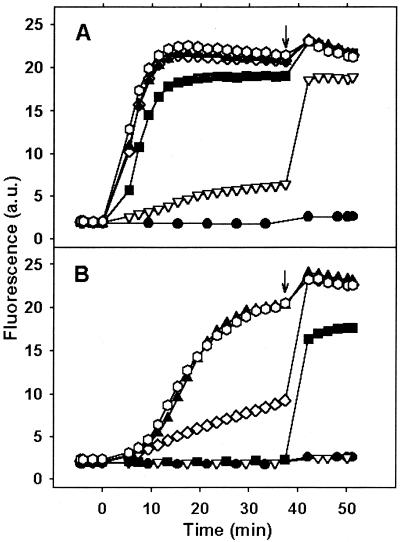
Furthermore, permeation of inner membrane was compared by two complementary approaches: (i) membrane depolarization, monitored by the increase in fluorescence of Disc3(5) (3, 3-dipropylthiadicarbocyanine iodide) (19), and (ii) influx of the membrane-impermeable probe SYTOX into the cytoplasm. Both assays were performed at 107 CFU/ml with a Hitachi F2000 spectrofluorometer. EDTA (10 mM) was included to allow Disc3(5) free access to the inner membrane. CA(1-18)M(1-18) caused a higher and faster permeabilization than PXB in both systems (Fig. 1 and 2), with a good correlation with bacterial killing, as reported for Escherichia coli (19). This was not the case for PXB, which permeabilized the membrane only at concentrations higher than the MIC (4). This fact reflects divergences in the respective lethal mechanisms, although both peptides share a self-promoted uptake when crossing the outer membrane (10, 13).
FIG. 1.
Depolarization of the inner membrane of A. baumannii strain Ac157 as assessed by Disc3(5) fluorescence (λex = 622, λem = 670 nm) in the presence of CA(1-8)M(1-18) (solid symbols) or PXB (open symbols). Peptide concentrations: control, circles; 0.05 μM, hexagons; 0.5 μM, squares; 1.0 μM, diamonds; 2.0 μM, triangles. Data are representative of three independent experiments. a.u., arbitrary units.
FIG. 2.
Inner membrane permeabilization in A. baumannii strain Ac157 by CA(1-8)M(1-18) and PXB measured by the increase in SYTOX green fluorescence (λex = 485, λem = 520 nm). (A) CA(1-8)M(1-18). (B) PXB. Peptide concentrations: control, circles; 0.2 μM, downward triangles; 0.5 μM, squares; 1.0 μM, diamonds; 2.0 μM, upward triangles; 5.0 μM, hexagons. Arrows indicate addition of 0.5% Triton X-100. Data are representative of three independent experiments.
Unexpectedly, PXB at sublethal concentrations inhibited the oxygen consumption rate (measured in a Clark electrode) of Acinetobacter (108 CFU/ml in Hanks+Glc) (Table 2), as expected for an inner membrane permeabilization process. A similar inhibition of respiration of Enterobacteriaceae by immobilized PXB has been described, suggesting that a mechanism other than pure membrane permeabilization is involved (9).
TABLE 2.
Inhibition of oxygen consumption of A. baumannii at 4 × 108 CFU/ml by CA(1-8)M(1-18) and PXB
| Peptide (μM) | Oxygen consumption ratea (nmol of O2 108 CFU−1 min−1)
|
|
|---|---|---|
| CA(1-8)M(1-18) | PXB | |
| Control | 21.0 ± 1.2 (0.0) | 21.0 ± 1.2 (0.0) |
| 1.25 | 8.3 ± 0.7 (60.4) | 8.9 ± 0.6 (57.6) |
| 2.5 | 5.2 ± 0.5 (75.2) | 5.8 ± 0.6 (72.3) |
| 5.0 | 3.2 ± 0.1 (84.7) | 5.3 ± 0.4 (74.7) |
| 10.0 | 0.3 ± 0.1 (98.5) | 3.9 ± 0.2 (81.4) |
| 20.0 | <0.1 | 0.5 ± 0.1 (97.6) |
Data are expressed as the mean of triplicate samples ± standard deviation. The percentage of inhibition with respect to that of the controls is included in parentheses.
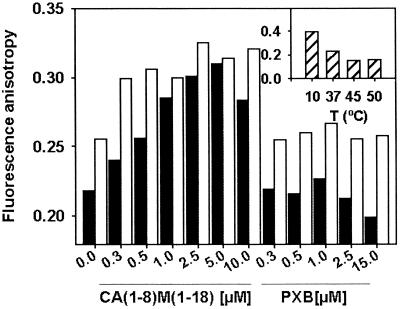
Only CA(1-8)M(1-18) produced a concentration-dependent increase in DPH (1,6-diphenyl-1,3,5-hexatriene) fluorescence anisotropy (0.7 μM; 107 CFU/ml in Hanks+Glc, 37°C), measured with a Fluorolog-3 spectrofluorometer (Longjumeau, France) (11) (Fig. 3). This parameter is inversely related to membrane fluidity, evidencing interaction of CA(1-8)M(1-18), but not PXB, with the hydrophobic core of the membrane, in agreement with the small increase in surface pressure of phospholipid monolayers after PXB penetration (20).
FIG. 3.
Effect of CA(1-8)M(1-18) and PXB on DPH anisotropy (λex =360, λem = 430 nm) on A. baumannii in the absence (solid bars) or presence (open bars) of 10 mM EDTA. Anisotropies at different temperatures (T) in the absence of peptide are included as internal controls in the inset. Data are representative of three independent experiments.
Thus, CA(1-8)M(1-18) constitutes a good alternative to PXB against Acinetobacter, because it is active on depolarized bacteria and lacks cytotoxicity on animal models (14). Furthermore, its microbicidal activity is faster than that of PXB. Although both peptides share self-promoted uptake, this does not imply parallel mechanisms of resistance. This agrees with such findings as the PXB resistance of certain E. coli and A. baumannii strains being overcome by cecropin B (15) and cecropin P1 (Urban et al., Letter), respectively, and supports a possible use of CA(1-8)M(1-18) and other membrane-active antibiotic peptides as alternatives to the predictable appearance of PXB resistance. On the downside, further research is required to unravel the systemic pharmacology of these peptides. This may in turn result in unexpected rewards. For instance, combination therapy based on synergy among CA(1-8)M(1-18) and antibiotic peptides with nonoverlapping targets, such as buforin II (7), is an unexplored possibility that deserves further attention.
Acknowledgments
This work was supported by grants from CAM (08/0029/1998) and FIS (99/0025) to M.L.B. and L.R., CAM Programa de Grupos Estratégicos to L.R., and CERBA, Generalitat de Catalunya to D.A. T.A. is recipient of a CAM postdoctoral fellowship.
† Present address: Department of Experimental and Health Sciences, Universitat Pompeu Fabra; E-08003 Barcelona, Spain.
REFERENCES
- 1.Alarcón, T., S. López-Hernández, D. Andreu, J. M. Saugar, L. Rivas, and M. López-Brea. 2001. In vitro activity of CA(1-8)M(1-18), a synthetic cecropin A-melittin hybrid peptide, against multiresistant Acinetobacter baumannii strains. Rev. Esp. Quimioter. 14:184-190. [PubMed] [Google Scholar]
- 2.Bergogne-Bérézin, E. 1996. Acinetobacter spp. as nosocomial pathogens: microbiological, clinical, and epidemiological features. Clin. Microbiol. Rev. 9:148-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boman, H. G., D. Wade, I. A. Boman, B. Wählin, and R. B. Merrifield. 1989. Antibacterial and antimalarial properties of peptides that are cecropin-melittin hybrids. FEBS Lett. 259:103-106. [DOI] [PubMed] [Google Scholar]
- 4.Daugelavičius, R., E. Bakienė, and D. H. Bamford. 2000. Stages of polymyxin B interaction with the Escherichia coli cell envelope. Antimicrob. Agents Chemother. 44:2969-2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evans, M. E., D. J. Feola, and R. P. Rapp. 1999. Polymyxin B sulfate and colistin: old antibiotics for emerging multiresistant gram-negative bacteria. Ann. Pharmacother. 33:960-967. [DOI] [PubMed] [Google Scholar]
- 6.Friedrich, C., M. G. Scott, N. Karunaratne, H. Yan, and R. E. Hancock. 1999. Salt-resistant alpha-helical cationic antimicrobial peptides. Antimicrob. Agents Chemother. 43:1542-1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giacometti, A., O. Cirioni, M. S. Del Prete, F. Barchiesi, A. M. Paggi, E. Petrelli, and G. Scalise. 2000. Comparative activities of polycationic peptides and clinically used antimicrobial agents against multidrug-resistant nosocomial isolates of Acinetobacter baumannii. J. Antimicrob. Chemother. 46:807-810. [DOI] [PubMed] [Google Scholar]
- 8.Gough, M., R. E. W. Hancock, and N. M. Kelly. 1996. Antiendotoxin activity of cationic peptide antimicrobial agents. Infect. Immun. 64:4922-4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.LaPorte, D. C., K. S. Rosenthal, and D. R. Storm. 1977. Inhibition of Escherichia coli growth and respiration by polymyxin B covalently attached to agarose beads. Biochemistry 16:1642-1648. [DOI] [PubMed] [Google Scholar]
- 10.Moore, R. A., N. C. Bates, and R. E. W. Hancock. 1986. Interaction of polycationic antibiotics with Pseudomonas aeruginosa lipopolysaccharide and lipid A studied by using dansyl-polymyxin. Antimicrob. Agents Chemother. 29:496-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Müller, S., S. Ullrich, A. Lösche, N. Loffhagen, and W. Babel. 2000. Flow cytometric techniques to characterise physiological states of Acinetobacter calcoaceticus. J. Microbiol. Methods 40:67-77. [DOI] [PubMed] [Google Scholar]
- 12.National Committee for Clinical Laboratory Standards. 2000. Approved standard M7-A5. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 5th ed. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 13.Piers, K. L., and R. E. Hancock. 1994. The interaction of a recombinant cecropin/melittin hybrid peptide with the outer membrane of Pseudomonas aeruginosa. Mol. Microbiol. 12:951-958. [DOI] [PubMed] [Google Scholar]
- 14.Piers, K. L., M. H. Brown, and R. E. W. Hancock. 1994. Improvement of outer membrane-permeabilizing lipopolysaccharide-binding activities of an antimicrobial cationic peptide by C-terminal modification. Antimicrob. Agents Chemother. 38:2311-2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vaara, M., and T. Vaara. 1994. Ability of cecropin B to penetrate the enterobacterial outer membrane. Antimicrob. Agents Chemother. 38:2498-2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Viljanen, P., P. Koski, and M. Vaara. 1988. Effect of small cationic leukocyte peptides (defensins) on the permeability barrier of the outer membrane. Infect. Immun. 56:2324-2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wade, D., A. Boman, B. Wahlin, C. M. Drain, D. Andreu, H. G. Boman, and R. B. Merrifield. 1990. All-D amino acid-containing channel-forming antibiotic peptides. Proc. Natl. Acad. Sci. USA 87:4761-4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Westphal, O., and K. Jann. 1965. Bacterial polysaccharides. Extraction with phenol-water and further application of the procedure, p. 83-91. In R. L. Whistler and J. N. Bemiller (ed.), Methods in carbohydrate chemistry, vol. 5. Academic Press, New York, N.Y. [Google Scholar]
- 19.Wu, M., E. Maier, R. Benz, and R. E. Hancock. 1999. Mechanism of interaction of different classes of cationic antimicrobial peptides with planar bilayers and with the cytoplasmic membrane of Escherichia coli. Biochemistry 38:7235-7242. [DOI] [PubMed] [Google Scholar]
- 20.Zhang, L., A. Rozek, and R. E. Hancock. 2001. Interaction of cationic antimicrobial peptides with model membranes. J. Biol. Chem. 276:35714-35722. [DOI] [PubMed] [Google Scholar]