Abstract
The in vivo activities of three bisphosphonates were determined against Leishmania donovani and Toxoplasma gondii. Alendronate was essentially inactive against both parasites. Pamidronate was active against L. donovani by intravenous administration. Risedronate had a 50% effective dosage of five 2.6-mg/kg of body weight intraperitoneal doses against L. donovani-infected mice but was less effective against T. gondii-infected mice.
Macrophages are the principal hosts or targets of the intracellular forms of the parasitic protozoa Leishmania donovani and Toxoplasma gondii, the causative agents of visceral leishmaniasis and toxoplasmosis, respectively. Macrophage-like cells, or osteoclasts, are also the targets of the bisphosphonate class of bone antiresorptive drugs, and in recent work we found that the bisphosphonates alendronate (ALE) (Fosamax; Merck), pamidronate (PAM) (Aredia; Novartis), and risedronate (RIS) (Actonel; Procter and Gamble) had considerable activity against both L. donovani and T. gondii in vitro (4). These protozoa are the cause of considerable mortality and morbidity worldwide, so the development of novel chemotherapeutic approaches to their control is of importance, and the use of bisphosphonates is of interest because they have already been developed for other clinical indications. The first in vivo results of the use of bisphosphonates against these two organisms are described here.
For L. donovani in vivo assays, the parasite strain used was MHOM/ET/67/L82. Female BALB/c mice (Tuck, United Kingdom), 20 g, were infected with 2 × 107 amastigotes via a lateral tail vein. Amastigotes were harvested from the spleen of a golden hamster (Charles Rivers Ltd., Margate, United Kingdom). Mice were randomly sorted into groups of five, and after 7 days the levels of infection were checked, all groups were weighed, and dosing commenced. In experiment 1, groups of mice received either RIS at 20 mg/kg of body weight/dose intraperitoneally (i.p.) or orally (p.o.), ALE at 20 mg/kg/dose i.p. or p.o., or PAM at 20 mg/kg/dose i.p. or p.o. or over a dose range of 10, 2, and 0.4 mg/kg/dose intravenously (i.v.). All doses were given consecutively on days 7 to 11 of infection. Another group of mice received sodium stibogluconate (Pentostam) at 15 or 45 mg of SbV/kg/dose subcutaneously (s.c.) for 5 days as a positive control. In experiment 2, infected mice received RIS at 15, 5, and 1.6 mg/kg/dose i.p. Positive and negative control groups were also included. In experiment 3, infected mice received RIS at 10, 3, and 1 mg/kg/dose i.p. or Pro-Phe-ALE or ALE at a dosage of five 10-mg/kg doses p.o. (10 mg/kg/dose p.o. × 5) to determine any oral activity of the former, a prodrug (3). Pro-Phe-ALE was the kind gift of Gershon Golomb, Hebrew University, Jerusalem, Israel. At 14 days postinfection, all groups were sacrificed and liver impression smears were prepared and counted as described elsewhere (2). For T. gondii in vivo assays, tissue cysts of strain C56 were obtained as previously described (1). Swiss Webster female mice (weight, 20 g at the beginning of each experiment) were infected orally with 10 cysts (1). The bisphosphonates were dissolved in phosphate-buffered saline (pH 6.8). Treatment was initiated at 3 days postinfection and drugs were administered once a day via i.p. injection for 10 days.
In the first set of experiments on the activities of the bisphosphonates in L. donovani, alendronate (ALE) proved inactive when administered by either the p.o. or the i.p. route, with toxic effects being observed following i.p. administration (Table 1, footnote a). With PAM, our results, together with i.v. results obtained with Trypanosoma cruzi-infected BALB/c mice (6), suggested that i.v. administration was more effective, since a 5-day treatment of 10-mg/kg doses i.v. gave a 91.8% suppression of liver amastigotes. When mice received doses of 2 mg/kg i.v. × 5 or less, the effect was greatly diminished. Using these doses, a 50% effective dose of ∼3.9 mg/kg was determined compared with a 50% effective dose of 15.3 mg/kg s.c. × 5 for the standard drug, sodium stibogluconate (Table 1). A first study with RIS indicated toxicity at 20 mg/kg, with two out of five mice dying after administrations of 20 mg/kg × 2 i.p. The remaining three mice received one further dose, and there was a 93.4% suppression of liver amastigotes in these animals (Table 1). Oral administration was ineffective at 20 mg/kg × 5, consistent with the typically low (∼0.5%) oral availability of bisphosphonates.
TABLE 1.
In vivo activity of nitrogen-containing bisphosphonates and a bisphosphonate prodrug against Leishmania donovani
| Drug | Dosing regimen | Inhibition (%) |
|---|---|---|
| Expt 1 | ||
| ALE | 20 mg/kg p.o. × 5 | 0 |
| 20 mg/kg i.p. × 4 | 14.7a | |
| PAM | 20 mg/kg p.o. × 3, 10 mg/kg i.p. × 2 | 13.5b |
| 20 mg/kg i.p. × 5 | 0 | |
| 10 mg/kg i.v. × 5 | 91.8 | |
| 2 mg/kg i.v. × 5 | 15.0 | |
| 0.4 mg/kg i.v. × 5 | 0 | |
| RIS | 20 mg/kg p.o. × 5 | 0 |
| 20 mg/kg i.p. × 3 | 93.4c | |
| Pentostam | 45 mg SbV/kg s.c. × 5 | 94.7 |
| 15 mg SbV/kg s.c. × 5 | 48.8 | |
| Expt 2 | ||
| RIS | 15 mg/kg i.p. × 3, 7.5 mg/kg i.p. × 2 | 99.1d |
| 5 mg/kg i.p. × 5 | 86.6 | |
| 1.6 mg/kg i.p. × 5 | 19.5 | |
| Pentostam | 15 mg SbV/kg s.c. × 5 | 51.73 |
| Expt 3 | ||
| Pro-Phe-ALE | 10 mg/kg p.o. × 5 | 9.2 |
| ALE | 10 mg/kg p.o. × 5 | 21.1 |
| RIS | 10 mg/kg i.p. × 2, 5 mg/kg i.p. × 3 | 99.6e |
| 3 mg/kg i.p. × 5 | 97.6 | |
| 1 mg/kg i.p. × 5 | 73.6 | |
| Pentostam | 15 mg SbV/kg s.c. × 5 | 17.1 |
One of five mice died after the final dose. The percentage of inhibition refers to the surviving mice.
Mice received 1/2 dose on 4th and 5th days of dosing due to apparent toxicity (excessive panting and horripilation).
Two of five mice died after two doses. The remaining three mice received three doses in total. The percentage of inhibition refers to the surviving mice.
This group received dosages of 15 mg/kg of body weight × 3 and then 7.5 mg/kg × 2, due to apparent toxicity.
Initially toxic to mice at 10 mg/kg; therefore, dose was halved for last 3 days.
In a second series of experiments, i.p. administration of RIS was again studied and an 86.6% decrease in parasite burden at 5 mg/kg i.p. × 5 was observed (Table 1). As toxicity (excessive panting, horripilation) was also apparent at higher doses, a split dose of 15 mg/kg × 3 followed by 7.5 mg/kg × 2 was then used, resulting in a 99.1% decrease in parasite load.
In a third study, oral delivery of ALE was compared with that of the ALE prodrug Pro-Phe-ALE (3), with small decreases in parasite burden being observed in both cases. A final study of RIS i.p. administered over a dose range extended the previous results and confirmed RIS as a lead compound for visceral leishmaniasis, since there was a 73.6% decrease in parasite burden at the lowest dosage of five 1-mg/kg i.p. doses, 97.6% at five 3-mg/kg i.p. doses, and a 99.6% decrease at 10 mg/kg × 2 plus 5 mg/kg × 3. In this study, some toxicity (horripilation, panting) was initially seen at the higher dose but no adverse reactions were seen at 3 mg/kg i.p. × 5. These results are of interest since they show that the nitrogen-containing bisphosphonate RIS, a known farnesylpyrophosphate synthase inhibitor (5), can cure infections caused by the parasitic protozoan L. donovani.
These results are consistent with in vitro parasite growth inhibition studies of L. donovani reported previously (4). Specifically, RIS has a 2.3 μM 50% inhibitory concentration (IC50) against L. donovani amastigotes, while ALE and PAM are much less effective (82.5 and >300 μM IC50s, respectively) (4). The toxicity effects of ALE, PAM, and RIS are also consistent with in vitro therapeutic index (T.I.) estimates, where the T.I. is defined as 50% lethal dose (LD50) (human nasopharynx carcinoma)/IC50 (L. donovani). LD50 refers to the concentration of drug (μM) causing a 50% reduction in viability in the human cell line (M. B. Martin, H. Kendrick, K. de Luca-Fradley, J. C. Lewis, J. S. Grimley, E. M. van Brussel, J. R. Olsen, S. L. Croft, and E. Oldfield, unpublished data), and IC50 represents the decrease in L. donovani growth in vitro (from reference 4). Specifically, ALE and PAM are the least effective drugs in the BALB/c mouse experiments (T.I. = 1.76 and < 0.52, respectively), while RIS is the most effective (T.I. = 108).
For Toxoplasma gondii, an initial series of experiments were conducted using ALE, PAM, or RIS doses ranging from 1 to 20 mg/kg i.p. × 10. All untreated control mice orally infected with tissue cysts died by day 14, with a mean survival time of 11.2 days. The administration of PAM and ALE offered no protection against death: all infected animals treated with either PAM or ALE died before the controls. However, RIS at 10 mg/kg i.p. × 10 provided a 40% survival rate (P < 0.01), with a mean survival time of 19.6 days. We therefore carried out three additional trials with RIS in order to verify the in vivo activity seen in the first trial. Figure 1 shows the cumulative results of all four in vivo RIS trials (n = a total of 40 treated mice) against T. gondii. All untreated control mice orally infected with tissue cysts died by day 16, with a mean survival time of 12.7 days (n = 20). RIS at 10 mg/kg i.p. × 10 (n = 40) provided a 35% survival rate (P < 6 × 10−7), with a mean survival time of 20.3 days, while RIS at 20 mg/kg i.p. × 10 (n = 40) provided a 55% survival rate (P < 4 × 10−4) with a mean survival time of 22.1 days.
FIG. 1.
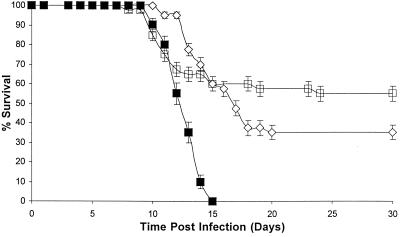
Cumulative survival results for T. gondii-infected mice after RIS administration in vivo for two different RIS concentrations. ▪, diluent (n = 20); ◊, 10 mg/kg (n = 40); □, 20 mg/kg (n = 40). Kaplan-Meier statistics were used to deduce standard error bars.
In these studies, the significant in vitro activities of PAM and ALE reported previously (4) did not translate into curative in vivo activities. The in vitro IC50 values for PAM and ALE are 35.6 μM and 25 μM, respectively (4). The LD50s of these compounds are both ∼150 μM in the mammalian cell model used, which translates into rather low therapeutic indices (5.8 for ALE and 4.4 for PAM), and in this murine model of toxoplasmosis, i.p. administration of either drug provided no protection against infection. However, in the case of RIS (IC50 = 0.49 μM), there was a significant increase in mouse survival following T. gondii infection if RIS was administered. This is consistent with the high therapeutic index (510) seen with RIS in the in vitro experiments.
These results indicate that the potent nitrogen-containing bisphosphonate drug RIS is a promising lead compound for development as a novel antiparasitic agent. RIS has a relatively broad spectrum of antiparasitic activity in in vitro testing (4) and has also been found to be the most potent inhibitor of L. donovani and T. gondii growth in vitro, in addition to having a relatively low overall cytotoxicity. The drug has a high therapeutic index and can give ≥99% decreases in parasite burden in L. donovani-infected mice. In cutaneous leishmaniases, other bisphosphonates may also be effective; for example, in a Leishmania mexicana amazonensis BALB/c model of infection, PAM (10 mg/kg i.p. × 5) effects a parasitological cure (N. Rodriguez, B. N. Bailey, M. B. Martin, E. Oldfield, J. A. Urbina, and R. Docampo, unpublished data), confirming that the nitrogen-containing bisphosphonates are a class of compounds worthy of further investigation as antiparasitic agents.
Acknowledgments
This work was supported in part by the United States Public Health Service (National Institutes of Health Grant GM-50694 to E.O. and AI04717 and N01-AI-35174 to F.G.A.), by the UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR) (E.O., V.Y., S.L.C., R.D.), and by the American Heart Association, Midwest Affiliate (E.O.) M.B.M. is an American Heart Association Predoctoral Fellow, Midwest Affiliate. S.N.J.M. is a Burroughs Welcome New Investigator in Molecular Parasitology.
We also thank Gershon Golomb for providing the Pro-Phe-ALE.
REFERENCES
- 1.Araujo, F. G., A. A. Khan, T. L. Slifer, A. Bryskier, and J. S. Remington. 1997. The ketolide antibiotics HMR 3647 and HMR 3004 are active against Toxoplasma gondii in vitro and in murine models of infection. Antimicrob. Agents Chemother. 41:2137-2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Croft, S. L., D. Snowdon, and V. Yardley. 1996. The activities of four anticancer alkyllysophospholipids against Leishmania donovani, Trypanosoma cruzi and Trypanosoma brucei. J. Antimicrob. Chemother. 38:1041-1047. [DOI] [PubMed] [Google Scholar]
- 3.Ezra, A., A. Hoffman, E. Breuer, I. S. Alferiev, J. Monkkonen, N. El Hanany-Rozen, G. Weiss, D. Stepenksy, I. Gati, H. Cohen, S. Tormalehto, G. L. Amidon, and G. Golomb. 2000. A peptide prodrug approach for improving bisphosphonate oral absorption. J. Med. Chem. 43:3641-3652. [DOI] [PubMed] [Google Scholar]
- 4.Martin, M. B., J. S. Grimley, J. C. Lewis, H. T. Heath III, B. N. Bailey, H. Kendrick, V. Yardley, A. Caldera, R. Lira, J. A. Urbina, S. N. J. Moreno, R. Docampo, S. L. Croft, and E. Oldfield. 2001. Bisphosphonates inhibit the growth of Trypanosoma brucei, Trypanosoma cruzi, Leishmania donovani, Toxoplasma gondii and Plasmodium falciparum: a potential route to chemotherapy. J. Med. Chem. 44:909-916. [DOI] [PubMed] [Google Scholar]
- 5.Montalvetti, A., B. N. Bailey, M. B. Martin, G. W. Severin, E. Oldfield, and R. Docampo. 2001. Bisphosphonates are potent inhibitors of Trypanosoma cruzi farnesyl pyrophosphate synthase. J. Biol. Chem. 276:33930-33937. [DOI] [PubMed] [Google Scholar]
- 6.Urbina, J. A., B. Moreno, S. Vierkotter, E. Oldfield, G. Payares, C. Sanoja, B. N. Bailey, W. Yan, D. A. Scott, S. N. Moreno, and R. Docampo. 1999. Trypanosoma cruzi contains major pyrophosphate stores, and its growth in vitro and in vivo is blocked by pyrophosphate analogs. J. Biol. Chem. 274:33609-33615. [DOI] [PubMed] [Google Scholar]


