Abstract
Ravuconazole (RCZ) was evaluated for efficacy in comparison to fluconazole (FCZ) and itraconazole (ITZ) in murine models of disseminated histoplasmosis. All regimens tested prolonged survival (P < 0.05 to 0.0001). At equivalent doses of 50 mg/kg of body weight, RCZ and ITZ were equally effective and RCZ was more effective than FCZ (P = 0.02). Clearance of fungal burden from the livers and spleens of mice showed RCZ and ITZ at doses of 50 mg/kg to be efficacious but not curative. These data indicate that RCZ should be studied further.
Disseminated histoplasmosis occurs in both healthy and immunocompromised patients. Therapy is necessary for these patients, with amphotericin B and itraconazole (ITZ) treatments being used most often. However, these are often noncurative therapies, and relapse and recrudescence can occur while patients are on therapy (2, 9, 15, 18, 24, 25). Improved therapeutic options are still necessary. As a result, over the last several years a number of experimental antifungal compounds have been tested in experimental models for their activities against Histoplasma capsulatum (4, 5, 10-12).
Recently, a new thiazole-containing triazole, ravuconazole (RCZ) (BMS 207147; Bristol-Myers Squibb, Princeton, N.J.), has been shown to be active both in vitro and in vivo against a variety of fungal pathogens (1, 6-8, 13, 14, 19, 20, 23, 26). This activity has been demonstrated in vivo against Candida albicans, Cryptococcus neoformans, and Aspergillus fumigatus (13, 14). To further examine the spectrum of activity of RCZ against endemic mycoses, we compared the efficacies of RCZ, fluconazole (FCZ), and ITZ for the treatment of experimental systemic histoplasmosis in mice. Our results indicate that the efficacy of RCZ is comparable or superior to those of FCZ and ITZ against histoplasmosis.
The in vitro activity of RCZ against H. capsulatum G217B was tested by NCCLS methods as described previously (22). An experimental model of systemic histoplasmosis was established as described previously (4, 5). Two experiments were done using 6-week-old female CD-1 mice (average weight, 22.8 or 24.4 g; Charles River Laboratories, Portage, Mich.), which were provided food and water ad libitum. In brief, the mice were infected intravenously with 2.18 × 106 (for the survival study) or 1 × 106 (for the CFU study) viable yeasts of H. capsulatum G217B. Therapy began 4 days after infection. In the survival study, groups of 10 mice were given diluent (10% dimethyl sulfoxide-5% Tween 80-saline), 1, 10, or 50 mg of RCZ in this diluent per kg of body weight, 10 or 50 mg of FCZ (Pfizer, Groton, Conn.) per kg, or 50 mg of ITZ (Janssen Pharmaceuticals, Beerse, Belgium) per kg. FCZ was suspended in 0.3% Noble agar, and ITZ was prepared in hydroxypropyl-β-cyclodextrin as described previously (16, 17); these diluents have been demonstrated in previous studies to have efficacies equivalent to that of no treatment (data not shown). All treatments were given in 0.1-ml doses by gavage once daily on days 4 through 19 of infection. In the CFU study, mice were given no treatment or 50 mg of RCZ or ITZ per kg beginning 4 days after infection for 12 consecutive days.
Numbers of deaths were tallied through 42 days (for the survival study) or 20 days (for the CFU study) of infection. All surviving mice were euthanized by CO2 asphyxiation, and their spleens and livers were removed aseptically. The number of CFU in each organ was determined by quantitative plating of organ homogenates as described previously and expressed as the log10 number of CFU per entire organ (4, 5). The lower limit of detection for determining the number of CFU in an entire organ is approximately 5 colonies.
Statistical analyses comparing the survival rates among the various treatment groups were done using a log rank test contained in Prism (version 3.02 for Windows; GraphPad Software, San Diego, Calif.). Comparison of residual burdens of H. capsulatum in the livers and spleens of surviving animals was done using the Mann-Whitney U test as previously described (4, 5, 21).
RCZ proved to have good in vitro activity against H. capsulatum, with an MIC of 0.024 μg/ml and a minimum fungicidal concentration (MFC) of 0.097 μg/ml. In comparison, FCZ had an MIC of 3.1 μg/ml and an MFC of 6.3 μg/ml (5) and ITZ had an MIC of 0.10 μg/ml and an MFC of 1.56 μg/ml for this strain of H. capsulatum. Thus, in terms of MICs, RCZ was about 4 times more active than ITZ and 129 times more active than FCZ; a comparison of MFCs showed RCZ to be 16 times and 65 times more active than ITZ and FCZ, respectively. However, H. capsulatum is considered susceptible to all three drugs and all three MFCs are below concentrations in serum achievable with the corresponding drug (3, 14, 16, 17).
The results of the long-term survival study indicate that the model of systemic histoplasmosis established with CD-1 mice was extremely acute and lethal (Fig. 1). All untreated and diluent-treated mice succumbed to infection within 7 days. All drug regimens proved efficacious in the prolongation of survival in comparison with either control regimen (P = 0.02 to 0.0001, depending on the comparison). Groups treated with 50 mg of RCZ or ITZ per kg had a 70 or 60% survival rate, respectively, versus a 20% survival rate for those treated with 50 mg of FCZ per kg (Fig. 1). Other regimens resulted in a survival rate of 30% or less. Comparisons showed that the efficacies of RCZ and ITZ at 50 mg/kg were equal. Both were superior to RCZ or FCZ at 10 mg/kg (P = 0.03 to 0.007, depending on the comparison); RCZ at 50 mg/kg was superior to FCZ at 50 mg/kg (P = 0.03), and RCZ at 1 or 10 mg/kg was equivalent to FCZ at 50 mg/kg. However, neither RCZ nor ITZ given at 50 mg/kg was significantly more effective than RCZ at 1 mg/kg.
FIG. 1.
Cumulative mortality rates of mice infected intravenously with H. capsulatum and left untreated or treated with RCZ, FCZ, ITZ, or RCZ-diluent (survival study).
Recovery of H. capsulatum from the spleens and livers of surviving mice in the survival study indicated that none of the mice given 50 mg of RCZ per kg were free of infection, whereas two of the six surviving mice given 50 mg of ITZ per kg were free of infection in both organs; all other mice had infections in both organs (data not shown). However, the results of these treatments were equivalent statistically.
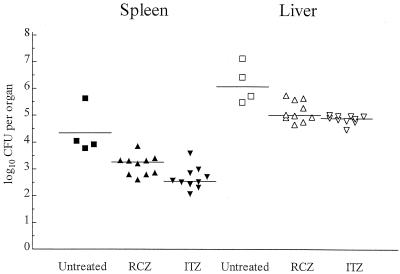
In the second experiment, the CFU study, a smaller infecting inoculum was used to reduce the overall mortality rate of the mice in the model in order to compare the efficacies of ITZ and RCZ in the clearance of infectious burden. No RCZ- or ITZ-treated mice died, whereas 7 of 11 control mice died between days 7 and 12. Statistical comparison showed that both RCZ and ITZ significantly prolonged survival (P = 0.003). Figure 2 shows the numbers of CFU recovered from the surviving animals. The mean burdens of H. capsulatum in the spleens and livers of untreated mice were significantly higher than they were after treatment with either RCZ or ITZ (P < 0.001). Comparison of the burdens of RCZ- and ITZ-treated mice showed that both treatments were equally effective in controlling infection in the livers (P > 0.05). In the spleens, ITZ-treated mice had a lower mean burden than RCZ-treated mice (P = 0.012). Neither treatment cured any animals of infection in either organ assayed.
FIG. 2.
Scattergram plot (CFU study) of individual CFU burdens on day 20 postinfection in the spleens and livers of surviving mice that were not treated or treated with RCZ or ITZ at 50 mg/kg. Horizontal lines represent mean numbers of CFU, closed symbols indicate numbers of CFU from the spleens, and open symbols indicate numbers of CFU from the livers. Only 4 of 11 untreated mice survived.
Both the survival study and the CFU study indicate that RCZ has efficacy in treating experimental systemic murine histoplasmosis. This efficacy was only partially dose responsive in the prolongation of survival in that RCZ at 50 mg/kg was significantly superior to RCZ at 10 mg/kg but not to RCZ at 1 mg/kg. In comparison with FCZ, RCZ was superior (a 50-mg dose of RCZ per kg was more effective than an equal dose of FCZ), perhaps as much as 50 times more potent in a milligram-per-kilogram comparison (1 mg of RCZ per kg was equivalent to 50 mg of FCZ per kg). The results of both studies indicate that RCZ and ITZ could be considered equal in efficacy at the 50-mg/kg doses. However, the CFU study showed that neither RCZ nor ITZ at 50 mg/kg was curative.
It is possible that a twice-daily dosing regimen would improve the efficacy of RCZ in this model. RCZ and FCZ have been reported to have half-lives (t1/2) of about 4 h in mice (3, 14), whereas ITZ has been reported to have a t1/2 of less than 2 h in mice (14). However, ITZ given in cyclodextrin appears to have a prolonged t1/2 (16) which would be at least equivalent to those of RCZ and FCZ. Peak levels of RCZ after the administration of a single dose occurred after 6 h and remained higher than the MICs reported here for H. capsulatum for at least 12 h (14). Both FCZ and ITZ have improved efficacies against histoplasmosis when they are given on a twice-daily schedule (4). Thus, additional studies are necessary to answer the questions of the comparative efficacies of those drugs when dosing is more frequent. The diluent formulation of RCZ used in our studies might not result in optimal bioavailability. It has been reported that use of a diluent of dimethyl sulfoxide-carboxymethyl cellulose results in a rate of RCZ absorption similar to that of ITZ in mice (14). Formulation of ITZ in cyclodextrin has been shown to dramatically improve the drug's pharmacokinetics and bioavailability, resulting in an improved therapeutic outcome (16, 17). Thus, testing different formulations to improve the bioavailability of RCZ should be considered. Overall, our studies clearly indicate that RCZ has efficacy against this infection and should be studied further for its activity against systemic histoplasmosis.
Acknowledgments
These studies were funded in part by a grant from Bristol-Myers Squibb.
REFERENCES
- 1.Bartroli, J., E. Turmo, M. Algueró, E. Boncompte, M. L. Vericat, L. Conte, J. Ramis, M. Merlos, J. García-Rafanell, and J. Forn. 1998. New azole antifungals. 3. Synthesis and antifungal activity of 3-substituted-4(3H)-quinazolinones. J. Med. Chem. 41:1869-1882. [DOI] [PubMed] [Google Scholar]
- 2.Bradsher, R. 1996. Histoplasmosis and blastomycosis. Clin. Infect. Dis. 22(Suppl. 2):S102-S111. [DOI] [PubMed] [Google Scholar]
- 3.Clemons, K. V., L. H. Hanson, A. M. Perlman, and D. A. Stevens. 1990. Efficacy of SCH39304 and fluconazole in a murine model of disseminated coccidioidomycosis. Antimicrob. Agents Chemother. 34:928-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clemons, K. V., M. Martinez, M. E. Homola, and D. A. Stevens. 1996. Therapy of systemic histoplasmosis in immunosuppressed mice with the triazole D0870. J. Med. Vet. Mycol. 34:241-246. [DOI] [PubMed] [Google Scholar]
- 5.Clemons, K. V., and D. A. Stevens. 1995. Efficacy of the triazole D0870 in a murine model of systemic histoplasmosis. Antimicrob. Agents Chemother. 39:778-780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diekema, D. J., M. A. Pfaller, S. A. Messer, A. Houston, R. J. Hollis, G. V. Doern, R. N. Jones, and the SENTRY Participants Group. 1999. In vitro activities of BMS-207147 against over 600 contemporary clinical bloodstream isolates of Candida species from the SENTRY Antimicrobial Surveillance Program in North America and Latin America. Antimicrob. Agents Chemother. 43:2236-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fung-Tomc, J. C., E. Huczko, B. Minassian, and D. P. Bonner. 1998. In vitro activity of a new oral triazole, BMS-207147 (ER-30346). Antimicrob. Agents Chemother. 42:313-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Georgopapadakou, N. H. 1999. BMS-207147. Curr. Opin. Anti-Infect. Investig. Drugs 1:373-376. [Google Scholar]
- 9.Graybill, J. R. 1988. Histoplasmosis and AIDS. J. Infect. Dis. 158:623-626. [DOI] [PubMed] [Google Scholar]
- 10.Graybill, J. R., L. Najvar, A. Fothergill, R. Bocanegra, and F. G. de las Heras. 1999. Activities of sordarins in murine histoplasmosis. Antimicrob. Agents Chemother. 43:1716-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Graybill, J. R., L. K. Najvar, R. Bocanegra, R. F. Hector, and M. F. Luther. 1998. Efficacy of nikkomycin Z in the treatment of murine histoplasmosis. Antimicrob. Agents Chemother. 42:2371-2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graybill, J. R., L. K. Najvar, E. M. Montalbo, F. J. Barchiesi, M. F. Luther, and M. G. Rinaldi. 1998. Treatment of histoplasmosis with MK-991 (L-743,872). Antimicrob. Agents Chemother. 42:151-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hata, K., J. Kimura, H. Miki, T. Toyosawa, M. Moriyama, and K. Katsu. 1996. Efficacy of ER-30346, a novel oral triazole antifungal agent, in experimental models of aspergillosis, candidiasis, and cryptococcosis. Antimicrob. Agents Chemother. 40:2243-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hata, K., J. Kimura, H. Miki, T. Toyosawa, T. Nakamura, and K. Katsu. 1996. In vitro and in vivo antifungal activities of ER-30346, a novel oral triazole with a broad antifungal spectrum. Antimicrob. Agents Chemother. 40:2237-2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hecht, F. M., J. Wheat, A. H. Korzun, R. Hafner, K. J. Skahan, R. Larsen, M. T. Limjoco, M. Simpson, D. Schneider, M. C. Keefer, R. Clark, K. K. Lai, J. M. Jacobson, K. Squires, J. A. Bartlett, and W. Powderly. 1997. Itraconazole maintenance treatment for histoplasmosis in AIDS: a prospective, multicenter trial. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 16:100-107. [DOI] [PubMed] [Google Scholar]
- 16.Hostetler, J. S., L. H. Hanson, and D. A. Stevens. 1992. Effect of cyclodextrin on the pharmacology of antifungal oral azoles. Antimicrob. Agents Chemother. 36:477-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hostetler, J. S., L. H. Hanson, and D. A. Stevens. 1993. Effect of hydroxypropyl-beta-cyclodextrin on efficacy of oral itraconazole in disseminated murine cryptococcosis. J. Antimicrob. Chemother. 32:459-463. [DOI] [PubMed] [Google Scholar]
- 18.Johnson, P. C., and G. A. Sarosi. 1994. Progressive disseminated histoplasmosis in patients with AIDS. HIV Adv. Res. Ther. 4:15-21. [Google Scholar]
- 19.Pfaller, M. A., S. A. Messer, S. Gee, S. Joly, C. Pujol, D. J. Sullivan, D. C. Coleman, and D. R. Soll. 1999. In vitro susceptibilities of Candida dubliniensis isolates tested against the new triazole and echinocandin antifungal agents. J. Clin. Microbiol. 37:870-872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pfaller, M. A., S. A. Messer, R. J. Hollis, R. N. Jones, G. V. Doern, M. E. Brandt, and R. A. Hajjeh. 1998. In vitro susceptibilities of Candida bloodstream isolates to the new triazole antifungal agents BMS-207147, Sch 56592, and voriconazole. Antimicrob. Agents Chemother. 42:3242-3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sokal, R. R., and F. J. Rohlf. 1981. Biometry, 2nd ed., p. 432-435. W. H. Freeman & Co., San Francisco, Calif.
- 22.Stevens, D. A., and B. H. Aristizabal. 1997. In vitro antifungal activity of novel azole derivatives with a morpholine ring, UR-9746 and UR-9751, and comparison with fluconazole. Diagn. Microbiol. Infect. Dis. 29:103-106. [DOI] [PubMed] [Google Scholar]
- 23.Tsuruoka, A., Y. Kaku, H. Kakinuma, I. Tsukada, M. Yanagisawa, K. Nara, and T. Naito. 1998. Synthesis and antifungal activity of novel thiazole-containing triazole antifungals. II. Optically active ER-30346 and its derivatives. Chem. Pharm. Bull. 46:623-630. [DOI] [PubMed] [Google Scholar]
- 24.Wheat, J. 1995. Endemic mycoses in AIDS: a clinical review. Clin. Microbiol. Rev. 8:146-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wheat, J., G. Sarosi, D. McKinsey, R. Hamill, R. Bradsher, P. Johnson, J. Loyd, and C. Kauffman. 2000. Practice guidelines for the management of patients with histoplasmosis. Infectious Diseases Society of America. Clin. Infect. Dis. 30:688-695. [DOI] [PubMed] [Google Scholar]
- 26.Yamazumi, T., M. A. Pfaller, S. A. Messer, A. Houston, R. J. Hollis, and R. N. Jones. 2000. In vitro activities of ravuconazole (BMS-207147) against 541 clinical isolates of Cryptococcus neoformans. Antimicrob. Agents Chemother. 44:2883-2886. [DOI] [PMC free article] [PubMed] [Google Scholar]