Abstract
Hygienic and microbiological examinations of watercourses are usually not carried out during heavy rainfall and runoff events. After rainfall or snowmelt, there are often massive increases in turbidity in flooding creeks in mountain ranges, which are frequently interpreted as an indication of microbial contamination. The aim of this study was to quantify the microbial loads of watercourses during such runoff events and to compare these loads with loads occurring during regular conditions. In a 14-month monitoring period we investigated the microbial loads of three tributaries of different drinking water reservoirs. A total of 99 water samples were taken under different runoff conditions and analyzed to determine physical, chemical, bacterial, and parasitic parameters. Thirty-two water samples were considered event samples during nine measuring series. The criteria for events, based on duration and intensity of precipitation, water depth gauge measurements, and dynamics, had been fixed before the investigation for each creek individually. Of the physical and chemical parameters examined, only the turbidity, pH, and nitrate values differed clearly from the values obtained for regular samples. Most of the bacteriological parameters investigated (colony, Escherichia coli, coliform, fecal streptococcal, and Clostridium perfringens counts) increased considerably during extreme runoff events. If relevant sources of parasitic contamination occurred in catchment areas, the concentrations of Giardia and Cryptosporidium rose significantly during events. The results show that substantial shares of the total microbial loads in watercourses and in drinking water reservoirs result from rainfall and extreme runoff events. Consequently, regular samples are considered inadequate for representing the microbial contamination of watercourse systems. The procedures for raw water surveillance in the context of multiple-barrier protection and risk assessment ought to include sampling during extreme runoff situations.
In some regions of Germany surface reservoirs are the main source of drinking water. In the state of North Rhine-Westphalia, 60% of the drinking water production in 1999 was based on surface water resources (2). Surface water bodies are presumed to be more vulnerable to fecal contamination than groundwater reservoirs due to the absence of natural soil protection and filtration and the possibly short distances between the occurrence of contamination and water extraction. It is argued that especially in the case of heavy rainfall the microbial loads of running waters may suddenly increase substantially and reach reservoir bodies very quickly (18, 19). For this reason monitoring microbiological raw water quality is an essential component of the protection strategy in catchment areas of surface drinking water reservoirs (8). In Germany, catchment areas of surface water supplies are usually protected by buffer zones, and the intensity of protection increases with proximity to the water body. Protection of entire catchment areas is the first step of the multiple-barrier protection concept (22, 33), which demands integrated water management from precipitation to the tap. As a second step, there are regular microbiological surveys of raw water before treatment. However, the samples may be taken without regard to meteorological and hydrological conditions, since sampling under extreme rainfall and runoff conditions is not required (12, 33). In fact, exceeded limits due to flooding, natural disasters, or unusual weather conditions are not taken into consideration (12, 30).
Some studies have shown that there are correlations between microbial loads of stream water and the conditions mentioned above (1, 9, 10, 28, 31), and the question is whether dry-weather sampling provides representative data for microbial contamination of reservoir tributaries throughout the year (5, 17, 31), since the (Enhanced) Surface Water Treatment Rule of the U.S. Environmental Protection Agency includes an action level of 30 parasites per 100 liters of raw water and no parasites per 100 liters of finished water (17, 20, 28). In this study we investigated the association of several parasitic and bacterial parameters with rainfall, runoff, and specific conditions in catchment areas of drinking water reservoirs in order to understand what factors are associated with peaks of contamination (4, 20).
MATERIALS AND METHODS
An investigation of the microbial loads of three tributaries of different drinking water reservoirs and the land use patterns in their drainage areas was performed. In a 14-month monitoring period (January 1997 to February 1998), 99 water samples were taken from the main tributaries under different runoff conditions and analyzed to determine physical, chemical, bacterial, and parasitic parameters. A total of 32 water samples, taken during nine sampling series, could be classified as event samples (ES). An analysis of the dynamics of these samples during extreme runoff conditions and a statistical comparison of ES with regular samples (RS) were carried out to assess the influence of flood periods on total annual contamination loads.
Selection of sample locations.
For this investigation, the main tributaries of three different drinking water reservoirs were chosen (Fig. 1). To guarantee precise sample reproducibility, the sampling points were located directly above the reservoirs, in the vicinity of water gauges.
FIG. 1.
Locations of the three drinking water reservoirs investigated.
The sampling points and tributary areas selected reflected different catchment area conditions. One watercourse represented a catchment area where to a large extent there was no anthropogenic influence (Nauholzbach). The second tributary represented a catchment area heavily influenced by pasture land for dairy farming (Kall), whereas the third tributary was chosen in order to monitor additional effects of treated wastewater discharge. Thus, the catchment areas of the tributaries investigated had very different geoecological structures, and they have been characterized in detail previously (19).
The catchment area of the Nauholzbach (3.27 km2), a tributary of the Obernau reservoir, is almost completely covered with forest (98%) and is rarely affected by agriculture or settlement. The density of fallow deer (15 animals/km2), however, exceeds the ecologically tolerable limit, 5 to 10 animals/km2.
The catchment area of the Kallbach, the main tributary of the Kall reservoir, extends over 19.7 km2 of hilly landscape. Moors and wetlands were formed in this area because of the prevailing climatic, geological, soil, and morphological conditions. Water flows mainly on or just beneath the surface. A wide drainage system has changed the runoff conditions substantially. As a result, during heavy rainfall runoff increases very rapidly. Agricultural land use (exclusively pasture land for dairy farming) comprises 56.3% of the Kall study area, whereas 19% is forest with a relatively low density of fallow deer (four to five animals/km2). Eighteen farms have local sewage treatment facilities. A total of 3,500 people live in the catchment area. All nonfarming households are connected to public sewage systems. The processed wastewater is piped out of the Kall catchment area. Ten percent of the area belongs to Belgium and because of this is not subject to national water protection regulations.
The Wahnbach study area comprises 45 km2. The soils are mainly formed by loess and are intensively used for agriculture (63% of the area). Erosion is a major problem. Woodland covers 21% of the catchment area. A total of 16,000 people live in numerous small settlements, some of which still are not connected to public wastewater treatment systems. Two sewage plants in the catchment area discharge their treated wastewater into the Wahnbach tributary right above the reservoir.
Criteria for sampling during extreme rainfall and runoff events.
For each site investigated, criteria for event sampling could be defined based on long-term flood-monitoring data. Continuous precipitation was used as a general criterion. However, a rapid increase in water depth exceeding a flood threshold value was decisive for obtaining an ES. For each creek, specific values for the summer and winter seasons could be derived from long-term water depth measurements. Additionally, the steepness of the water level increase played an important role in defining flood events, because the level is affected by the kind, amount, and course of precipitation, as well as by site-specific environmental factors. Frequently, water levels rise more rapidly after a heavy rainfall in the summer than in the winter.
Meteorological and hydrological parameters.
Rainfall and air temperature data recorded at representative weather stations and continuous runoff records for the creeks investigated were utilized for characterization of the site-specific water balance. The data were used to calculate weekly sums (precipitation) or weekly arithmetic means (temperature, runoff). Long-term monthly means for rainfall and air temperature were used to evaluate the hydrologic and meteorological situation during the study period. In addition, air temperature, rainfall, water depth, and runoff velocity were recorded during sampling. In the case of event sampling, supplementary records for rainfall and runoff conditions with high temporal resolution (0.5-h records) were obtained retrospectively from the reservoir operating authorities.
Water sampling, preparation, and analysis.
At the onset of an event with heavy rainfall and runoff, an ES series was initiated. One series included up to five single water samples obtained at 1- to 2-h intervals. When an RS (RS were taken every 2 weeks) coincided with an event, the sample was considered an ES.
Parasitological sampling was carried out by using a wound polypropylene cartridge filter with a nominal pore size of 1 μm. The filter case was connected to a water pump which pumped about 500 liters of water through the filter. The flow rate was adjusted to 2 liters/min. When the flow rate fell below 2 liters/min, sampling was stopped. Turbidity and pH values were obtained on site. For further chemical and bacteriological analyses, water was pumped into suitable receptacles. The material was cooled, immediately transported into the laboratory, and processed on the same day.
Physical and chemical parameters were analyzed according to the German regulations for water, sewage, and sludge analysis (32). General colony counts at 20 and 37°C (CC 20°C and CC 37°C, respectively) were obtained by the pour plate method (12), whereas coliforms, Escherichia coli, Clostridium perfringens, and fecal streptococci were identified by membrane filtration (11, 30). The parasites Giardia lamblia and Cryptosporidium spp. were recovered quantitatively by using the method of Her Majesty's Stationery Office comprising filtration, density flotation, and immunofluorescence (15).
Statistical methods.
To compare the ES and RS results, arithmetic means and parameter maxima were calculated separately for RS and ES obtained from each creek. The ES and RS results were assumed to be t distributed and were analyzed for independence from the other samples by using the one-sided t test for two spot checks with different variances (24). The level of significance [P(α)] was calculated after determination of the degrees of freedom. A P(α) value of <0.05 was considered significant.
To assess the contributions of chemical, bacterial, and parasitic loads during heavy rainfall and runoff events to the total loads in each drinking water reservoir, theoretical quantitative loads during an average event (means for all ES; medians for bacteriological and parasite parameters) were compared to loads determined for regular conditions (means for all RS; medians for bacteriological and parasite parameters). For the calculations, an event was assumed to last 12 h. The mean load for a 12 h-event was determined from the ES mean values. The contribution of this average 12-h event load to a theoretical annual load without events was calculated. This theoretical share was divided by the 12-h value expected under the assumption that runoff and parameter concentrations were constant throughout the year, as follows: 12-h expected value (EV12 h) = 1/(365.24 × 24/12) × 100 = 0.137%. Finally EV12 h was corrected by considering the increased runoff under event conditions. In this way the factor EV12hCorr was obtained, which quantified the additional load due to the increases in parameter concentrations.
RESULTS
During a 14-month investigation in which there were nine extreme rainfall and runoff events, a total of 32 ES were collected (Table 1). Within the framework of ES collections seven ES series were obtained. In two cases (E.1 and F.1) ES collections coincided with RS collections. The results obtained with 67 RS collected at the same sites during the same time period were available for comparison.
TABLE 1.
Synopsis of ES series
| Series | Date (mo/day/yr) | Creek | Samples | Type of runoff |
|---|---|---|---|---|
| A | 10/09/1997 | Nauholzbach | A.1-A.3 | (First) outgoing flood |
| B | 12/12/1997 | Nauholzbach | B.1-B.4 | Rising flood |
| C | 02/12/1997 | Kall | C.1-C.5 | Peak |
| D | 03/19/1997 | Kall | D.1-D.5 | Peak |
| E | 10/09/1997 | Kall | E.1 | Outgoing flood |
| F | 02/05/1997 | Wahnbach | F.1 | Rising flood |
| G | 10/09/1997 | Wahnbach | G.1-G.4 | Peak |
| H | 10/10/1997 | Wahnbach | H.1-H.5 | Outgoing flood |
| I | 12/11/1997 | Wahnbach | I.1-I.4 | Outgoing flood |
Comparison with RS.
The results of the comparisons of ES and RS are shown in Table 2.
TABLE 2.
Data for RS and ES obtained in 1997 and 1998
| Location | Samples | No. of samples | Arithmetic mean (maximum)
|
Median (maximum)
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Turbidity (NTU) | pH | Runoff (liters/s) | Nitrate (mg/liter) | Nitrite (mg/liter) | Ammonia (mg/liter) | Total phosphorus (mg/liter) | Borate (mg/liter) | CC 20°C (CFU/ml) | CC 37°C (CFU/ml) | E. coli (CFU/100 ml) | Coliforms (CFU/100 ml) | Fecal streptococci (CFU/100 ml) | C. perfringens (CFU/100 ml) | Giardia (cysts/100 liters) | Cryptosporidium (oocysts/100 liters) | |||
| Nauholzbach | RS | 16 | 1.30 (3.80) | 7.18 (7.50) | 49.29 (190.00) | 6.84 (10.90) | 0.00 (0.02) | 0.00 (0.00) | 0.01 (0.05) | 0.00 (0.04) | 444.50 (1,730.00) | 69.50 (339.00) | 22.00 (148.00) | 1,110.00 (15,000.00) | 7.50 (400.00) | 1.50 (31.00) | 0.00 (1.10) | 2.40 (52.20) |
| ES | 7 | 47.38 (93.80) | 6.98 (7.30) | 139.06 (317.00) | 11.43 (13.90) | 0.00 (0.00) | 0.00 (0.00) | 0.09 (0.40) | 0.02 (0.04) | 3,480.00 (10,629.00) | 327.00 (849.00) | 190.00 (650.00) | 12,200.00 (42,000.00) | 130.00 (620.00) | 82.00 (155.00) | 0.00 (0.00) | 11.40 (35.50) | |
| Kall | RS | 26 | 3.57 (10.20) | 7.43 (7.70) | 290.78 (1,783.32) | 13.94 (22.20) | 0.01 (0.09) | 0.04 (0.19) | 0.01 (0.10) | 0.07 (0.11) | 595.00 (46,300.00) | 185.00 (12,000.00) | 133.00 (11,000.00) | 1,880.00 (400,000.00) | 54.00 (11,000.00) | 22.50 (198.00) | 0.95 (11.10) | 2.65 (10.30) |
| ES | 11 | 28.25 (54.30) | 7.05 (7.40) | 1,276.78 (2,732.00) | 10.58 (15.40) | 0.07 (0.13) | 0.24 (0.46) | 0.08 (0.30) | 0.11 (0.18) | 50,500.00 (114,000.00) | 5,550.00 (37,000.00) | 1,300.00 (3,800.00) | 19,800.00 (63,000.00) | 540.00 (4,600.00) | 250.00 (900.00) | 0.00 (21.00) | 14.30 (65.60) | |
| Wahnbach | RS | 25 | 11.45 (36.00) | 7.55 (8.00) | 459.54 (2,118.00) | 17.22 (33.90) | 0.10 (0.40) | 0.15 (1.46) | 0.15 (0.62) | 0.13 (0.27) | 1,110.00 (16,500.00) | 332.00 (1,850.00) | 490.00 (5,100.00) | 3,800.00 (74,000.00) | 130.00 (2,200.00) | 130.00 (900.00) | 1.10 (9.10) | 1.30 (23.10) |
| ES | 14 | 56.99 (125.30) | 7.33 (7.50) | 1,040.46 (2,747.00) | 10.43 (20.10) | 0.09 (0.14) | 0.34 (1.70) | 0.25 (0.50) | 0.18 (0.35) | 37,400.00 (77,000.00) | 8,010.00 (14,700.00) | 13,700.00 (34,500.00) | 88,000.00 (1,180,000.00) | 13,600.00 (77,000.00) | 430.00 (2,500.00) | 12.40 (21.60) | 17.05 (147.10) | |
(i) Physical and chemical parameters.
At the Nauholzbach the most conspicuous changes in concentration compared to the RS concentrations occurred with regard to the turbidity. While the turbidity never exceeded 3.8 nephelometric turbidity units (NTU) in 16 RS, it increased to a maximum of 93.8 NTU during the events; the arithmetic mean for seven ES was 47.4 NTU (arithmetic mean for RS, 1.3 NTU). The nitrate concentrations for ES always exceeded the mean of the nitrate concentrations for RS, and the nitrate concentrations for 57% of the ES exceeded the maximum nitrate concentration observed in RS.
At the Kall there was also a massive increase in turbidity compared to the RS values. The mean increased from 3.57 to 28.25 NTU. Clear increases in concentration were also observed for nitrite, ammonia, total phosphorus, and borate. On the other hand, the means and maxima for pH and nitrate concentration were below the corresponding values obtained for the RS.
At the Wahnbach, the mean turbidity (11.45 to 57 NTU) and the maximum turbidity (36.0 to 125.3 NTU) increased to extreme values during events. In contrast, the pH and nitrate concentration were clearly less than the corresponding values for the RS. The concentrations of nitrite, ammonia, total phosphorus, and borate changed only slightly.
(ii) Bacteriological parameters.
At the Nauholzbach, the concentrations of quantitatively identified bacteriological parameters (CC 20°C, CC 37°C, E. coli, coliforms, fecal streptococci, C. perfringens) increased during extreme rainfall and runoff events; the medians increased about 1 to 2 log10, and the maximum values increased about 1 log10.
At the Kall and Wahnbach, the median and maximum concentrations of quantitatively identified bacteriological parameters increased about 1 to 2 log10.
(iii) Parasitological parameters.
At the Nauholzbach, the median Cryptosporidium concentrations in ES reached 11.4 oocysts/100 liters, in contrast to the 2.4 oocysts/100 liters in RS. Giardia cysts were not regularly recovered.
At the Kall, the median concentrations of Cryptosporidium oocysts (ES, 14.3 oocysts/100 liters; RS, 2.65 oocysts/100 liters) and the maximum concentrations of Cryptosporidium oocysts (ES, 65.6 oocysts/100 liters; RS, 10.3 oocysts/100 liters) increased substantially during events, whereas the frequencies of recovery of Giardia cysts were low and no recognizable tendencies were identified.
At the Wahnbach, the median concentrations of Cryptosporidium oocysts (ES, 17 oocysts/100 liters; RS, 1.3 oocysts/100 liters) and Giardia cysts (ES, 12.4 cysts/100 liters; RS, 1.1 cysts/100 liters) in ES were clearly higher than those in the corresponding RS. The maximum concentrations also occurred in ES. In ES the Cryptosporidium concentrations reached 147.1 oocysts/100 liters (RS, 23.1 oocysts/100 liters), and the Giardia concentrations reached 21.6 cysts/100 liters (RS, 9.1 cysts/100 liters).
(iv) Student's t test for significant differences between ES and RS.
At the Nauholzbach during extreme rainfall and runoff events, the turbidity and nitrate concentration increased significantly, but the pH was significantly lower. The total bacteriological parameters showed significant increases, whereas the parasite load did not increase significantly due to the high standard deviation for the RS (Table 3).
TABLE 3.
Analysis of assumed t-distributed RS and ES at the Nauholzbach gauge for independence from each other, using the one-sided t-test for two spot checks with different variances (1997 data)
| Parameter | RS (n = 16)
|
ES (n = 7)
|
t test t (empirical) | df (empirical) | P(α) (one sided)a | ||||
|---|---|---|---|---|---|---|---|---|---|
| Arithmetic mean | Maximum | SD | Arithmetic mean | Maximum | SD | ||||
| Turbidity (NTU) | 1.30 | 3.80 | 0.92 | 47.38 | 93.80 | 37.22 | 3.275 | 6.004 | 0.008b |
| pH | 7.18 | 7.50 | 0.19 | 6.98 | 7.30 | 0.25 | 1.819 | 10.311 | 0.049c |
| Runoff (liters/s) | 49.29 | 190.00 | 56.25 | 139.06 | 317.00 | 113.84 | 1.983 | 7.748 | 0.044b |
| Nitrate (mg/liter) | 6.84 | 10.90 | 2.70 | 11.43 | 13.90 | 2.34 | 4.128 | 15.217 | 0.000b |
| Nitrite (mg/liter) | 0.00 | 0.02 | 0.01 | 0.00 | 0.00 | 0.00 | 1.000 | 15.000 | 0.167 |
| Ammonia (mg/liter) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |||
| Total phosphorus (mg/liter) | 0.01 | 0.05 | 0.02 | 0.09 | 0.40 | 0.16 | 1.325 | 6.068 | 0.117 |
| Borate (mg/liter) | 0.00 | 0.04 | 0.01 | 0.02 | 0.04 | 0.02 | 1.605 | 9.049 | 0.071 |
| CC 20°C (CFU/ml) | 673.06 | 1,730.00 | 534.50 | 5,509.86 | 10,629.00 | 3,746.37 | 3.401 | 6.143 | 0.007b |
| CC 37°C (CFU/ml) | 100.94 | 339.00 | 93.64 | 439.43 | 849.00 | 205.85 | 4.166 | 7.477 | 0.002b |
| E. coli (CFU/100 ml) | 33.81 | 148.00 | 36.87 | 235.71 | 650.00 | 190.69 | 2.779 | 6.263 | 0.016b |
| Coliforms (CFU/100 ml) | 3,300.75 | 15,000.00 | 4,464.67 | 17,114.29 | 42,000.00 | 11,844.61 | 2.994 | 7.009 | 0.010b |
| Fecal streptococci (CFU/100 ml) | 41.56 | 400.00 | 99.27 | 207.14 | 620.00 | 205.48 | 2.031 | 7.670 | 0.041b |
| C. perfringens (CFU/100 ml) | 4.06 | 31.00 | 7.75 | 66.71 | 155.00 | 65.95 | 2.506 | 6.097 | 0.023b |
| Giardia (cysts/100 liters) | 0.11 | 1.10 | 0.30 | 0.00 | 0.00 | 0.00 | 1.396 | 15.000 | 0.091 |
| Cryptosporidium (oocysts/100 liters) | 7.18 | 52.20 | 13.18 | 15.30 | 35.50 | 11.85 | 1.461 | 14.709 | 0.083 |
Values were considered significantly different if the P(α) value was <0.05.
The ES values were significantly greater than the RS values.
The ES values were significantly less than the RS values.
At the Kall, the values for some physical and chemical parameters (turbidity and nitrite, ammonia, total phosphorus, and borate concentrations) increased significantly during events; the pH and the nitrate concentration, however, decreased significantly. Of the bacteriological parameters, only CC 20°C, CC 37°C, and the C. perfringens count increased significantly, as the concentration of Cryptosporidium did, whereas there was not a significant change in the G. lamblia concentration (Table 4).
TABLE 4.
Analysis of assumed t-distributed RS and ES at the Kall gauge for independence from each other, using the one-sided t test for two spot checks with different variances (1997 data)
| Parameter | RS (n = 26)
|
ES (n = 11)
|
t test t (empirical) | df (empirical) | P(α) (one sided)a | ||||
|---|---|---|---|---|---|---|---|---|---|
| Arithmetic mean | Maximum | SD | Arithmetic mean | Maximum | SD | ||||
| Turbidity (NTU) | 3.57 | 10.20 | 2.53 | 28.25 | 54.30 | 15.99 | 5.092 | 10.254 | 0.000b |
| pH | 7.43 | 7.70 | 0.15 | 7.05 | 7.40 | 0.17 | 6.362 | 18.468 | 0.000c |
| Runoff (liters/s) | 290.78 | 1,783.32 | 363.55 | 1,276.78 | 2,732.00 | 948.54 | 5.685 | 11.515 | 0.000b |
| Nitrate (mg/liter) | 13.94 | 22.20 | 3.15 | 10.58 | 15.40 | 4.16 | 2.405 | 16.061 | 0.014c |
| Nitrite (mg/liter) | 0.01 | 0.09 | 0.02 | 0.07 | 0.13 | 0.03 | 5.169 | 16.120 | 0.000b |
| Ammonia (mg/liter) | 0.04 | 0.19 | 0.06 | 0.24 | 0.46 | 0.18 | 3.632 | 11.167 | 0.002b |
| Total phosphorus (mg/liter) | 0.01 | 0.10 | 0.03 | 0.08 | 0.30 | 0.10 | 2.299 | 10.969 | 0.022b |
| Borate (mg/liter) | 0.07 | 0.11 | 0.02 | 0.11 | 0.18 | 0.03 | 3.961 | 15.217 | 0.001b |
| CC 20°C (CFU/ml) | 3,623.50 | 46,300.00 | 9,864.96 | 49,109.09 | 114,000.00 | 30,359.10 | 4.862 | 11.084 | 0.000b |
| CC 37°C (CFU/ml) | 787.42 | 12,000.00 | 2,321.39 | 10,797.27 | 37,000.00 | 11,589.17 | 2.841 | 10.409 | 0.009b |
| E. coli (CFU/100 ml) | 929.38 | 11,000.00 | 2,316.65 | 1,340.91 | 3,800.00 | 1,040.45 | 0.745 | 36.958 | 0.230 |
| Coliforms (CFU/100 ml) | 27,396.38 | 400,000.00 | 85,304.60 | 20,890.91 | 63,000.00 | 21,107.60 | 0.363 | 31.788 | 0.359 |
| Fecal streptococci (CFU/100 ml) | 601.38 | 11,000.00 | 2,156.15 | 919.09 | 4,600.00 | 1,259.27 | 0.559 | 33.771 | 0.290 |
| C. perfringens (CFU/100 ml) | 37.96 | 198.00 | 48.28 | 316.00 | 900.00 | 240.12 | 3.808 | 10.412 | 0.002b |
| Giardia (cysts/100 liters) | 1.67 | 11.10 | 2.55 | 3.08 | 21.00 | 6.27 | 0.722 | 11.708 | 0.243 |
| Cryptosporidium (oocysts/100 liters) | 3.03 | 10.30 | 2.80 | 22.32 | 65.60 | 17.75 | 3.585 | 10.253 | 0.002b |
Values were considered significantly different if the P(α) value was <0.05.
The ES values were significantly greater than the RS values.
The ES values were significantly less than the RS values.
At the Wahnbach, the turbidity and the borate concentration increased significantly in ES; the pH and nitrate concentration, however, decreased significantly. All of the bacteriological and parasitological concentrations increased significantly (Table 5).
TABLE 5.
Analysis of assumed t-distributed RS and ES at the Wahnbach gauge for independence from each other, using the one-sided t test for two spot checks with different variances (1997 data)
| Parameter | RS (n = 25)
|
ES (n = 14)
|
t test t (empirical) | df (empirical) | P(α) (one sided)a | ||||
|---|---|---|---|---|---|---|---|---|---|
| Arithmetic mean | Maximum | SD | Arithmetic mean | Maximum | SD | ||||
| Turbidity (NTU) | 11.45 | 36.00 | 8.19 | 56.99 | 125.30 | 31.12 | 5.372 | 14.173 | 0.000b |
| pH | 7.55 | 8.00 | 0.25 | 7.33 | 7.50 | 0.16 | 3.404 | 38.362 | 0.001c |
| Runoff (liters/s) | 459.54 | 2,118.00 | 448.31 | 1,040.46 | 2,747.00 | 608.07 | 3.130 | 22.227 | 0.002b |
| Nitrate (mg/liter) | 17.22 | 33.90 | 6.82 | 10.43 | 20.10 | 5.27 | 3.466 | 35.378 | 0.001c |
| Nitrite (mg/liter) | 0.10 | 0.40 | 0.09 | 0.09 | 0.14 | 0.04 | 0.469 | 38.337 | 0.321 |
| Ammonia (mg/liter) | 0.15 | 1.46 | 0.35 | 0.34 | 1.70 | 0.61 | 1.061 | 18.610 | 0.151 |
| Total phosphorus (mg/liter) | 0.15 | 0.62 | 0.16 | 0.25 | 0.50 | 0.18 | 1.610 | 26.392 | 0.060 |
| Borate (mg/liter) | 0.13 | 0.27 | 0.06 | 0.18 | 0.35 | 0.08 | 2.218 | 23.985 | 0.018b |
| CC 20°C (CFU/ml) | 2,132.88 | 16,500.00 | 3,449.77 | 34,542.86 | 77,000.00 | 22,355.75 | 5.389 | 13.401 | 0.000b |
| CC 37°C (CFU/ml) | 468.40 | 1,850.00 | 378.73 | 8,547.14 | 14,700.00 | 3,344.97 | 9.005 | 13.216 | 0.000b |
| E. coli (CFU/100 ml) | 913.28 | 5,100.00 | 1,252.98 | 19,471.43 | 34,500.00 | 11,870.37 | 5.832 | 13.187 | 0.000b |
| Coliforms (CFU/100 ml) | 13,714.80 | 74,000.00 | 20,136.04 | 264,428.57 | 1,180,000.00 | 417,695.21 | 2.244 | 13.039 | 0.021b |
| Fecal streptococci (CFU/100 ml) | 266.40 | 2,200.00 | 443.30 | 20,457.14 | 77,000.00 | 25,138.54 | 3.005 | 13.005 | 0.005b |
| C. perfringens (CFU/100 ml) | 190.92 | 900.00 | 226.01 | 880.00 | 2,500.00 | 900.59 | 2.814 | 14.065 | 0.007b |
| Giardia (cysts/100 liters) | 2.13 | 9.10 | 2.48 | 10.60 | 21.60 | 7.25 | 4.232 | 14.980 | 0.000b |
| Cryptosporidium (oocysts/100 liters) | 3.60 | 23.10 | 6.37 | 27.48 | 147.10 | 37.69 | 2.352 | 13.482 | 0.018b |
Values were considered significantly different if the P(α) value was <0.05.
The ES values were significantly greater than the RS values.
The ES values were significantly less than the RS values.
Dynamics of parameters during extreme rainfall and runoff events.
ES series were used to record the various runoff situations during different phases of the events (Table 1). Sometimes the rising flood was registered, and sometimes the outgoing flood was registered. However, in all events, samples were immediately connected to the first wave and sometimes included the peak of the flood (Fig. 2 to 4).
FIG. 2.
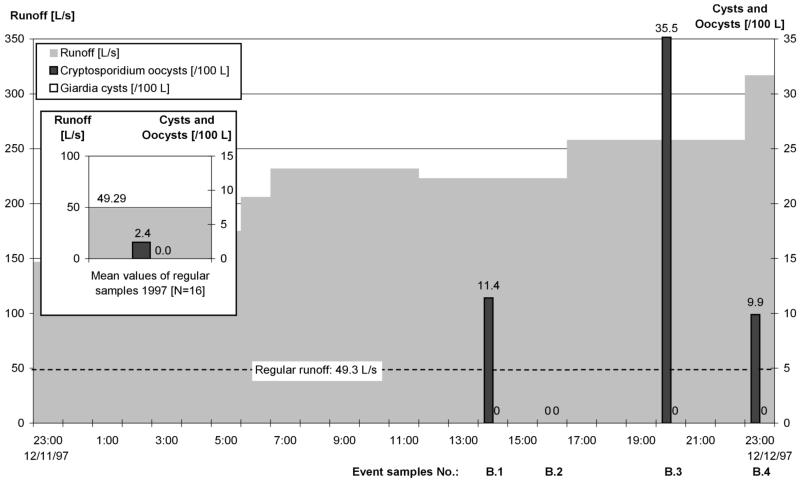
Parasite concentrations at the Nauholzbach gauge during ES series B (12 December 1997).
FIG. 4.
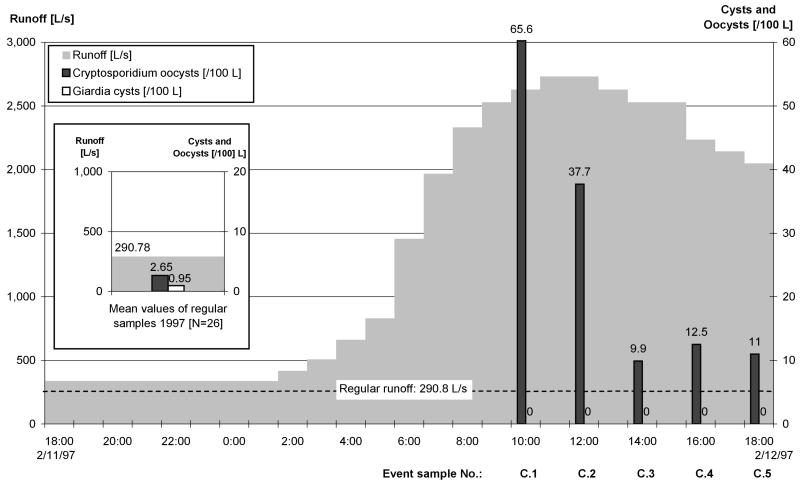
Parasite concentrations at the Wahnbach gauge during ES series G (9 October 1997).
(i) Physical and chemical parameters.
Most of the parameters (pH and nitrate, nitrite, ammonia, total phosphorus, and borate concentrations) remained relatively constant during an extreme rainfall and runoff event. Except for turbidity, the ranges of values remained narrow. At the Nauholzbach, the turbidity in ES series A was highest at the beginning of the sampling period, and the turbidity in ES series B was highest at the end of the sampling period. At the Kall, the turbidity was highest at the beginning of the ES series C sampling period and in the middle of the ES series D sampling period. At the Wahnbach, turbidity decreased in ES series H, which correlated with the outgoing flood. Therefore, in all ES series the highest turbidity values were recorded temporally close to the peak of the flood.
(ii) Bacteriological parameters.
At the Nauholzbach the values for CC 20°C, CC 37°C, E. coli, coliforms, and fecal streptococci increased slightly but continuously in ES series A. C. perfringens was not detected. There was no visible trend at all in ES series B.
At the Kall, the values for all bacteriological parameters examined remained nearly constant in ES series C. In ES series D, the maximum values for CC 20°C, E. coli, and coliforms were reached close to the peak, but there was no uniformity or meaningful trend.
At the Wahnbach, the variance was very low. However, for ES series H the lowest values for most of the parameters occurred at the beginning, immediately after the peak was passed.
(iii) Parasitological parameters.
At the Nauholzbach, the concentration of Cryptosporidium oocysts increased continuously in ES series A and reached the maximum value when runoff became greater again. During ES series B, the maximum concentration occurred in the rising flood (Fig. 2). Giardia cysts were not recovered.
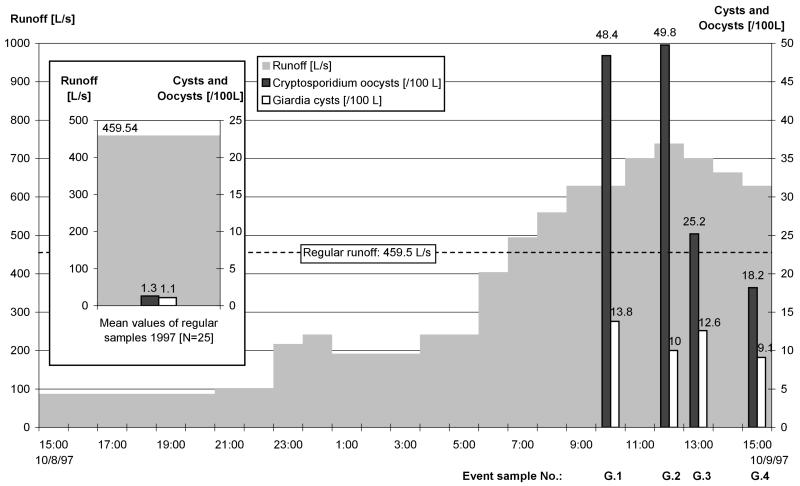
At the Kall, the concentration of Cryptosporidium oocysts decreased in ES series C with the outgoing flood, and Giardia cysts were not found (Fig. 3). In ES series D, the Cryptosporidium concentration decreased while the peak of the wave passed. Giardia cysts were detected discontinuously; the highest concentration occurred at the peak of the flood.
FIG. 3.
Parasite concentrations at the Kall gauge during ES series C (12 February 1997).
At the Wahnbach, the highest Cryptosporidium concentrations in ES series G and H occurred close to the peak of the flood, and the concentrations decreased rapidly thereafter. Giardia cysts were recovered discontinuously without any trend (Fig. 4). In ES series I, the concentrations of Cryptosporidium oocysts and Giardia cysts decreased with the rising flood; the maximum concentrations were found in the first ES collected in this ES series.
Giardia cysts were regularly detected only at the Wahnbach, whereas Cryptosporidium oocysts were recovered at each creek. Most maximum values occurred immediately before the peak of the flood was reached.
Contributions of heavy rainfall and runoff events to the total loads in drinking water reservoirs.
The contributions of heavy rainfall and runoff events to the total loads in drinking water reservoirs are shown in Table 6.
TABLE 6.
Calculated contributions of an assumed average runoff event to the annual loads (1997) of the creeks investigated within 12 h
| Location | Parameter | Assumed annual loada | Load of a 12-h eventb | Contribution of a 12-h event to the annual load (%)c | Estimated value/ expected valued | Estimated value/corrected expected valuee |
|---|---|---|---|---|---|---|
| Nauholzbachf | Runoff (liters) | 1.56×109 | 1.20×107 | 0.77 | 5.64 | 1.00 |
| Nitrate (tons) | 10.63 | 0.14 | 1.29 | 9.44 | 1.67 | |
| Nitrite (kg) | 1.94 | 0.00 | 0.00 | 0.00 | 0.00 | |
| Ammonia (kg) | 0.00 | 0.00 | ||||
| Total phosphorus (kg) | 9.72 | 1.02 | 10.50 | 76.73 | 13.60 | |
| CC 20°C (CFU) | 6.91×1014 | 4.18×1013 | 6.05 | 44.17 | 7.83 | |
| CC 37°C (CFU) | 1.08×1014 | 3.93×1012 | 3.63 | 26.55 | 4.71 | |
| E. coli (CFU) | 3.42×1011 | 2.28×1010 | 6.67 | 48.73 | 8.64 | |
| Coliforms (CFU) | 1.73×1013 | 1.47×1012 | 8.49 | 62.01 | 10.99 | |
| Fecal streptococci (CFU) | 1.17×1011 | 1.56×1010 | 13.39 | 97.80 | 17.33 | |
| C. perfringens (CFU) | 2.33×1010 | 9.85×109 | 42.22 | 308.44 | 54.67 | |
| Giardia cysts | 0 | 0 | ||||
| Cryptosporidium oocysts | 3.73×107 | 1.37×106 | 3.67 | 26.80 | 4.75 | |
| Kallg | Runoff (liters) | 9.18×109 | 5.52×107 | 0.60 | 4.39 | 1.00 |
| Nitrate (tons) | 127.94 | 0.58 | 0.46 | 3.33 | 0.76 | |
| Nitrite (kg) | 127.05 | 3.81 | 3.00 | 21.91 | 4.99 | |
| Ammonia (kg) | 321.16 | 13.19 | 4.11 | 29.99 | 6.83 | |
| Total phosphorus (kg) | 119.99 | 4.46 | 3.72 | 27.17 | 6.19 | |
| CC 20°C (CFU) | 5.46×1015 | 2.79×1015 | 51.02 | 372.67 | 84.87 | |
| CC 37°C (CFU) | 1.70×1015 | 3.06×1014 | 18.03 | 131.73 | 30.00 | |
| E. coli (CFU) | 1.22×1013 | 7.17×1011 | 5.88 | 42.92 | 9.77 | |
| Coliforms (CFU) | 1.73×1014 | 1.09×1013 | 6.33 | 46.24 | 10.53 | |
| Fecal streptococci (CFU) | 4.96×1012 | 2.98×1011 | 6.01 | 43.91 | 10.00 | |
| C. perfringens (CFU) | 2.06×1012 | 1.38×1011 | 6.68 | 48.79 | 11.11 | |
| Giardia cysts | 8.72×107 | 0 | 0.00 | 0.00 | 0.00 | |
| Cryptosporidium oocysts | 2.43×108 | 7.89×108 | 3.24 | 23.69 | 5.40 | |
| Wahnbachh | Runoff (liters) | 1.45×1010 | 4.49×107 | 0.31 | 2.26 | 1.00 |
| Nitrate (tons) | 249.72 | 0.47 | 0.19 | 1.37 | 0.61 | |
| Nitrite (kg) | 1,450.16 | 4.05 | 0.28 | 2.04 | 0.90 | |
| Ammonia (kg) | 2,175.25 | 15.15 | 0.70 | 5.09 | 2.25 | |
| Total phosphorus (kg) | 2,233.25 | 11.17 | 0.50 | 3.65 | 1.61 | |
| CC 20°C (CFU) | 1.61×1016 | 1.68×1015 | 10.44 | 76.29 | 33.69 | |
| CC 37°C (CFU) | 4.81×1015 | 3.60×1014 | 7.48 | 54.63 | 24.13 | |
| E. coli (CFU) | 7.11×1013 | 6.16×1012 | 8.67 | 63.30 | 27.96 | |
| Coliforms (CFU) | 5.51×1014 | 3.96×1013 | 7.18 | 52.43 | 23.16 | |
| Fecal streptococci (CFU) | 1.89×1013 | 6.11×1012 | 32.43 | 236.86 | 104.62 | |
| C. perfringens (CFU) | 1.89×1013 | 1.93×1011 | 1.03 | 7.49 | 3.31 | |
| Giardia cysts | 1.60×108 | 5.57×106 | 3.49 | 25.52 | 11.27 | |
| Cryptosporidium oocysts | 1.89×108 | 7.66×106 | 4.07 | 29.69 | 13.12 |
Estimate for the average load of RS.
Estimate for the average load of ES.
Assumed value for an annual load without events (expected value, 0.137%).
Assumed value for regular runoff.
Corrected value considering the increased runoff.
For Nauholzbach 16 RS and 7 ES were used.
For Kall 26 RS and 11 ES were used.
For Wahnbach 25 RS and 14 ES were used.
At the Nauholzbach, within 12 h under assumed average event conditions (n = 7), 0.77% of the theoretical annual runoff (calculated for a year without any runoff events) drained into the reservoir. The additional nitrate load was very small, whereas the share of total phosphorus was 10.5% (13.6 times the EV12hCorr). Some of the additional bacterial load was much higher (42.2% for clostridia [54.7 times the EV12hCorr]). The Cryptosporidium load also exceeded the expected value (3.7% [4.75 times the EV12hCorr]).
At the Kall, within 12 h under assumed average event conditions (n = 11), 0.6% of the theoretical annual runoff drained into the reservoir. Except for nitrate and Giardia cysts, the values for the parameters were many times the expected values. Dramatic excesses were found for CC 20°C and CC 37°C (51 and 18% [84.9 and 30 times the EV12hCorr, respectively).
At the Wahnbach, within 12 h under assumed average event conditions (n = 14), 0.31% of the theoretical annual runoff drained into the reservoir. While the values for chemical parameters were only slightly higher or even lower (nitrate, nitrite) than the assumed values, the bacterial and parasite loads were dramatically higher (bacteria, 20 to 100 times the EV12hCorr; parasites, >10 times the EV12hCorr).
These results demonstrate that both absolutely and when values are corrected for runoff conditions, considerable additional loads are transported to drinking water reservoirs during heavy rainfall and runoff events.
DISCUSSION
The RS bacteriological analysis of the Kall and Wahnbach areas resulted in similar concentrations of fecal indicator bacteria, while the lowest level of bacterial contamination was seen at the Nauholzbach. Cryptosporidium spp. oocysts were frequently detected in all samples from the different tributaries; Giardia cysts, however, were detected less frequently (18). The ES indicated that the values and dynamics of turbidity and the bacterial and parasite loads were heavily influenced by specific hydrological conditions at the sites of the tributaries. The values for microbial parameters, especially the parasite load, increased to the maximum levels during heavy rainfall and runoff events. Even water samples from the Nauholzbach that were not affected by agriculture and settlement contained higher levels of microorganisms when there was precipitation.
The dynamics of the floods during the runoff events corresponded well with drastic increases in turbidity, whereas the changes in the concentrations of chemical parameters were nonspecific and some of these changes were not more than moderate. In most cases the concentrations of bacteriological parameters increased about 1 to 2 log10, with high variances. In the ES series, the dynamics, however, were low, in contrast to concentrations of parasite (oo)cysts, which followed the runoff dynamics. When parasites were detected in RS, the concentrations of the parasites in ES increased considerably.
Statistical analyses showed that there were significant differences between RS and ES which corresponded well with site-specific environmental factors. At the Nauholzbach with its forests, significant increases in the nitrate and microorganism loads indicated that there was erosion and resuspension of river bottom and drain sediment, whereas the parasite load did not reach the levels in the other tributaries despite the fact that it increased. At the Kall, increases in the values for chemical parameters (except nitrate concentration) confirmed that water drained from pasture land and drainage systems were activated. The relatively small amount of Giardia cysts was assumed to result from processed sewage piped out of the Kall catchment area. At the Wahnbach, however, heavy loads of bacteria and both parasites reflected the influence of combined sewage overflows and discharges from sewage treatment plants on the creek.
Only moderate correlations between parasites and other parameters, such as fecal microorganisms, especially C. perfringens (1, 6, 9, 27, 28), turbidity (1, 9), or rainfall and runoff, which increased the concentrations of several feces-borne microorganisms (1, 9, 20), have been reported. Many studies did not detect any statistically significant correlations between parasites and other microbial, chemical, and hydrological parameters (4, 16, 29, 31). No significant correlation was found between Cryptosporidium oocyst concentrations and rainfall or river flow at 10 sites in the United Kingdom (3). Hsu et al. investigated small water systems in Taiwan for correlations with altitude and water quality parameters but found significant correlations only between Giardia and Cryptosporidium concentrations (16). Thurman et al. analyzed reservoirs and creeks at different sites in Australia for interactions among land use, rainfall, and water quality parameters but found no significance even though parasite and E. coli concentrations peaked after heavy rainfall (31).
Relationships between meteorological parameters and microbial loads of fecal origin have been described. The concentrations of E. coli, coliform bacteria, and enterococci, as well as turbidity, increased after rainfall (1, 9, 10, 28, 31). The concentrations of Cryptosporidium oocysts were found to be lower during dry periods, such as summer (14, 28). Additional loading from non-point-source runoff (5, 7, 10), as well as from river bottom resuspension, was determined to be responsible for the increases (6, 9, 13, 23, 25). As most of the Giardia cysts observed had a nonviable morphology, resuspension of stream bottom sediment or suspension of soil and aged fecal material was assumed to be the major contributor to rainfall-induced increases in parasite concentrations (1, 31). However, because of combined sewage overflows and reduced treatment of discharged wastewater during extreme runoff situations, sewage plants should not be excluded as point sources of parasite contamination (1, 5, 9, 14, 20, 21, 29).
It has been argued that the lack of significant associations between different environmental variables and the parasite loads detected in previous studies was due to inadequate study design; the parasite assay may have been inappropriate, the number of parasite samples may have been too limited, the sampling scheme may not have been designed to detect correlations, or extreme rainfall situations may not have been included (1). However, the intensity of correlations could also have been due to site-specific environmental factors or differences in parasite source contributions (5).
The design of our investigation was geared to the additional bacterial and parasite loads that occur during flood events and therefore took previous experience into account, as described below.
Relationships between concentrations of Cryptosporidium oocysts and Giardia cysts have been investigated in several studies. It has been demonstrated that Cryptosporidium oocysts occur almost ubiquitously at concentrations that correlate strongly with dairy farming and densities of fallow deer in the catchment area. In contrast, the occurrence of Giardia cysts is principally associated with the presence of sewage and beavers (1, 5, 14, 17, 20, 26, 28, 29). For this reason the geoecological situations in the catchment areas of the three tributaries were subjected to a small-scale systematic analysis using geographical information systems (18, 19). This geoecological characterization revealed that no area is entirely unaffected by human activity and that each catchment area of the surface reservoirs is differentiated by environmental conditions.
Relatively small but typical creeks that are used as sources for drinking water production in central Europe were selected because of their high sensitivity to changing conditions in the catchment area. Our results for small tributaries showed that there were significant relationships between parasite and bacterial loads and turbidity, as well as substantial loads added to the total annual loads during heavy rainfall and runoff events, in contrast to results obtained for some bigger rivers (1, 14, 29).
For event sampling, exact criteria were defined beforehand to allow specific sampling instead of continuous but nonspecific monitoring of water systems (1).
Several runoff events were studied by using close-mesh ES series to obtain information about runoff dynamics.
In addition to ES, RS were used for comparisons under identical sampling conditions.
To eliminate seasonal effects (14, 28), RS were collected during a 1-year period. Preparedness to take ES was maintained during the same period.
The numbers of ES and comparative RS were large enough so that we could perform separate analyses of rivers with different site characteristics (3, 31).
Floods make extremely large contributions to the bacterial and parasite loads of drinking water reservoirs. So far, this has been taken into account only minimally, particularly when the annual microbial load is considered. Accordingly, inclusion of ES in assessments of pressures on the quality of surface water used for drinking water production is imperative to get an impression of the watershed-specific potential microbial loads of tributaries. In this way, a realistic microbial risk assessment can be made to estimate maximum loads in raw water for drinking water treatment in case of a short circuit in a reservoir. To stay on the safe side, if treatment procedures do not manage maximum load situations, water authorities should not take raw source water during high-water situations or they should use facilities for bypassing reservoirs during flood events (20). Finally, national guidelines and international recommendations (e.g., World Health Organization guidelines) ought to integrate heavy rainfall and runoff events into the risk assessment process.
If there is a basic parasite load in watercourses, the concentrations of parasites increase to extreme values during flood situations. Parasite loads correspond well with runoff but cannot be adequately indicated by chemophysical or bacteriological parameters because turbidity is nonspecific and because fecal indicators do not necessarily correlate well with the occurrence of pathogens such as parasites. For this reason it has been concluded that reliance on the coliform group creates serious problems in measuring environmental quality and in assessing risks for public health (9). Consequently, regular examination of surface water used for drinking water production should include parasitological tests for risk characterization.
Natural conditions, as well as human activities, in catchment areas of surface reservoirs significantly affect the quality and safety of running and stored water (4, 5, 20, 26). This study shows that for every situation at a watercourse an individual analysis has to be carried out, taking into account geoecological conditions in catchment areas as well as variability in precipitation and runoff. It is important to evaluate systematically the environmental conditions of the catchment areas and their roles in microbial contamination of surface water, in addition to routine analytical monitoring of chemical and microbial parameters of water samples.
Acknowledgments
We are grateful to the Department of Environment, North Rhine-Westphalia, and to the Drinking Water Reservoirs Association (Arbeitsgemeinschaft Trinkwassertalsperren e.V.), which supported this work through a research grant.
In addition, we thank the personnel of the reservoir operating authorities (WdKA, WTV, WVS), who made the event sampling possible through their commitment.
REFERENCES
- 1.Atherholt, T. B., M. W. LeChevallier, W. D. Norton, and J. S. Rosen. 1998. Effect of rainfall on Giardia and crypto. J. Am. Water Works Assoc. 90:66-80. [Google Scholar]
- 2.Bundesverband der Deutschen Gas- und Wasserwirtschaft e.V. (ed.). 2001. Wasserstatistik 1999/ 2000. VGW, Bonn, Germany.
- 3.Carrington, E. G., and D. G. Miller. 1993. The occurrence and origins of Cryptosporidium oocysts in source waters. Water Supply 11:91. [Google Scholar]
- 4.Chauret, C., N. Armstrong, J. Fisher, R. Sharma, S. Springthorpe, and S. Sattar. 1995. Correlating Cryptosporidium and Giardia with microbial indicators. J. Am. Water Works Assoc. 87:76-84. [Google Scholar]
- 5.Crockett, C. S., and C. N. Haas. 1997. Understanding protozoa in your watershed. J. Am. Water Works Assoc. 89:62-73. [Google Scholar]
- 6.Davies, C. M., J. A. H. Long, M. Donald, and N. J. Ashbolt. 1995. Survival of fecal microorganisms in marine and freshwater sediments. Appl. Environ. Microbiol. 61:1888-1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis, E. M., D. M. Casserly, and J. D. Moore. 1977. Bacterial relationships in stormwaters. Water Resour. Bull. 13:895-905. [Google Scholar]
- 8.Exner, M., and G. J. Tuschewitzki. 1993. Aktuelle hygienisch-mikrobiologische Aspekte der Trinkwasserhygiene. Forum Staedte-Hyg. 45:57-63. [Google Scholar]
- 9.Ferguson, C. M., B. G. Coote, N. J. Ashbolt, and I. M. Stevenson. 1996. Relationships between indicators, pathogens and water quality in an estuarine system. Water Res. 30:2045-2054. [Google Scholar]
- 10.Geldreich, E. E., C. C. Best, B. A. Kenner, and D. J. Van Donsel. 1968. The bacteriological aspects of stormwater pollution. J. Water Pollut. Control Fed. 40:1816-1872. [PubMed] [Google Scholar]
- 11.German Ministry of Health. 1984. Verordnung über natürliches Mineralwasser, Quellwasser und Tafelwasser (Mineral- und Tafelwasser-Verordnung; MTVO) vom 01 August 1984. BGBl. I:1036.
- 12.German Ministry of Health. 1990. Verordnung über Trinkwasser und über Lebensmittelbetriebe (Trinkwasserverordnung-TrinkwV) vom 12 Dezember 1990. BGBl. I:2613.
- 13.Grimes, D. J. 1975. Release of sediment-bound fecal coliforms by dredging. Appl. Microbiol. 29:109-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hansen, J. S., and J. E. Ongerth. 1991. Effects of time and watershed characteristics on the concentration of Cryptosporidium oocysts in river water. Appl. Environ. Microbiol. 57:2790-2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Her Majesty's Stationery Office. 1989. Isolation and identification of Giardia cysts, Cryptosporidium oocysts and free living pathogenic amoebae in water etc. Her Majesty's Stationery Office, London, United Kingdom.
- 16.Hsu, B.-M., C. Huang, and C. L.-L. Hsu. 2001. Analysis for Giardia cysts and Cryptosporidium oocysts in water samples from small water systems in Taiwan. Parasitol. Res. 87:163-168. [DOI] [PubMed] [Google Scholar]
- 17.Karanis, P., and H. M. Seitz. 1996. Vorkommen und Verbreitung von Giardia und Cryptosporidium im Roh- und Trinkwasser von Oberflächenwasserwerken. Gas- Wasserfach Wasser-Abwasser 137:94-100. [Google Scholar]
- 18.Kistemann, T., F. Dangendorf, C. Koch, R. Fischeder, and M. Exner. 1998. Mikrobielle Belastung von Trinkwassertalsperren-Zuläufen in Abhängigkeit vom Einzugsgebiet. Gas- Wasserfach Wasser-Abwasser 139:17-22. [Google Scholar]
- 19.Kistemann, T., F. Dangendorf, and M. Exner. 2001. A geographical information system (GIS) as a tool for microbial risk assessment in catchment areas of drinking water reservoirs. Int. J. Hyg. Environ. Health 203:225-233. [DOI] [PubMed] [Google Scholar]
- 20.LeChevallier, M. W., W. D. Norton, and R. G. Lee. 1991. Occurrence of Giardia and Cryptosporidium spp. in surface water supplies. Appl. Environ. Microbiol. 57:2610-2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.LeChevallier, M. W., and W. D. Norton. 1995. Giardia and Cryptosporidium in raw and finished water. J. Am. Water Works Assoc. 87:54-68. [Google Scholar]
- 22.LeChevallier, M. W., W. D. Norton, and T. B. Atherholt. 1997. Protozoa in open reservoirs. J. Am. Water Works Assoc. 89:84-96. [Google Scholar]
- 23.Marino, R. P., and J. J. Gannon. 1991. Survival of fecal coliforms and fecal streptococci in storm drain sediment. Water Res. 25:1089-1098. [Google Scholar]
- 24.McPherson, G. 2001. Applying and interpreting statistics: a comprehensive guide, 2nd ed. Springer, New York, N.Y.
- 25.Medema, G. J., M. Bahar, and F. M. Schets. 1997. Survival of Cryptosporidium parvum, Escherichia coli, faecal enterococci and Clostridium perfringens in river water: influence of temperature and autochthonous microorganisms. Water Sci. Technol. 35:249-252. [Google Scholar]
- 26.Ong, C., W. Moorehead, A. Ross, and J. Isaac-Renton. 1996. Studies of Giardia spp. and Cryptosporidium spp. in two adjacent watersheds. Appl. Environ. Microbiol. 62:2798-2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Payment, P., and E. Franco. 1993. Clostridium perfringens and somatic coliphages as indicators of the efficiency of drinking water treatment for viruses and protozoan cysts. Appl. Environ. Microbiol. 59:2418-2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Payment, P., A. Berte, M. Prévost, B. Ménard, and B. Barbeau. 2000. Occurrence of pathogenic microorganisms in the Saint Laurence River (Canada) and comparison of health risks for populations using it as their source of drinking water. Can. J. Microbiol. 46:565-576. [PubMed] [Google Scholar]
- 29.States, S., K. Stadterman, L. Ammon, P. Vogel, J. Baldizar, D. Wright, L. Conley, and J. Sykora. 1997. Protozoa in river water: sources, occurrence, and treatment. J. Am. Water Works Assoc. 89:74-83. [Google Scholar]
- 30.The European Council. 1976. Council directive 76/160/EEC of 8 December 1975 concerning the quality of bathing water. Official Journal L 031:1-7. [Google Scholar]
- 31.Thurman, R., B. Faulkner, D. Veal, G. Cramer, and M. Meiklejohn. 1998. Water quality in rural Australia. J. Appl. Microbiol. 84:619-626. [DOI] [PubMed] [Google Scholar]
- 32.Wasserchemische Gesellschaft and DIN e.V. (ed.). 2001. Deutsche Einheitsverfahren zur Wasser-, Abwasser- und Schlammuntersuchung. Wiley-VCH Verlag, Weinheim, Germany.
- 33.World Health Organization. 1993. Guidelines for drinking-water quality, vol. 1. Recommendations, 2nd ed. World Health Organization, Geneva, Switzerland.