Abstract
Rhodococcus rhodochrous S-2 produces extracellular polysaccharides (S-2 EPS) containing d-glucose, d-galactose, d-mannose, d-glucuronic acid, and lipids, which is important to the tolerance of this strain to an aromatic fraction of (AF) Arabian light crude oil (N. Iwabuchi, N. Sunairi, H. Anzai, M. Nakajima, and S. Harayama, Appl. Environ. Microbiol. 66:5073-5077, 2000). In the present study, we examined the effects of S-2 EPS on the growth of indigenous marine bacteria on AF. Indigenous bacteria did not grow significantly in seawater containing AF even when nitrogen, phosphorus, and iron nutrients were supplemented. The addition of S-2 EPS to seawater containing nutrients and AF resulted in the emulsification of AF, promotion of the growth of indigenous bacteria, and enhancement of the degradation of AF by the bacteria. PCR-denaturing gradient gel electrophoresis analyses show that addition of S-2 EPS to the seawater containing nutrients and AF changed the composition of the bacterial populations in the seawater and that bacteria closely related to the genus Cycloclasticus became the major population. These results suggest that Cycloclasticus was responsible for the degradation of hydrocarbons in AF. The effects of 15 synthetic surfactants on the degradation of AF by indigenous marine bacteria were also examined, but enhancement of the degradation of AF was not significant. S-2 EPS was hence the most effective of the surfactants tested in promoting the biodegradation of AF and may thus be an attractive agent to use in the bioremediation of oil-contaminated marine environments.
Petroleum is one of the major pollutants of marine environments, a vast amount of petroleum hydrocarbons discharged from industries or from accidental oil spills being continuously released into the ocean. These compounds subsequently undergo modification by either physicochemical or biological processes. Microbial activities allow the conversion of some petroleum components into CO2 and H2O, and microbial transformation is considered a major route for the complete degradation of petroleum components (15). In the marine environment, the growth and activities of hydrocarbon-degrading microorganisms are limited by available nitrogen and phosphorus nutrients, and the addition of nitrogen and phosphorus fertilizers to an oil-contaminated marine environment is required to stimulate the biodegradation of spilled oil. This notion has been applied to the removal of spilled oil in marine environments since the accident of the Exxon-Valdez in Alaska in 1989 (1, 15). However, even in the presence of sufficient amounts of nitrogen and phosphorus nutrients, only half of the petroleum constituents could be biodegraded in a period of from one to several months, while the other half was resistant to biodegradation (8, 12, 14). To expand the possibilities of bioremediation to cope with future oil spills, techniques to stimulate marine bacteria that are capable of degrading the resistant constituents of petroleum are necessary.
Rhodococci are a group of bacteria exhibiting a diverse range of metabolic activities. Some of them have the ability to degrade a variety of organic compounds, including man-made xenobiotics such as polychlorinated biphenyls, while others are capable of degrading numerous aliphatic and aromatic hydrocarbons (2, 6, 17, 20). We have previously reported a close relationship between mucoid colony morphology and the ability to grow on crude oil in various Rhodococcus strains. Mucoid strains but not rough strains of rhodococci exhibited resistance to petroleum hydrocarbons and utilized the hydrocarbons as sources of carbon and energy. Mucoid strains of Rhodococcus rhodochrous S-2 produce an extracellular polysaccharide (EPS) of several million daltons in size, which consists of d-glucose, d-galactose, d-mannose, d-glucuronic acid, stearic acid, palmitic acid, and oleic acid (M. Urai et al., unpublished data). In the presence of the extracellular polysaccharides produced by R. rhodochrous S-2 (S-2 EPS), the rough strains of rhodococci acquired resistance to the hydrocarbons and then grew on them (10). These data indicate that S-2 EPS played an important role in the tolerance of Rhodococcus strains to the hydrocarbons.
In the present study, the effects of S-2 EPS on the degradation of crude-oil hydrocarbons in seawater by natural microbial populations were examined. The addition of S-2 EPS stimulated the growth of certain bacteria and enhanced crude-oil biodegradation.
MATERIALS AND METHODS
Seawater, media, cultures, and bacterial growth.
Seawater was collected from Heita Bay at a depth of 1 m. To prepare natural seawater medium (NSW), 800 ml of natural seawater without filtration was mixed with 200 ml of an autoclaved solution containing nitrogen, phosphorus, and iron nutrients (5.0 g of NH4NO3, 0.1 g of FeC6H5O7·nH2O, 0.1 g of K2HPO4 in 200 ml of Milli-Q water). The pH of the medium was adjusted to pH 7.8. Microorganisms inhabiting the natural seawater were used as the inoculum. The aromatic fraction (AF) derived from Arabian light crude oil (10) was added to the NSW at a final concentration of 10 mg/ml, this medium being hereafter called NSW-A.
When required, S-2 EPS prepared by the procedure described previously (10) or a surfactant (Table 1; Detergent Starter Kit II, Dojin Chemicals) was added to the medium at a final concentration of 100 μg/ml prior to adding AF. Then 10 ml of cultures thus prepared was incubated at 30°C in tubes (2.4 cm in diameter and 30 cm long) with shaking (110 rpm) for 15 days. The bacterial growth of the cultures was followed by determining the CFU number after incubation at 30°C for 8 days on Bacto Marine Agar 2216 plates (Difco) or on 1/5-strength Bacto Marine Agar 2216 plates diluted with filtered seawater; these plates are hereafter called MA and 1/5 MA, respectively. The bacterial growth in each culture was also followed by determining the total direct count by fluorescence microscopy after staining with 4′,6′-diamidino-2-phenylindole (DAPI), as described previously (19). When required, samples were diluted by filter-sterilized seawater prior to the DAPI staining.
TABLE 1.
Effects of synthetic surfactants on emulsification and degradation of AFa
| Surfactantb | Emulsification | Oil wt (mg) |
|---|---|---|
| S-2 EPS | +++ | 52.2 ± 6.7 |
| MEGA-9 | − | 76.2 ± 9.2 |
| Digitonin | +++ | 82.3 ± 12.8 |
| CHAPS | +++ | 85.4 ± 1.1 |
| Sucrose monolaurate | + | 89.1 ± 1.4 |
| n-Octyl-β-d-glucoside | − | 89.1 ± 17.7 |
| Sodium dodecyl sulfate | ++ | 90.3 ± 8.3 |
| BIGCHAP | ++ | 92.4 ± 20.2 |
| MEGA-8 | − | 94.1 ± 13 |
| Deoxy-BIGCHAP | + | 95.7 ± 11.7 |
| n-Dodecyl-β-d-maltoside | +++ | 96.3 ± 1.8 |
| Sucrose monocaprate | ++ | 97 ± 12.2 |
| CHAPSO | − | 101.3 ± 16.3 |
| Sodium cholate | − | 101.4 ± 2.8 |
| n-Heptyl-β-d-thioglucoside | − | 118.4 ± 24.7 |
| No surfactant | − | 99.3 ± 7.1 |
| No bacteria | − | 99.2 ± 9.1 |
A nonsterilized NSW medium supplemented with AF (10 mg/ml) and a surfactant (100 μg/ml) was incubated at 30°C with shaking for 15 days. +++, ++, and + indicate very intense, intense, and weak emulsification, respectively, while − indicates no emulsification after incubation for 15 days. The emulsification occurred in 1 day in cultures supplemented with S-2 EPS and digitonin, while approximately 10 days was required for emulsification in other cultures. Oil was extracted from the culture, and its weight was determined.
S-2 EPS, extracellular polysaccharide from R. rhodochrous S-2; MEGA-9, n-nonanoyl-N-methylglucamide; CHAPS, 3-[(3-cholamidotrophyl)-dimethylammonio]-1-propanesulfonate hydrate; BIGCHAP, N,N-bis(3-d-gluconamidopropyl)cholamide; MEGA-8, n-octanoyl-N-methylglucamide; deoxy-BIGCHAP, N,N-bis(3-d-gluconamidopropyl)deoxycholamide; CHAPSO, 3-[(3-cholamidopropyl)dimethylammonio]-2-hydroxypropanesulfonic acid; no bacteria, filter-sterilized seawater was used to prepare the medium.
Extraction and quantification of AF.
Oil was extracted from each culture by one-half volume of dichloromethane, the extraction procedure being repeated until the dichloromethane layer had become clear. The extract was then dried in vacuo, and the residual material was dissolved in chloroform. Each sample was subsequently analyzed by gas chromatography-mass spectrometry (GC-MS). The GC-MS analysis was performed with a QP-2000A instrument (Shimadzu) fitted with a fused silica capillary column (DB-5; 30 m long, 0.25 mm diameter; J&W Scientific). The operating temperature of the flame ionization detector was 300°C and that of the injector was 230°C. The column temperature was set at 50°C for 2 min, increased to 300°C at a rate of 6°C/min, and then kept at 300°C for 15 min. The carrier gas was helium at a flow rate of 1 ml/min, and naphthalene was added to each sample as an internal standard. The GC-MS instrument was operated in the selected ion-monitoring mode.
Total DNA extraction.
Recombinant DNA techniques described by Sambrook et al. (16) were used. Total DNA was extracted from 10 ml of a culture or from fresh seawater by the method described by Watanabe et al. (18) with slight modifications. Briefly, 10 ml of a phenol-chloroform solution was added to a sample, mixed, and transferred into a tube (tube A). The mixture was centrifuged at 10,000 × g for 10 min at 4°C, and the aqueous layer was transferred to a new tube (tube B). Five milliliters of a lysing solution (100 mM Tris-HCl [pH 8.0], 20 mM EDTA [pH 8.0], 0.3 M NaCl, 2% [wt/vol] sodium dodecyl sulfate, and 2% [wt/vol] 2-mercaptoethanol) was added to tube A, and the contents were mixed. The mixture was then centrifuged at 10,000 × g for 10 min at 4°C before the aqueous layer was transferred to tube B. This step was conducted three times. DNA in tube B was precipitated by ethanol and then dissolved in TE (Tris-EDTA) buffer. RNase A was added to the DNA solution at a final concentration of 10 μg/ml, and the DNA solution was incubated for 1 h at 37°C. After the incubation, the DNA solution was extracted by a phenol-chloroform solution, precipitated by ethanol, and dissolved in TE buffer. The DNA solution was used for PCR amplification of the V3 region of bacterial 16S ribosomal DNA (rDNA) (13).
PCR-DGGE analysis.
PCR primers P2 and P3 (containing 40 bp of a GC clamp) (13) were used to amplify the V3 region of bacterial 16S rDNA (corresponding to positions 341 to 534 in the Escherichia coli sequence) under the conditions described previously (19).
Denaturing gradient gel electrophoresis (DGGE) was performed with a D-Code gel system (16 by 16 cm) of 1.0 mm gel thickness (Bio-Rad), which was maintained at a constant temperature of 58°C in 7 liters of 1× TAE (Tris-acetate-EDTA) buffer. The gel contained 10% acrylamide with a urea-formamide gradient of 30 to 70%. Each gel was run at 200 V for 3.5 h, stained with SYBR Gold (FMC Bioproducts) or ethidium bromide for 30 min, and finally photographed.
Extraction of DNA from the acrylamide gel and DNA sequencing.
Intense bands of DGGE were excised and transferred into new microtubes containing 200 μl of TE buffer, and the tubes were gently shaken at 37°C for 16 h. The gel slice was removed, and the DNA fragment was recovered by ethanol precipitation. The DNA fragment was dissolved in 20 μl of TE buffer, and a portion of this DNA solution was applied to a second PCR under the same conditions as those for the first PCR to check the purity of the isolated DGGE band. Furthermore, another second PCR was conducted with primers GC-2 and GC-2P (19). The PCR products were purified with a Gene Clean Kit II (Bio 101). The nucleotide sequence of the PCR products amplified with GC-2 and GC-2P was determined in both orientations with a dye terminator cycle sequencing kit (Perkin-Elmer) and analyzed with an ABI Prism model 377 automatic sequencer (Perkin-Elmer). The search for sequences in DNA databases was performed by using the BLASTN facility of the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/BLAST).
RESULTS
Effects of S-2 EPS on degradation of AF by marine bacteria.
We analyzed the effect of S-2 EPS on the degradation of the AF of Arabian light crude oil by native marine bacteria. When S-2 EPS was added to NSW-A, AF was immediately emulsified, whereas AF either adhered to the inner surface of the culture tubes or formed oil clumps in the liquid when S-2 EPS was not added.
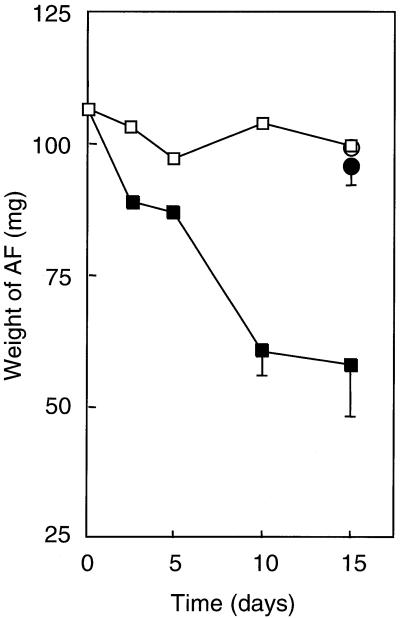
The biodegradation of AF by native bacteria in seawater was gravimetrically examined by determining the amount of AF remaining in a culture. At the start of the determination, the dry weight of the extracted oil was 106.6 ± 5.2 mg. Time course plots demonstrate that the oil weight decreased significantly in the presence of S-2 EPS (Fig. 1). In the absence of S-2 EPS, however, the oil weight after a 15-day cultivation was 99.2 ± 9.1 mg, which is almost the same as that of the control sample incubated in filter-sterilized seawater. These results indicate that the biodegradation of AF by bacteria indigenous to seawater was difficult, except that the addition of S-2 EPS enhanced the biodegradation of AF.
FIG. 1.
Degradation of AF in the presence and absence of S-2 EPS. □, weight of oil extracted from cultures of NSW containing no EPS; ▪, weight of oil extracted from cultures of NSW containing S-2 EPS; ○, weight of oil extracted from filter-sterilized NSW medium; •, weight of oil extracted from filter-sterilized NSW medium containing S-2 EPS. Each value is the average of at least two independent experiments, and the standard deviation is indicated if it is larger than 10% of the mean value.
Degradation of AF constituents by marine bacteria.
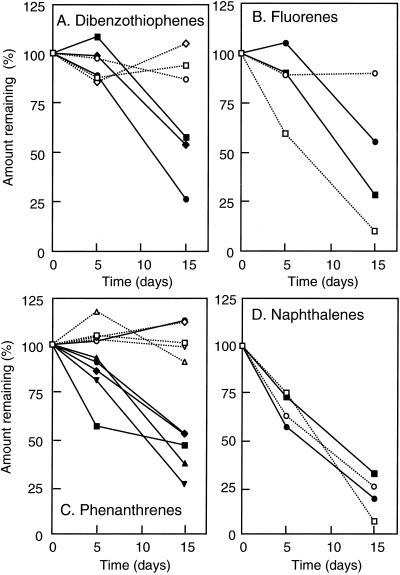
The degradation of some of the constituents of AF, i.e., naphthalene, phenanthrene, fluorene, dibenzothiophene, and their alkyl-substituted derivatives, was next determined by GC-MS, with the results shown in Fig. 2. In the absence of S-2 EPS, C0-fluorene, C3-naphthalene, and C4-naphthalene were degraded, but the degradation of C2-fluorene, phenanthrenes, and dibenzothiophenes was not significant. In the presence of S-2 EPS, the degradation of C0-fluorene, C3-naphthalene, and C4-naphthalene was not affected; however, the degradation of all other polycyclic aromatic hydrocarbons (PAHs) was strongly accelerated in the presence of S-2 EPS.
FIG. 2.
Effects of S-2 EPS on degradation of AF components. (A) Dibenzothiophenes (DBTs): squares, C2-DBT (Cn indicates the carbon number in the side chain; C0 indicates no alkyl substitution); diamonds, C3-DBT; circles, C4-DBT. (B) Fluorenes: squares, C0-fluorene; circles, C2-fluorene. (C) Phenanthrenes: squares, C3-phenanthrene; diamonds, C4-phenanthrene; circles, C5-phenanthrene; triangles, C6-phenanthrene; inverted triangles, C7-phenanthrene. (D) Naphthalenes: squares, C3-naphthalene; circles, C4-naphthalene. Open symbols show the amounts in the absence of S-2 EPS; solid symbols show the amounts in the presence of S-2 EPS. Each value is the average of at least two independent experiments. The standard deviation is not indicated for clarity. It was less than 25% of the average value for all the data at day 15, one exception being that of C7-phenanthrene, which was 60% of the average value.
Effects of S-2 EPS on growth of marine bacteria on AF.
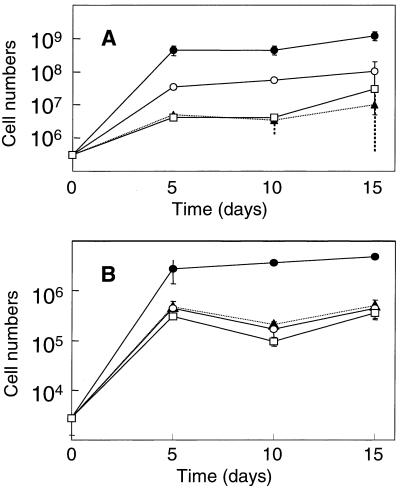
The growth of marine bacteria on AF in the presence or absence of S-2 EPS was then examined. The total bacterial count at day zero was 3.1 (± 0.9) × 105/ml. In NSW containing no AF, the bacterial count increased nearly 10-fold in 5 days and reached a plateau. A very similar growth response was observed when S-2 EPS at a concentration of 100 μg/ml was added to NSW containing no AF. Thus, S-2 EPS alone did not stimulate the growth of bacteria. When AF was supplemented, the bacterial count increased more than 100-fold at day 5, and the addition of S-2 EPS further increased the number to almost 109/ml.
The numbers of viable cells were also determined. The counts of each sample when using MA and 1/5 MA were almost identical: at day zero, for example, they were, respectively, 1.0 (± 1.6) × 104 CFU/ml and 2.9 (± 1.6) × 103 CFU/ml on the MA and 1/5 MA plates. The growth in NSW, that in NSW supplemented with EPS, and that in NSW supplemented with AF were very similar and reached a plateau in 5 days. The addition of S-2 EPS in NSW containing AF increased the growth yield (Fig. 3). We conclude from these results that the addition of S-2 EPS together with minerals enhanced the degradation of some components in AF by marine bacteria, which resulted in their growth.
FIG. 3.
Effects of S-2 EPS on the growth of marine bacteria on AF. (A) DAPI counts. (B) CFU on 1/5 MA. Symbols: ▴, in NSW; □, in NSW containing S-2 EPS; ○, in NSW containing AF; •, in NSW containing AF and S-2 EPS. The standard deviation is also indicated.
PCR-DGGE analysis of the bacterial community structure.
To analyze the effects of adding S-2 EPS on the bacterial community structure in seawater, the V3 region of small-subunit rRNA was amplified by PCR, and the amplified products were analyzed by DGGE. Three independent experiments were carried out, and except for the profile of the day 0 samples (natural seawater), the DGGE profiles were highly reproducible among these three experiments: the patterns of major bands were always identical, while those of minor bands were not necessarily identical.
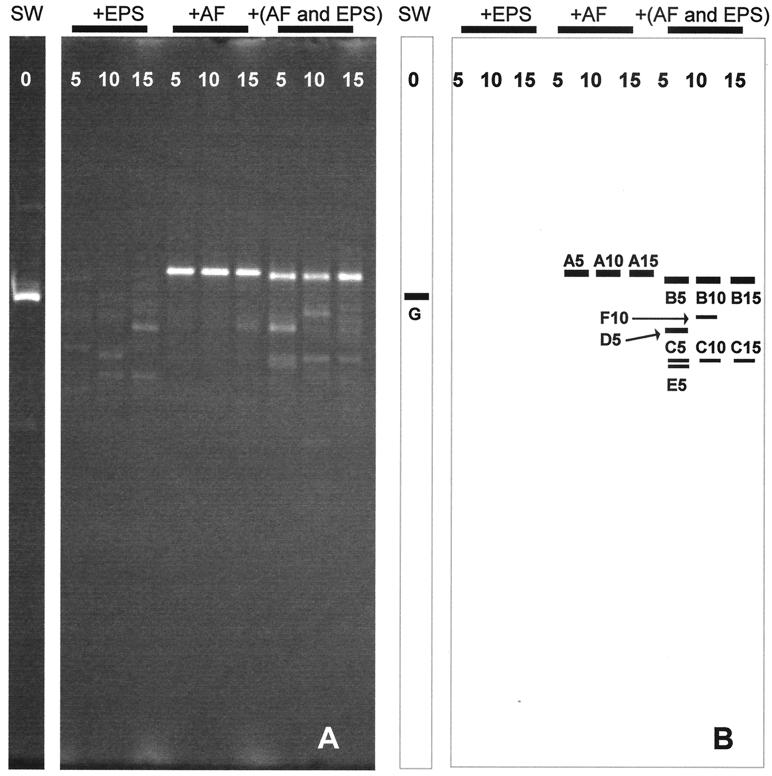
A typical electrophoregram is shown in Fig. 4A, with some of the major bands being excised and sequenced (Fig. 4B). The major DGGE bands with the same mobility, i.e., A5, A10, and A15; B5, B10, and B15; and C5, C10, and C15, were identical in their nucleotide sequences. The sequences in the databases exhibiting the greatest similarity to the sequences of the DGGE bands are shown in Table 2. In this experiment, Sphingomonas paucimobilis was detected as a major population in seawater (lane SW). However, this was a particular result: bacteria belonging to Cytophaga-Flavobacteria and α-Proteobacteria are generally predominant in seawater (11). In the absence of S-2 EPS, major bands (A5, A10, and A15) closely related to Alcanivorax vorkumensis DSM11573 appeared between day 5 and day 15. When NSW-A was incubated in the presence of S-2 EPS, no such Alcanivorax band was apparent, but different bands appeared. Among these bands, B5, B10, and B15 were the most intense at each sampling time, and the database search showed the sequence to be the same as that of Cycloclasticus pugetti PS-1 (Table 2). Minor band C (C5, C10, and C15) was commonly observed during the experimental term, this band being closely related to α-Proteobacteria strain KT0221 (5).
FIG. 4.
Effects of S-2 EPS on the bacterial community structure established in the NSW cultures. (A) DGGE profiles of PCR-amplified partial 16S rDNA fragments. (B) Drawing of the DGGE gel (panel A). The bands used for the sequence analyses are shown. SW, seawater sample at day 0. +EPS, NSW was supplemented with S-2 EPS; +AF, NSW was supplemented with AF; +(AF and EPS), NSW was supplemented with AF and S-2 EPS. Cultures were withdrawn on days 5, 10, and 15 for DNA extraction.
TABLE 2.
16S rDNA sequences most closely related to the major bacterial populations detected by DGGE shown in Fig. 4 and 5
| Figure | Band | Closest sequence | Accession no. | % Identity |
|---|---|---|---|---|
| 4 | A5-15 | Alcanivorax vorkumensis DSM11573 | AF062642 | 100 |
| B5-15 | Cycloclasticus pugetii PS-1 | U12624 | 100 | |
| C5-15 | α-Proteobacteria strain KT0221 | AF235122 | 100 | |
| D5 | Cycloclasticus oligotrophus | AF148215 | 96 | |
| E5 | Uncultured bacteria | AF268243 | 100 | |
| F10 | Marinobacter sp. strain MR-6 | AF264687 | 99 | |
| G | Sphingomonas paucimobilis ATCC 29837 | U37337 | 100 | |
| 5 | 1 | Sphingomonas parapaucimobilis IFO15100 | D13724 | 96 |
| 5B-1 | Sphingomonas sp. strain K101 | AJ009706 | 99 | |
| 5B-2 | Sphingomonas paucimobilis | U20776 | 97 | |
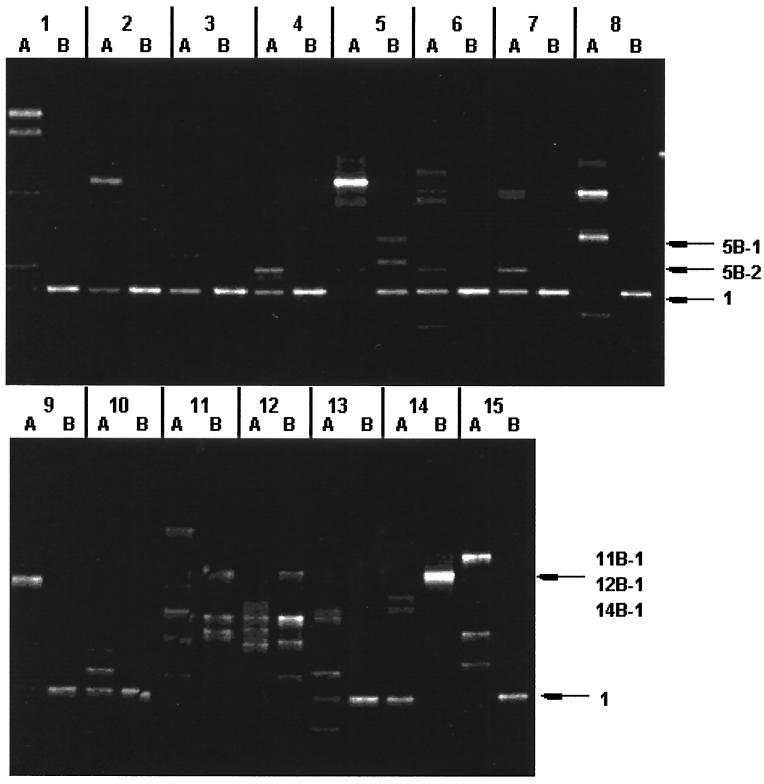
| 11B-1 | Alteromonas distincta | AF043742 | 98 | |
| 12B-1 | Alteromonas distincta | AF043742 | 97 | |
| 14B-1 | Cycloclasticus oligotrophus | AF148215 | 96 |
Effects of synthetic surfactants on degradation of AF by marine bacteria.
We analyzed the effect of synthetic surfactants on the degradation of AF by native marine bacteria (Table 1). The addition of S-2 EPS and digitonin to NSW-A resulted in the emulsification of AF in 1 day, while such other surfactants as 3-[(3-cholamidotrophyl)-dimethylammonio]-1-propanesulfonate hydrate (CHAPS) and n-dodecyl-β-maltoside resulted in the emulsification of AF only after a prolonged (e.g., 10-day) cultivation. In the latter cases, the emulsification may not have resulted from the added surfactants, but from biosurfactants produced in these cultures. The addition of some surfactants, exemplified by nonyl-N-methylglucamide (MEGA-9), did not cause any emulsification of the cultures. Only three surfactants, MEGA-9, digitonin, and CHAPS, significantly stimulated the biodegradation of AF (Table 1). The decrease in oil weight in cultures with these surfactants, however, was much lower than that in the S-2 EPS-amended culture.
The effects of the synthetic surfactants on the structure of the marine bacterial consortium were also examined by PCR-DGGE. Three independent experiments were carried out, a typical electrophoregram being shown in Fig. 5. We were specifically interested in detecting bacteria capable of degrading components of AF in NSW supplemented with a specific synthetic surfactant. Such bacterial populations would become dominant in NSW containing both AF and the specific surfactant. Several bands responding to the addition of AF were detected, and they were related to Sphingomonas, Alteromonas, and Cycloclasticus (Fig. 5, Table 1). However, the results in Fig. 5 were not always reproducible. Neither Sphingomonas nor Alteromonas was detected as major DGGE bands in the second and third experiments. In the second experiment, Cycloclasticus was a major population responding to the addition of AF in the presence of all the synthetic surfactants except sucrose monocaprate and sodium cholate, but this group of bacteria was not detected in the third experiment except in NSW containing AF and digitonin. Thus, the growth of Cycloclasticus in NSW containing both AF and digitonin was reproducibly observed.
FIG. 5.
Bacterial community structure in the presence of a synthetic surfactant. The NSW either containing AF or not was supplemented with a surfactant and cultivated at 30°C. DNA was isolated from each culture after incubation for 15 days. Partial 16S rDNA fragments were PCR amplified from the DNA and separated by DGGE. The bands used for the sequence analyses are shown, and the sequences are listed in Table 2. Lane 1, CHAPS; lane 2, CHAPSO; lane 3, BIGCHAP; lane 4, deoxy-BIGCHAP; lane 5, n-octyl-β-d-glucoside; lane 6, n-heptyl-β-d-thioglucoside; lane 7, n-octyl-β-d-thioglucoside; lane 8, n-dodecyl-β-d-maltoside; lane 9, MEGA-8; lane 10, MEGA-9; lane 11, sucrose monocaprate; lane 12, sucrose monolaurate; lane 13, sodium cholate; lane 14, digitonin; lane 15, sodium dodecyl sulfate. In each lane, A shows the band pattern of cultures grown on the NSW supplemented with a surfactant, while B shows the band pattern of cultures grown on the NSW supplemented with a surfactant and AF (10 mg/ml). See Table 1 for the chemical names of the surfactants.
In Fig. 5, band 1 was detected in many samples, and it was related to Sphingomonas. However, the corresponding band was not detected in the second and third experiments. Alcanivorax, which was a dominant population in the absence of surfactant (Fig. 4), was not detected in the presence of any of the synthetic surfactants.
DISCUSSION
We examined in this study the effects of S-2 EPS on the biodegradation of AF by native marine bacteria and have shown that the addition of S-2 EPS promoted the emulsification of AF in seawater, the growth of bacteria, and the degradation of AF. S-2 EPS certainly enhanced the abilities of native marine bacteria to degrade some components of AF.
Harayama et al. (8) have reported that the Alcanivorax group became predominant in oil-contaminated seawater when nutrients were supplemented. Under such conditions, saturates of crude oil, i.e., alkanes, decreased rapidly, whereas other major components of crude oil, aromatics, resins, and asphaltenes, decreased at much slower rates (3). We tested in this study the biodegradation in seawater of hydrocarbons in AF prepared from Arabian light crude oil. Even using AF from which the saturates have been substantially removed, the Alcanivorax group still became predominant in seawater after the addition of mineral nutrients (Fig. 4, lanes 7 to 9), while the degradation of AF was moderate (Fig. 1). Alcanivorax strains cannot utilize PAHs for their growth (unpublished data), and therefore this organism may use either alkanes contaminating AF and/or alkyl side chains of PAH as carbon and energy sources.
The addition of S-2 EPS together with mineral nutrients rapidly emulsified and biodegraded the AF hydrocarbons. Under such conditions, the Cycloclasticus group became predominant in the microbial community (Fig. 4, lanes 10 to 12), while no band related to the Alcanivorax group could be detected. These data suggest that the bioavailability of hydrocarbons in AF was limited in the absence of S-2 EPS and that only the Alcanivorax group was able to grow on AF to a low extent. However, the addition of S-2 EPS seems to have increased the hydrocarbon availability to some of the indigenous marine bacteria capable of degrading aromatics, e.g., Cycloclasticus (4, 7), and the relative population size of Alcanivorax diminished. S-2 EPS emulsified AF, and the initial emulsification of AF seems to have been essential for the overgrowth of Cycloclasticus, because the predominance of Cycloclasticus was only observed in the presence of two surfactants, S-2 EPS and digitonin, which allowed the emulsification of AF in 1 day. We detected Cycloclasticus as a predominant population grown on AF in the presence of S-2 EPS. However, it is possible that other bacteria that are difficult to lyse escaped detection.
Cycloclasticus strains degrade aromatic hydrocarbons, including naphthalene, methylnaphthalenes, phenanthrene, fluorene, and anthracene (7). We also isolated Cycloclasticus strains and demonstrated that these strains degrade (methyl)naphthalenes, (methyl)dibenzothiophenes, (methyl)phenanthrenes, and (methyl)fluorenes in various petroleum products (Y. Kasai et al., unpublished data). The substrate specificities of Cycloclasticus thus account for the rapid degradation of PAH in AF in cultures supplemented by S-2 EPS.
The data presented here thus suggest that EPS produced by R. rhodochrous S-2 could be useful for the biodegradation of spilled oil in marine environments, and especially for the bioremediation of polyaromatic hydrocarbons that remain in the environment even after a traditional bioremediation treatment. Further studies are necessary on the application of EPS to the bioremedation of oil-polluted marine environments.
Acknowledgments
We thank H. Kasai and Y. Kasai for valuable discussions and encouragement. We also acknowledge the technical assistance provided by M. Nozawa, A. Katsuta, and N. Hayashi.
This study was supported by a grant from the Industrial Science and Technology Frontier Program of the New Energy and Industrial Technology Development Organization (NEDO), by a research grant for assistants and young researchers in Nihon University (research grant for 2001), and by a grant-in-aid for Scientific Research B (10059660) of the Japan Society for the Promotion of Science. Life Science Research Center of Nihon University is supported by the High-Tech Research Center Project of the Ministry of Education, Culture, Sports, Science and Technology of Japan.
REFERENCES
- 1.Bartha, R. 1993. Microbial ecology: fundamentals and applications, 3rd ed. Addison Wesley Longman Publishers, Amsterdam, The Netherlands.
- 2.Bell, K. S., J. C. Philip, D. W. J. Aw, and N. Christofi. 1998. The genus Rhodococcus. J. Appl. Microbiol. 85:195-210. [DOI] [PubMed] [Google Scholar]
- 3.Dutta, T. K., and S. Harayama. 2000. Fate of crude oil by the combination of photooxidation and biodegradation. Environ. Sci. Technol. 34:1500-1505. [Google Scholar]
- 4.Dyksterhouse, S. E., J. P. Gray, R. P. Herwig, J. C. Lara, and J. T. Staley. 1995. Cycloclasticus pugetii gen. nov., sp. nov., an aromatic hydrocarbon-degrading bacterium from marine sediments. Int. J. Syst. Bacteriol. 45:116-123. [DOI] [PubMed] [Google Scholar]
- 5.Eilers, H., J. Pernthaler, F. O. Glockner, and R. Amann. 2000. Culturability and in situ abundance of pelagic bacteria from the North Sea. Appl. Environ. Microbiol. 66:3044-3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finnerty, W. R. 1992. The biology and genetics of the genus Rhodococcus. Annu. Rev. Microbiol. 46:193-218. [DOI] [PubMed]
- 7.Geiselbrecht, A. D., B. P. Hedlund, M. A. Tichi, and J. T. Staley. 1998. Isolation of marine polycyclic aromatic hydrocarbon (PAH)-degrading Cycloclasticus strains from the Gulf of Mexico and comparison of their PAH degradation ability with that of Puget Sound Cycloclasticus strains. Appl. Environ. Microbiol. 64:4703-4710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harayama, S., H. Kishira, Y. Kasai, and K. Shutsubo. 1999. Petroleum biodegradation in marine environments. J. Mol. Microbiol. Biotechnol. 1:63-70. [PubMed] [Google Scholar]
- 9.Henson, J. M., and S. S. Hayasaka. 1982. Effects of the water-soluble fraction of microbiologically or physically altered crude petroleum on the heterotrophic activity of marine bacteria. Mar. Environ. Res. 6:205-214. [Google Scholar]
- 10.Iwabuchi, N., M. Sunairi, H. Anzai, M. Nakajima, and S. Harayama. 2000. Relationships between colony morphotypes and oil tolerance in Rhodococcus rhodochrous. Appl. Environ. Microbiol. 66:5073-5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kasai, Y., H. Kishira, K. Syutsubo, and S. Harayama. 2001. Molecular detection of marine bacterial populations on beaches contaminated by the Nakhodka tanker oil-spill accident. Environ. Microbiol. 3:246-255. [DOI] [PubMed] [Google Scholar]
- 12.Mackay, D., and C. D. MacAuliffe. 1988. Fate of hydrocarbons discharged at sea. Chem. Pollut. 5:1-20. [Google Scholar]
- 13.Muyzer, G., E. C. de Waal, and A. G. Uitterlinden. 1993. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl. Environ. Microbiol. 59:695-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Payne, J. R., and C. R. Phillips. 1985. Photochemistry of petroleum in water. Environ. Sci. Technol. 19:569-579. [DOI] [PubMed] [Google Scholar]
- 15.Prince, R. C. 1993. Petroleum spill bioremediation in marine environments. Crit. Rev. Microbiol. 19:217-242. [DOI] [PubMed] [Google Scholar]
- 16.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 17.Warhurst, A. M., and C. A. Fewson. 1994. Biotransformations catalyzed by the genus Rhodococcus. Crit. Rev. Biotechnol. 14:29-73. [DOI] [PubMed] [Google Scholar]
- 18.Watanabe, K., S. Yamamoto, S. Hino, and S. Harayama. 1998. Population dynamics of phenol-degrading bacteria in activated sludge determined by gyrB-targeted quantitative PCR. Appl. Environ. Microbiol. 64:1203-1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Watanabe, K., M. Teramoto, H. Futamata, and S. Harayama. 1998. Molecular detection, isolation, and physiological characterization of functionally dominant phenol-degrading bacteria in activated sludge. Appl. Environ. Microbiol. 64:4396-4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whyte, L. G., J. Hawari, E. Zhou, L. Bourbonniere, W. E. Inniss, and C. W. Greer. 1998. Biodegradation of variable-chain-length alkanes at low temperatures by a psychrotrophic Rhodococcus sp. Appl. Environ. Microbiol. 64:2578-2584. [DOI] [PMC free article] [PubMed] [Google Scholar]