Abstract
In the present study, we developed a quick, highly specific method for detection of Shigella species by combining immunocapturing of the bacteria and a universal primer PCR. The method drastically enhances test sensitivity, and it can be used not only for identification of Shigella species in the environment but also for rapid detection of other pathogens.
Dysentery caused by Shigella species is one of the most common infectious diseases in developing countries and in travelers to tropical countries (18, 19). One statistic showed that about 50% of Spanish travelers who visited developing countries developed dysentery, and Shigella species were among the main etiological agents (3, 4). In the People's Republic of China, Shigella species are the second leading cause of intestinal infectious diseases. The dysentery bacilli include four Shigella species, S. dysenteriae, S. flexneri, S. sonnei, and S. boydii; S. sonnei, S. dysenteriae, S. flexneri, and S. boydii have 15, 10, 8, and 1 serotypes, respectively. The symptoms of shigellosis are mild or severe depending on the species causing the infection. Therefore, early rapid identification of Shigella species and their serotypes is very important for public health, epidemiological investigations, and clinical treatment.
Traditionally, identification of pathogenic bacteria has been based on phenotypic characteristics and mainly involves analyses of differential metabolic properties and reactions of specific antibodies. Recently, molecular analysis of phylogenetic markers has been recognized as a very useful tool for identification of bacterial genera, species, or subspecies (2, 4, 14, 17). Among these markers, 16S rRNAs are particularly useful because these molecules are present in every living cell and their function is highly conserved. However, an approach based on utilization of universal primer PCR (UPPCR) for conserved regions, such as 16S rRNA genes, can be used to study almost all bacteria (5, 8). The bacteria have to be characterized further by subsequent steps, including restriction fragment length polymorphism analysis, single-strand conformation polymorphism analysis, or sequencing analysis (4, 10, 11, 12). These extra steps make the detection procedure more complex and tedious.
In this paper, we report development of a new technique for rapid and efficient detection and differentiation of dysentery bacilli in environmental sewage. The new method, termed immunocapture UPPCR (iUPPCR), employs UPPCR amplification to detect bacteria captured by specific antibodies coupled to polystyrene 96-well plates. The specificity of coating antibodies distinguishes specific cell types, while the conserved 16S rRNA contributes to the universality of bacterial detection. We believe that this method will have broad application for detection and differentiation of pathogenic organisms in the environment.
The bacteria used in this study included S. dysenteriae serotype 1, S. flexneri serotypes 1a, 2a, 3a, 4, 5, and Y variant, S. sonnei, and S. boydii serotype 1; these organisms were purchased from the Public Health Station of Fujian Province, People's Republic of China. Cultures were grown at 37°C in L-broth medium. Then immune capture and conventional treatment of bacteria were carried out. For immunocapturing procedures, monoclonal antibodies (purchased from Institute of Lanzhou Biological Products, Lanzhou, People's Republic of China) against S. dysenteriae 1, S. flexneri 1a, 2a, 3a, 4, 5, and Y variant, S. sonnei, and S. boydii 1 were separately coupled to polystyrene 96-well plates using 0.05 M carbonic acid buffer (pH 9.6) at 4°C for 18 h. The plates were then washed with phosphate-buffered saline containing 0.05% Tween 20 and incubated with 20-μl portions of bacterial cultures at 37°C for 1 h. Following washing, the wells were incubated with 20 μl of sterile double-distilled H2O and heated at 100°C for 5 min to denature the bacterial DNA templates. For conventional procedures, 20-μl portions of bacterial cultures were separately centrifuged at 3,000 × g for 20 min following one wash with sterile 0.85% NaCl. The pellets were each dissolved in 20 μl of sterile double-distilled H2O and were heated at 100°C for 5 min to denature the bacterial DNA templates. Eighteen microliters of a suspension resulting from the immunocapturing or conventional treatment was used as the template for UPPCR amplification.
Each UPPCR mixture (total volume, 25 μl) consisted of 2.5 μl of 10× PCR buffer, 3 mM MgCl2, each deoxynucleoside triphosphate at a concentration of 200 μM, each primer at a concentration of 250 nM, 1 U of Taq DNA polymerase (MBI Fermentas, Inc., Amherst, N.Y.), and 18 μl of DNA template in a thin-wall 600-μl tube. The UPPCR mixtures were subjected to 40 cycles of 94°C for 1 min (denaturation), 51°C for 1 min (annealing), and 70°C for 2.5 min (extension). The amplified DNA products were separated by electrophoresis on 2.5% agarose gels. DNA was visualized by ethidium bromide staining. Oligonucleotide primers were designed by using the conserved regions of the 16S rRNA sequence from bp 909 to 1410. The primer sequences were 5′-AAACTCAAAGGAATTGAC-3′ for the forward primer and 5′-GACGGGCGGTGTGTACAA-3′ for the reverse primer. The expected DNA fragments (about 500 bp) were amplified by this method. The fidelity of PCR was further checked by digestion of PCR products with HaeIII. The lengths of the restriction fragments were identical to the expected lengths. No PCR product was obtained with the control samples.
Specificity and sensitivity tests were repeated three times. Differences between groups were tested for significance by using a chi-square test with Yates's continuity correction for small numbers, and two levels of significance (P < 0.05 and P < 0.01) were used.
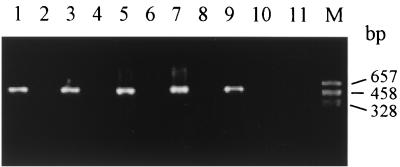
Neutralization and coating antibody replacement tests were used to check the specificity of iUPPCR. In the neutralization test, cell suspensions (20 μl) containing 20 CFU were separately mixed with equal volumes of monoclonal antibodies against dysentery species (50, 100, and 200 μg/ml) prior to incubation at 37°C for 1 h and were then added to wells. The results of the neutralization test showed that all otherwise positive samples were negative. In the second test, monoclonal antibodies against dysentery species were replaced with monoclonal antibody against hepatitis B surface antigen as a coating antibody. No PCR products were detected in the positive samples. Moreover, two types of cross-reactive tests to determine the specificity of iUPPCR were used in this study. One test was designed for cross-reactive detection of S. dysenteriae 1, S. flexneri 1a, S. sonnei, and S. boydii 1. Monoclonal antibodies against S. dysenteriae 1, S. flexneri 1a, S. sonnei, and S. boydii 1 were separately coated in polystyrene 96-well plates. Then S. dysenteriae 1, S. flexneri 1a, S. sonnei, and S. boydii 1 were separately added to the wells coated with the four different antibodies. The other test was used for cross-reactive detection of S. flexneri serotypes 1a, 2a, 3a, 4, 5, and Y variant. Monoclonal antibodies against S. flexneri 1a, 2a, 3a, 4, and 5 were separately coated and reacted with S. flexneri 1a, 2a, 3a, 4, 5, and Y variant. The results of the two types of cross-reaction tests showed that PCR products were detected only in the wells coat ed with antibodies to which cells containing the corresponding antigens were added (Fig. 1 and Tables 1 and 2).
FIG. 1.
Representative cross-reaction analysis showing the specificity of iUPPCR for identification of serotypes of S. flexneri. Lane M, PGEM-7zf(+)/HaeIII marker; lane 1, S. flexneri 1a added to well coated with antibody against S. flexneri 1a; lane 2, S. flexneri 2a added to well coated with antibody against S. flexneri 1a; lane 3, S. flexneri 2a added to well coated with antibody against S. flexneri 2a; lane 4, S. flexneri 3a added to well coated with antibody against S. flexneri 2a; lane 5, S. flexneri 3a added to well coated with antibody against S. flexneri 3; lane 6, S. flexneri 4 added to well coated with antibody against S. flexneri 3; lane 7, S. flexneri 4 added to well coated with antibody against S. flexneri 4; lane 8, S. flexneri 5 added to well coated with antibody against S. flexneri 4; lane 9, S. flexneri 5 added to well coated with antibody against S. flexneri 5; lane 10, S. flexneri Y variant added to well coated with antibody against S. flexneri 5; lane 11, S. flexneri Y variant added to well coated with antibody against S. flexneri 6.
TABLE 1.
Cross-reactivities of S. dysenteriae 1, S. sonnei, S. flexneri 4, and S. boydii 1, as determined by iUPPCR
| Antibody | Cross-reactivity
|
|||
|---|---|---|---|---|
| S. dysenteriae 1 | S. sonnei | S. flexneri 4 | S. boydii 1 | |
| Anti-S.-dysenteriae 1 | + | − | − | − |
| Anti-S. sonnei | − | + | − | − |
| Anti-S. flexneri 4 | − | − | + | − |
| Anti-S. boydii 1 | − | − | − | + |
TABLE 2.
Cross-reactivity used for identification of S. flexneri 1a, 2a, 3a, 4, 5, and Y variant by iUPPCR
| Antibody | Cross-reactivities with S. flexneri serotypes
|
|||||
|---|---|---|---|---|---|---|
| 1a | 2a | 3a | 4 | 5 | Y variant | |
| Anti-S. flexneri 1a | + | − | − | − | − | − |
| Anti-S. flexneri 2a | − | + | − | − | − | − |
| Anti-S. flexneri 3a | − | − | + | − | − | − |
| Anti-S. flexneri 4 | − | − | − | + | − | − |
| Anti-S. flexneri 5 | − | − | − | − | + | − |
| Anti-S. flexneri 6 | − | − | − | − | − | − |
Two methods were used to compare the sensitivities of iUPPCR and conventional UPPCR (cUPPCR). All tests were repeated three times. First, a bacterial pellet was diluted to obtain concentrations of 20, 50, 500, and 5,000 CFU per 20 μl. All reaction tubes were positive as determined by iUPPCR or cUPPCR. Of the 120 tubes containing 10 or 5 CFU/20 μl, which were divided into two groups, however, 60 tubes were found to be positive by using iUPPCR; cUPPCR was used to examine the other tubes, and in three tests 6, 6, and 7 of the 10 tubes containing 10 CFU/20 μl and 2, 3, and 3 tubes of the 10 tubes containing 5 CFU/20 μl were positive. Second, bacteria were diluted to obtain a concentration of 20 CFU per 360 μl, evenly distributed into 20 wells for immunocapture, and then amplified by the two methods. The results indicated that when iUPPCR was used, 5, 4, and 4 of 10 tubes were positive in three tests, whereas all 10 tubes were negative in each test when cUPPCR was used. Samples without bacteria were used as controls, and all of them were negative. Table 3 shows the data obtained for the three tests. There were significant differences between the two methods for samples containing 10, 5, or 1 CFU/20 μl (P < 0.01).
TABLE 3.
Comparison of sensitivities of iUPPCR and cUPPCR based on detection of 10, 5, and 1 CFU per 20 μla
| Method | No. tested | No. positive (%) with:
|
||
|---|---|---|---|---|
| 10 CFU/20 μl | 5 CFU/20 μl | 1 CFU/20 μl | ||
| iUPPCR | 30 | 30 (100.0) | 30 (100.0) | 13 (43.3) |
| cUPPCR | 30 | 19 (63.3) | 7 (23.3) | 0 |
| Control | 15 | 0 | 0 | 0 |
Comparison of rates of positive results between iUCCPR and cUPPCR at 10 CFU (χ2 = 13.47; P < 0.01), at 5 CFU (χ2 = 37.29; P < 0.01), and at 1 CFU (χ2 = 13.47; P < 0.01) per 20 μl is shown.
We further validated our method by performing a stool environment simulation test. Stool (1 g) from a healthy individual was diluted with 1 ml of saline. The suspension was centrifuged at 2,500 × g for 10 min. The supernatant was used for dilution of Shigella species. A dilution containing 200 CFU in 400 μl was added to 20 wells for iUPPCR or cUPPCR analysis. Of 20 tubes divided into two groups, 8 were positive and 2 were negative as determined by iUPPCR, whereas 10 were negative when cUPPCR was used. The difference between the results was significant (P < 0.01).
Sewage samples were collected from sewage ditches near hospitals and in inhabited areas. Of 35 samples, 15 were collected from places around hospitals, and the other 20 were collected from residential areas. Five milliliters of each sewage sample was removed and kept in a sterile tube for 2 h. Then 20 μl of the supernatant was used for iUPPCR, and 40 μl was plated for conventional bacterial culture. Bacteria on agar plates were identified by using monoclonal antisera, colony lifting, and antibody reactions, followed by visualization with a microscope. Table 4 shows the prevalence of Shigella species in sewage as detected by the two different methods. Of the 35 samples, 23 were found to be positive by iUPPCR, whereas 5 samples were positive as determined by bacterial culture. The difference between the results was significant (P < 0.01). In the iUPPCR procedure, monoclonal antibodies against different serotypes of Shigella species were used. S. dysenteriae 1, S. sonnei, S. flexneri 2, and S. flexneri 3 were shown to be positive by iUPPCR, and the positive rates were 5.7% (2 of 35 samples), 8.6% (3 of 35 samples), 42.9% (15 of 35 samples), and 8.6% (3 of 35 samples), respectively. Only S. flexneri 2 was found to be positive by bacterial culture, and its positive rate was 14.3% (5 of 35 samples). There was a significant difference in the positive rates for S. flexneri 2 between the two methods (P < 0.01).
TABLE 4.
Identification of Shigella species in 35 sewage samples by iUPPCR and bacterial culture
| Taxon | No. positive (%) as determined by:
|
|
|---|---|---|
| iUPPCR | Bacterial culture | |
| S. dysenteriae 1 | 2 (5.7) | 0 |
| S. dysenteriae 2 | 0 | 0 |
| S. sonnei | 3 (8.6) | 0 |
| S. flexneri 1 | 0 | 0 |
| S. flexneri 2 | 15 (42.9) | 5 (14.3)a |
| S. flexneri 3 | 3 (8.6) | 0 |
| S. flexneri 4 | 0 | 0 |
| S. flexneri 5 | 0 | 0 |
| S. flexneri 6 | 0 | 0 |
| S. boydii 1-6 | 0 | 0 |
| S. boydii 7-11 | 0 | 0 |
| S. boydii 12-15 | 0 | 0 |
| S. boydii 16-18 | 0 | 0 |
| Total | 23 (65.8) | 5 (14.3)a |
S. boydii serotypes 1 to 6, 7 to 11, 12 to 15, and 16 to 18 were identified by antisera against S. boydii serotypes 1 to 6, 7 to 11, 12 to 15, and 16 to 18, respectively. Values are significantly different between iUPPCR and bacterial culture of S. flexneri 2 (χ2 = 8.10; P < 0.01) and from total S. flexneri 2 (χ2 = 16.06; P < 0.01).
The iUPPCR detection method for Shigella species is at least four times more sensitive than the cUPPCR method, which is currently the conventional method used internationally. More importantly, the specificity provided by coated monoclonal antibodies makes the use of restriction fragment length polymorphism analysis, single-strand conformation polymorphism analysis, or sequencing analysis unnecessary and shortens the diagnosis time.
Similar procedures coupling immunocapturing to PCR have been reported for detecting virions and both DNA and RNA viruses; however, universal primers are not used in these procedures (1, 6, 7, 9, 15). We previously reported a new method termed immunocapture reverse transcription-PCR (iRT-PCR) for detection of the virions present in the circulating immune complexes of hepatitis patients and for analysis of specific immunoglobulin-bound hepatitis C virions (13). In this study, the iUPPCR method was used to detect serotypes of diarrheal pathogens or Shigella. Because the primers are universal, the iUPPCR used for detection of bacteria has advantages over the similar immunocapture PCR used for detection of viruses and the iRT-PCR used for detection of virions coupled to antibodies. Moreover, the iRT-PCR method was designed for detection of viruses bound with isotypes of immunoglobulin, while the iUPPCR technique for bacteria was developed for identification of free bacteria. Samples from sewage inevitably contained dead bacteria, and bacteria from different sample sources were unevenly distributed so that samples with fewer bacteria may have resulted in negative culture detection but positive detection by iUPPCR. These reasons may contribute to the fact that the iUPPCR is more sensitive for detection of bacteria in sewage samples than bacterial culture. It was reported previously that specific antibodies could determine whether target bacteria were alive or dead (16). By replacing a coating antibody with a monoclonal antibody that was specific for live cells, we could distinguish live bacteria from dead bacteria by this new method.
The new method described here provided increased sensitivity, specificity, and convenience for clinical diagnosis and epidemiological investigation of bacillary dysentery. More importantly, this method could be used directly to detect bacteria in environmental samples with no pretreatment, and the test results were obtained within 5 h.
Acknowledgments
This work was supported by grants from the Foundation of Chinese Education Ministry for Excellent Young Teachers, the State Foundation of China for Natural Science (grant 39770585), and IFS (grant A/2338-2).
REFERENCES
- 1.Bariana, H. S., A. L. Shannon, P. W. G. Chu, and P. M. Waterhouse. 1994. Detection of five seedborne legume viruses in one sensitive multiplex polymerase chain reaction test. Phytopathology 84:1201-1205. [Google Scholar]
- 2.Carroll, N. M., E. E. M. Jaeger, S. Choudhury, A. A. S. Dunlop, M. M. Matheson, P. Adamson, N. Okhravi, and S. Lightman. 2000. Detection of and discrimination between gram-positive and gram-negative bacteria in intraocular samples by using nested PCR. J. Clin. Microbiol. 38:1753-1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gascon, J., J. Vila, M. E. Valls, L. Ruiz, J. Vidal, M. Corachan, G. Prats, and M. T. Jimenez de Anta. 1993. Etiology of traveller's diarrhea in Spanish travellers to developing countries. Eur. J. Epidemiol. 9:217-223. [DOI] [PubMed] [Google Scholar]
- 4.Gascon, J., M. Vargas, L. Quinto, M. Corachon, M. T. Jimenez de Anta, and J. Vila. 1998. Enteroaggregative Escherichia coli strains as a cause of traveler's diarrhea: a case-control study. J. Infect. Dis. 177:1409-1412. [DOI] [PubMed] [Google Scholar]
- 5.Greisen, K., M. Loeffelholz, A. Purohit, and D. Leong. 1994. PCR primers and probes for the 16S rRNA gene of most species of pathogenic bacteria, including bacteria found in cerebrospinal fluid. J. Clin. Microbiol. 32:335-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harper, G., G. Dahal, G. Thottappilly, and R. Hull. 1999. Detection of episomal banana streak badnavirus by IC-PCR. J. Virol. Methods 79:1-8. [DOI] [PubMed] [Google Scholar]
- 7.Henrard, D. R., W. F. Mehaffey, and J. P. Allain. 1992. A sensitive viral capture assay for detection of plasma viremia in HIV-infected individuals. AIDS Res. Hum. Retrovir. 8:47-52. [DOI] [PubMed] [Google Scholar]
- 8.Marchesi, J. R., T. Sato, A. J. Weightman, T. A. Martin, J. C. Fry, S. J. Hiom, and W. G. Wade. 1998. Design and evaluation of useful bacterium-specific PCR primers that amplify genes coding for bacterial 16S rRNA. Appl. Environ. Microbiol. 64:795-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mumford, R. A., and S. E. Seal. 1997. Rapid single-tube immuno-capture RT-PCR for the detection of two yam potyviruses. J. Virol. Methods 69:73-79. [DOI] [PubMed] [Google Scholar]
- 10.Osorio, C. R., M. D. Collins, A. E. Toranzo, J. L. Barja, and J. L. Romalde. 1999. 16S rRNA gene sequence analysis of Photobacterium damselae and nested PCR method for rapid detection of the causative agent of fish pasteurellosis. Appl. Environ. Microbiol. 65:2942-2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peng, X. X., H. Gao, S. Y. Wang, and W. Z. Zheng. 2000. Universal primer PCR with SSCP and RFLP for identification of fish disease pathogens. J. Fish. China 24:345-348. [Google Scholar]
- 12.Peng, X. X., Y. Zhou, S. Y. Wang, and Y. L. Zhang. 2000. Quick identification for pathogenic vibrio of aquacultivated creatures by UPPCR-SSCP. J. Oceanogr. Taiwan Strait 19:460-463. [Google Scholar]
- 13.Peng, X. X., H. Zhu, J. Y. Zhang, and S. Y. Wang. 2001. Analysis of HCV-immunoglobulin isotype complexes by a novel immuno-capture RT-PCR method. Scand. J. Immunol. 54:409-413. [DOI] [PubMed] [Google Scholar]
- 14.Rossi, M. C., A. Gori, G. Zehender, G. Marchetti, G. Ferrario, C. De Maddalena, L. Catozzi, A. Bandera, A. D. Esposti, and F. Franzetti. 2000. A PCR-colorimetric microwell plate hybridization assay for detection of Mycobacterium tuberculosis and M. avium from culture samples and Ziehl-Neelsen-positive smears. J. Clin. Microbiol. 38:1772-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharman, M., J. E. Thomas, and R. G. Dietzgen. 2000. Development of a multiplex immunocapture PCR with colourimetric detection for viruses of banana. J. Virol. Methods 89:75-88. [DOI] [PubMed] [Google Scholar]
- 16.Sølve, M., J. Boel, and B. Nørrung. 2000. Evaluation of a monoclonal antibody able to detect live Listeria monocytogenes and Listeria innocua. Int. J. Food Microbiol. 57:219-224. [DOI] [PubMed] [Google Scholar]
- 17.Song, Y. L., N. Kato, C. X. Liu, Y. Matsumiya, H. Kato, and K. Watanabe. 2000. Rapid identification of 11 human intestinal Lactobacillus species by multiplex PCR assays using group and species specific primers derived from the 16S-23S rRNA intergenic spacer region and its flanking 23S rRNA. FEMS Microbiol. Lett. 187:167-173. [DOI] [PubMed] [Google Scholar]
- 18.Tauxe, R. V., N. D. Puhr, J. G. Wells, N. Hargrett-Bean, and P. A. Blake. 1990. Antimicrobial resistance of Shigella isolates in the USA: the importance of international travelers. J. Infect. Dis. 162:1107-1111. [DOI] [PubMed] [Google Scholar]
- 19.Vila, J., J. Gascon, S. Abdalla, J. Gomez, F. Marco, A. Moreno, M. Corachan, and T. Jimenez de Anta. 1994. Antimicrobial resistance of Shigella isolates causing traveler's diarrhea. Antimicrob. Agents Chemother. 38:2668-2670. [DOI] [PMC free article] [PubMed] [Google Scholar]