Abstract
Escherichia coli O157:H7 cells survived for up to 77, >226, and 231 days in manure-amended autoclaved soil held at 5, 15, and 21°C, respectively. Pathogen populations declined more rapidly in manure-amended unautoclaved soil under the same conditions, likely due to antagonistic interactions with indigenous soil microorganisms. E. coli O157:H7 cells were inactivated more rapidly in both autoclaved and unautoclaved soils amended with manure at a ratio of 1 part manure to 10 parts soil at 15 and 21°C than in soil samples containing dilute amounts of manure. The manure-to-soil ratio, soil temperature, and indigenous microorganisms of the soil appear to be contributory factors to the pathogen's survival in manure-amended soil.
Animal waste in the form of raw manure or composted manure is routinely applied to the land as a crop fertilizer and/or soil amendment. Compost also is used as a growth medium in home gardening, ornamental nurseries, and greenhouses (9). A potential risk arising from the disposal of animal waste of fecal origin is the spread of enteric pathogens (15). Animals from which food is derived are recognized as reservoirs of many significant food-borne pathogens, including Escherichia coli O157:H7, Salmonella spp., and Campylobacter spp. (6, 7). Many outbreaks or cases of E. coli O157:H7 infection have been associated with water or food directly or indirectly contaminated with animal manure (1-4). For example, an outbreak of E. coli O157:H7 infection among members of four families was associated with food fertilized with cattle manure on the farm (3). In another instance, a woman acquired E. coli O157:H7 infection associated with eating inadequately washed vegetables that were obtained from a garden fertilized with bovine manure (4).
Previous studies on the fate of E. coli O157:H7 in bovine feces revealed that the pathogen survived for 42 to 49 days at 37°C, for 49 to 56 days at 22°C, and for 63 to 70 days at 5°C (17). Another study of E. coli O157:H7 in manure heaps revealed that the pathogen could survive for up to 47 days, 4 months, and 21 months in bovine, aerated ovine, and nonaerated ovine manure, respectively (12). Himathongkham et al. (11), based on their research findings, recommended that cow manure should be held for 105 days at 4°C or 45 days at 37°C to achieve a 5-log10 reduction of both E. coli O157:H7 and Salmonella enterica serovar Typhimurium. These results indicate that E. coli O157:H7 can persist in bovine feces for an extended period of time and that bovine feces are a potential vehicle for transmitting the pathogen to cattle, food, and the environment.
Cross-contamination of produce from manure or improperly composted manure used as a soil amendment may be a source of pathogen contamination during preharvest. Although competition with soil microorganisms and adverse environmental conditions can influence pathogen survival, there is little information regarding the degree to which E. coli O157:H7 cells can survive in manure-amended soils. In this study, our objective was to determine the fate of E. coli O157:H7 cells in cow manure applied to soil at different manure application rates and held at different temperatures.
A five-strain mixture of E. coli O157:H7 cells (strains E0143 [meat isolate], C7927 [human isolate], K262 [human isolate], C0083 [cattle feces isolate], and E0139 [beef jerky isolate]) was used as the inoculum. In order to facilitate enumeration of these isolates, all five strains of E. coli O157:H7 were labeled with jellyfish green fluorescent protein (GFP) according to the protocol described by Delazari et al. (5), with slight modification. The competent bacterial cells were electroporated in a Gene Pulser II (Bio-Rad) with plasmid vector pGFPuv (ClonTech, Palo Alto, Calif.), and an electrical pulse (τ ∼ 1.7 msec) was applied at 2.5 kV and 25 μF with the pulse controller adjusted to 200 Ω. Transformants were selected from isolated colonies grown on Luria-Bertani (LB) plates containing 100 μg of ampicillin/ml. The resulting ampicillin-resistant transformants emitted bright green fluorescence under a handheld UV light.
Each strain of GFP-labeled E. coli O157:H7 was inoculated into 10 ml of Tryptic soy broth (TSB; Becton Dickinson, Sparks, Md.) containing 100 μg ampicillin/ml (TSB-A) and was incubated at 37°C for 16 to 18 h with agitation (150 rpm). A 0.5-ml suspension of each isolate was transferred to 100 ml of TSB containing ampicillin (100 μg/ml) and incubated for 16 to 18 h with agitation (150 rpm). The bacteria were harvested by centrifugation (4,000 × g for 20 min) and were washed three times in 0.1% peptone water. Each strain was adjusted with 0.1% peptone water to an optical density (OD) at 630 nm of 0.7 (ca. 109 CFU/ml) and combined in equal volume to obtain a five-strain mixture prior to the inoculation of the manure. Cell populations of E. coli O157:H7 in the five-strain mixture were determined by plating on Tryptic soy agar (TSA; Becton Dickinson) plates containing 100 μg of ampicillin/ml (TSA-A).
Soil (Tifton sandy loam soil) samples were collected from Tifton, Ga. A five-strain mixture of GFP-labeled E. coli O157:H7 cells were inoculated into cow manure by spraying 10 ml of the culture mixture with a spray bottle (Sprayco, Detroit, Mich.; sanitized by soaking in 70% ethanol) on the surface of 1000 g of manure and then mixing for 5 min with a 40-ml sterile spoon to homogeneously distribute the E. coli O157:H7. The inoculated manure was held at 23°C in sterile polypropylene trays (26 by 16 by 6.4 cm) supported by test tube racks in a plastic box (45 by 42 by 30 cm) with ca. 2.5 cm of tap water on the bottom to retain moisture in the manure. After 20 to 24 h of incubation, the inoculated manure was mixed with unautoclaved or autoclaved (121°C for 20 min, on three successive days) soil at a ratio of 1 part of manure to 10, 25, 50, or 100 (wt/wt) parts of soil, i.e., 1:10, 1:25, 1:50, or 1:100, respectively. The manure-amended soil was then held inside a polyethylene plastic box with lid (59 by 42 by 30 cm) at 21, 15, and 5°C.
Duplicate soil samples (ca. 25 g each) were obtained from each treatment tray at each sampling interval for determination of E. coli O157:H7 counts, pH, and moisture content. Each sample (5 g) was added to 45 ml of 0.1% peptone water in a sterile Whirl-Pak bag and macerated in a Seward Stomacher for 1 min at medium speed. Dilutions were prepared using 0.1% peptone water, and 0.1-ml portions of each dilution were spread onto TSA-A plates which were incubated at 37°C for 48 h for the determination of E. coli O157:H7 counts. The GFP-labeled E. coli O157:H7 colonies were counted under an UV light at 365 nm.
When E. coli O157:H7 could not be detected by the direct plating method (detection limit of 50 CFU/g), a selective enrichment culture method was used. A 1-g manure-amended soil sample was added to 99 ml of selective enrichment broth, i.e., TSB-A and incubated at 37°C for 24 h with agitation (150 rpm). Dilutions of cultures were surface plated on TSA-A plates. Randomly selected colonies that were green and fluoresced under UV light were confirmed as E. coli O157 by an E. coli O157 latex agglutination test (Oxoid Limited, Hampshire, England). The pH of manure-amended soil was determined by adding 5 g of soil to 250 ml of distilled water. The suspension was stirred for 5 min and then allowed to settle for 5 min. The pH of the liquid was determined with an Accumet Basic pH meter (Fisher Scientific). Moisture content of manure-amended soil was determined by drying 5 g of soil at 105°C for 24 h in a Precision oven (Precision Scientific) and then weighing the residual.
Microbiological data were converted to log CFU/gram for statistical analysis. An analysis of variance (ANOVA) for a completely randomized design with repeated measures across dates was conducted to determine if general differences existed among the four treatment means. Specific comparisons between any pair of treatment means at any date were accomplished with the Fisher's least-significant difference (LSD) test. All calculations were performed using the general linear model (GLM) procedure of the Statistical Analysis System (SAS 2001, Cary, N.C.). The analysis was repeated for each of the six temperature and soil conditions.
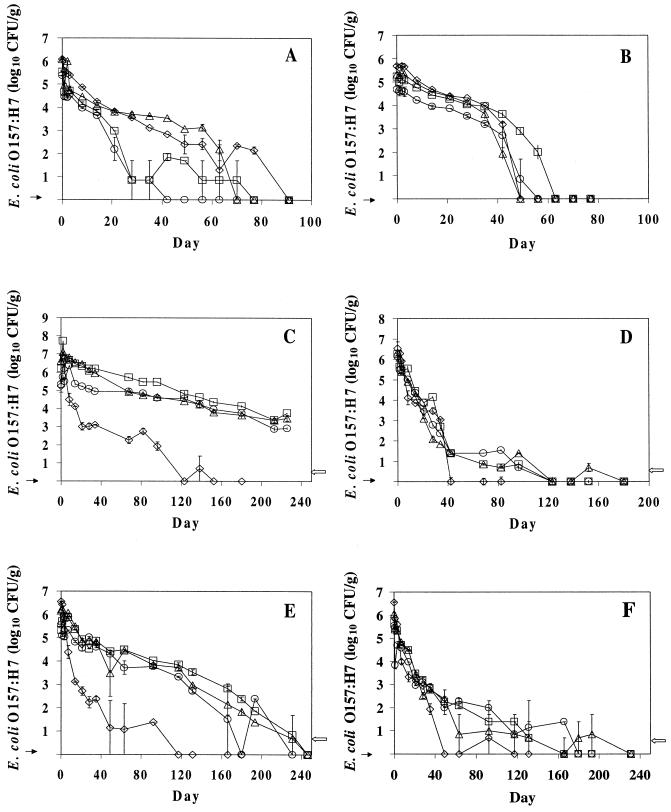
E. coli O157:H7 populations in manure-amended autoclaved soil at 5°C for 3 days decreased by 0.5 to 1.0 log10 CFU/g compared to no change in manure-amended unautoclaved soil (Fig. 1A and B). During the following 3 weeks, E. coli O157:H7 populations in all samples decreased at approximately the same rate. However, during the initial 50 days, E. coli O157:H7 populations declined more rapidly in manure-amended autoclaved soil formulated at 1:50 and 1:100 than in samples formulated at 1:25 and 1:10. The pathogen survived for up to 77 days at 5°C in 1:10 samples. E. coli O157:H7 in manure-amended unautoclaved soil was inactivated during the initial 50 days more rapidly in combinations of 1:10 and 1:25 samples than in 1:50 and 1:100 samples. The pathogen was not detected by the 63rd day in manure-amended unautoclaved soil samples. At 15°C, E. coli O157:H7 populations within 7 days increased by ca. 0.5 to 2 log10 CFU/g in manure-amended autoclaved soil, whereas populations declined by 3 log10 CFU/g in manure-amended unautoclaved soil (Fig. 1C and D). During the following weeks, E. coli O157:H7 populations in manure-amended unautoclaved soil declined considerably more rapidly than in manure-amended autoclaved soil. In unautoclaved soil samples, E. coli O157:H7 populations decreased by 5 log10 CFU/g within 42 days in samples of all four combinations and was no longer detected thereafter in the 1:10 samples. However, the pathogen was detected only by enrichment culture in samples formulated at 1:25, 1:50, and 1:100 for more than 109 days. E. coli O157:H7 survived in 1:25, 1:50, and 1:100 manure-amended autoclaved soil samples at 15°C for greater than 226 days, at which time there was a 3- to 4- log10 CFU/g reduction in cell populations. E. coli O157:H7 in 1:10 samples of manure-amended autoclaved soil decreased to undetectable populations (>6 log10 CFU/g reduction) at 152 days at 15°C. Similar survival patterns for E. coli O157:H7 were observed for the 1:25, 1:50, and 1:100 formulations of manure-amended autoclaved soil samples held at 21°C, with only a ca. 1.5 to 2 log10 CFU/g reduction during 63 days of storage (Fig. 1E). The pathogen died off more rapidly in the 1:10 formulation, with a ca. 5 log10 CFU/g reduction within 40 days (Fig. 1E). The pathogen died off more rapidly in manure-amended unautoclaved soil than in the autoclaved soil at 21°C (Fig. 1F). The pathogen was no longer detectable in 1:10, 1:25, 1:50, and 1:100 formulated samples at 117, 231, 180, and 145 days, respectively. Overall, E. coli O157:H7 survived longer in manure-amended unautoclaved soil held at 21°C than at 15°C. E. coli O157:H7 at 15 or 21°C, depending on the manure-to-soil ratio, survived for more than 226 days in manure-amended autoclaved soil (Table 1), whereas in manure-amended unautoclaved soil, the pathogen decreased to undetectable populations within 165 days at 15°C and within 231 days at 21°C (Fig. 1D and E).
FIG. 1.
Survival of E. coli O157:H7 at 5°C in manure-amended autoclaved (A) and unautoclaved (B) soil, at 15°C in manure-amended autoclaved (C) and unautoclaved (D) soil, and at 21°C in manure-amended autoclaved (E) and unautoclaved (F) soil. E. coli O157: H7 was enumerated on TSA-A plates from manure-to-soil formulations of 1:10 (◊), 1:25 (▵), 1:50 (□), and 1:100 (○). →, not detected by enrichment culture; ←, detection by enrichment culture only. Each point represents the average of duplicate trials, and the bars at each point indicate the standard deviation of results obtained at each sampling time.
TABLE 1.
Survival of E. coli O157:H7 in manure-amended autoclaved or unautoclaved soil held at different temperatures
| Samples | Days of survival at storage temp (°C) of:
|
||
|---|---|---|---|
| 5 | 15 | 21 | |
| Manure:autoclaved soil | |||
| 1:10 | 77a | 138 | 103 |
| 1:25 | 63 | >226 | 231 |
| 1:50 | 70 | >226 | 231 |
| 1:100 | 35 | >226 | 193 |
| Manure:unautoclaved soil | |||
| 1:10 | 42 | 34 | 103 |
| 1:25 | 42 | 152 | 193 |
| 1:50 | 56 | 109 | 174 |
| 1:100 | 49 | 109 | 131 |
Maximum day at which E. coli O157:H7 was detected by either direct plating or enrichment culture methods.
The E. coli O157:H7 survival data in Fig. 1 were analyzed statistically across all dates to determine if general differences existed among the four treatment means. The LSD values (P < 0.05) between any pair of treatment means at any date were 0.9595, 0.4368, 0.3418, 0.786, 0.9498, and 0.9775 log CFU/g for Fig. 1A, B, C, D, E, and F, respectively. E. coli O157:H7 cells died off significantly (P < 0.05) more rapidly in 1:10 samples than in 1:25, 1:50, and 1:100 samples in autoclaved soil held at 15 and 21°C.
Tifton sandy loam soil, which had a pH of 7.0 and a 7% moisture content (MC) and contained P, K, Ca, and Mg at 140, 123, 724, and 85 lb/acre, respectively, was used for the study. After adding manure and E. coli O157:H7 to both autoclaved and unautoclaved soil, the 1:10 samples had the highest MC and pH, whereas the 1:100 samples had the lowest MC and pH at the onset. Over time, there was a gradual loss of moisture from all samples. The moisture content of the manure-amended soils, either autoclaved or unautoclaved, decreased to 0.6 to 3% by 68 days of storage. The initial aerobic plate count in soil was 1.9 × 1010 CFU/g, and no ampicillin-resistant bacteria were detected. After storage at 21°C, the total aerobic plate counts in both autoclaved and unautoclaved manure-amended soil decreased steadily from ca. 1010 to ca. 107 CFU/g at 120 days and then remained at that level for up to 230 days.
During the first 2 weeks at 5°C, the pH of all manure-amended soil samples increased ca. 0.5 but thereafter decreased with increased storage time. The 1:10 manure-amended autoclaved soil retained a slightly higher pH than the other soil samples. The pH of the manure-amended unautoclaved soil samples was lower than the autoclaved equivalents after storage for more than 2 months at 5°C. Apparently, the relatively higher pH in the soil favored the survival of E. coli O157:H7 at 5°C. The pH of manure-amended unautoclaved soil held at 15°C increased slightly during storage for 1 week and for 2 weeks in autoclaved soil. The pH change in manure-amended autoclaved or unautoclaved soil varied by approximately ±1 pH unit during storage at 21°C. This variability could in part be attributed to variation in the distribution of manure-amended soil ingredients as well as variation that occurs at different locations during natural composting metabolic activities.
Properties of soil influencing the survival or inactivation of pathogenic bacteria include soil composition, pH, water activity, oxidation-reduction potential, presence of a rhizosphere, and microbial interactions (8, 16). Under field conditions, other variables, such as solar radiation and dryness, may also affect the survival of human pathogens. In our study, autoclaved soil was used as a control to minimize the influence of indigenous soil microorganisms on the survival and growth of E. coli O157:H7. Cow manure normally contains more than 109 CFU of indigenous bacteria/g. In samples in which inoculated cow manure was mixed with autoclaved soil, ca. 107 to 108 CFU of manure bacteria/g were introduced into the autoclaved soil. The antagonistic effect of manure microorganisms was likely a factor in killing E. coli O157:H7 cells in our manure-amended soil studies at 15 and 21°C. E. coli O157:H7 cells in soil containing both plentiful manure nutrients and the highest population of manure microorganisms (1 part manure per 10 parts soil) died off more rapidly at growth temperatures in autoclaved soil than in soil formulations (1:25, 1:50, and 1:100) containing less manure microorganisms and nutrients. Other contributing factors may have included the pH and moisture content of the manure-amended soil, with the 1:10-formulated soil preparation maintaining conditions that were more favorable for growth of competitive manure microorganisms.
In addition to apparent antimicrobial activities of microorganisms in manure to E. coli O157:H7, indigenous soil microorganisms also appear to contribute to inactivation of E. coli O157:H7. E. coli O157:H7 was inactivated more rapidly in unautoclaved soil than in autoclaved soil at all three temperatures and with an equivalent amount of manure added. However, in unautoclaved soil, small numbers of the pathogen, which were only detected by enrichment culture, survived for very long periods of time at 15 and 21°C.
In the manure-amended autoclaved soil, E. coli O157:H7 populations increased from 0.3 to 2 log10 CFU/g within 3 days at 15 and 21°C in manure-to-soil formulations of 1:25, 1:50, and 1:100. Gagliardi and Karns (10) also determined in a study simulating conditions of rainfall that E. coli O157 cells grew well in soil cores that were treated with manure. However, E. coli O157:H7 growth did not occur in unautoclaved soil in our study, likely because of the antagonistic activity of soil bacteria that were present. Variability in the growth of E. coli O157:H7 that occurred in manure-amended soil of different types is likely attributed to variation in the antimicrobial activity of different microbes present in different soil.
Our study revealed that E. coli O157:H7 can survive for extended periods of time in manure-amended soil, even under very dry conditions, i.e., less than 1% moisture content in the soil. Maule (14) reported survival of E. coli O157:H7 for 130 days at 18°C when inoculated into a laboratory-prepared soil and grass microcosm. Fenlon et al. (8) applied E. coli O157:H7 cells (3 × 101 CFU/100 ml) inoculated in cattle slurry to arable and grass plots on a clay loam soil and determined that E. coli O157 was detected only in the soil and on the grass during the first week after land application. In contrast, Maule (13) observed prolonged survival of E. coli O157 with very high application rates of manure-containing E. coli O157 on soil cores with a grass cover.
We determined rates of inactivation of E. coli O157:H7 in soil containing different amounts of manure and held at different temperatures. Intensive application of manure to soil (e.g., 1 part manure to 10 parts soil versus 1:25, 1:50 or 1:100) generally resulted in greater inactivation of E. coli O157:H7 with a possible exception at 5°C, which is a temperature at which microorganisms antagonistic to E. coli O157:H7 may be less competitive because of slow or no growth or failure to produce significant amounts of antimicrobials against E. coli O157:H7. Microorganisms naturally occurring in soil appear to have a major influence on rates of inactivation of E. coli O157:H7 at 15 and 21°C, as the rates of pathogen inactivation were considerably less at these temperatures in manure-amended autoclaved soil than in manure-amended unautoclaved soil. Results from these studies provide useful information in identifying manure handling practices to reduce the risk of E. coli O157:H7 transmission to foods produced in the presence of animal waste. Additional studies that would be beneficial include identifying and characterizing the soil microorganisms that inactivate E. coli O157:H7 and further elucidating the environmental conditions that are optimal to kill pathogens in manure and soil.
Acknowledgments
This study was funded in part by a grant from the International Life Sciences Institute-North America and by a special grant from the USDA-CSREES for the Alliance for Food Protection.
We thank Gary Gascho, at the University of Georgia, Tifton campus, for assistance in obtaining soil samples.
REFERENCES
- 1.Centers for Disease Control and Prevention. 2001. Outbreaks of Escherichia coli O157:H7 infections among children associated with farm visits-Pennsylvania and Washington, 2000. Morb. Mortal. Wkly. Rep. 50:293-297. [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. 1996. Outbreak of Escherichia coli O157:H7 infections associated with drinking unpasteurized commercial apple juice—British Columbia, California, Colorado, and Washington, October 1996. Morb. Mortal. Wkly. Rep. 45:975. [PubMed] [Google Scholar]
- 3.Chapman, P. A., C. A. Siddons, J. Manning, and C. Cheetham. 1997. An outbreak of infection due to verocytotoxin-producing Escherichia coli O157 in four families: the influence of laboratory methods on the outcome of the investigation. Epidemiol. Infect. 119:245-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cieslak, P. R., T. J. Barrett, P. M. Griffin, K. F. Gensheimer, G. Beckett, J. Buffington, and M. G. Smith. 1993. Escherichia coli O157:H7 infection from a manured garden. Lancet 342:367. [DOI] [PubMed] [Google Scholar]
- 5.Delazari, I., S. T. Iaria, H. Riemann, D. Cliver, and N. Jothikumar. 1998. Removal of Escherichia coli O157:H7 from surface tissues of beef carcasses inoculated with wet and dry manure. J. Food Prot. 61:1265-1268. [DOI] [PubMed] [Google Scholar]
- 6.Doyle, M. P., T. Zhao, J. Meng, and S. Zhao. 1997. Escherichia coli O157:H7, p. 171-191. In M. P. Doyle, L. R. Beuchat, and T. J. Montville (ed.), Food microbiology: fundamentals and frontiers. ASM Press, Washington, D.C.
- 7.Elder, R. O., J. E. Keen, G. R. Siragusa, G. A. Barkocy-Gallagher, M. Koohmaraie, and W. W. Laegreid. 2000. Correlation of enterohemorrhagic Escherichia coli O157:H7 prevalence in feces, hides and carcasses of beef cattle during processing. Proc. Natl. Acad. Sci. USA 97:2999-3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fenlon, D. R., I. D. Ogden, A. Vinten, and I. Svoboda. 2000. The fate of Escherichia coli and E. coli O157:H7 in cattle slurry after application to land. J. Appl. Microbiol. Symp. Suppl. 88:149S-156S. [DOI] [PubMed] [Google Scholar]
- 9.Freeman, T. M., and D. L. Cawthon. 1999. Use of composted dairy cattle solid biomass, poultry litter and municipal biosolids as greenhouse growth media. Compost Sci. Utilization 3:66-71. [Google Scholar]
- 10.Gagliardi, J. V., and J. S. Karns. 2000. Leaching of Escherichia coli O157:H7 in diverse soils under various agricultural management practices. Appl. Environ. Microbiol. 66:877-883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Himathongkham, S., S. Bahari, H. Riemann, and D. Cliver. 1999. Survival of Escherichia coli O157:H7 and Salmonella Typhimurium in cow manure and cow manure slurry. FEMS Microbiol. Lett. 178:251-257. [DOI] [PubMed] [Google Scholar]
- 12.Kudva, I. T., K. Blanch, and C. J. Hovde. 1998. Analysis of Escherichia coli O157:H7 survival in ovine or bovine manure and manure slurry. Appl. Environ. Microbiol. 64:3166-3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maule, A. 2000. Survival of verocytotoxigenic Escherichia coli O157:H7 in soil, water and on surfaces. J. Appl. Microbiol. Symp. Suppl. 88:71S-78S. [DOI] [PubMed] [Google Scholar]
- 14.Maule, A. 1997. Survival of the verotoxigenic strain Escherichia coli O157:H7 in laboratory-scale microcosms, p. 61-65. In D. Kay and C. Fricker (ed.), Coliforms and E. coli: problem or solution? Royal Society of Chemistry, London, England.
- 15.Pell, A. N. 1997. Manure and microbes: public and animal health problem? J. Dairy Sci. 80:2673-2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tamasi, G. 1981. Factor influencing the survival of pathogenic bacteria in soils. ACTA Vet. Acad. Sci. Hungaricae 29:119-126. [PubMed] [Google Scholar]
- 17.Wang, G., T. Zhao, and M. P. Doyle. 1996. Fate of enterohemorrhagic Escherichia coli O157:H7 in bovine feces. Appl. Environ. Microbiol. 62:2567-2570. [DOI] [PMC free article] [PubMed] [Google Scholar]