Abstract
An adhesion-promoting protein involved in the binding of Lactobacillus fermentum strain 104R to small intestinal mucus from piglets and to partially purified gastric mucin was isolated and characterized. Spent culture supernatant fluid and bacterial cell wall extracts were fractionated by ammonium sulfate precipitation and gel filtration. The active fraction was purified by affinity chromatography. The adhesion-promoting protein was detected in the fractions by adhesion inhibition and dot blot assays and visualized by polyacrylamide gel electrophoresis (PAGE), sodium dodecyl sulfate-PAGE, and Western blotting with horseradish peroxidase-labeled mucus and mucin. The active fraction was characterized by estimating the relative molecular weight and by assessing the presence of carbohydrates in, and heat sensitivity of, the active region of the adhesion-promoting protein. The purified protein was digested with porcine trypsin, and the peptides were purified in a SMART system. The peptides were tested for adhesion to horseradish peroxidase-labeled mucin by using the dot blot adhesion assay. Peptides which bound mucin were sequenced. It was shown that the purified adhesion-promoting protein on the cell surface of L. fermentum 104R is extractable with 1 M LiCl and low concentrations of lysozyme but not with 0.2 M glycine. The protein could be released to the culture supernatant fluid after 24 h of growth and had affinity for both small intestinal mucus and gastric mucin. In the native state this protein was variable in size, and it had a molecular mass of 29 kDa when denatured. The denatured protein did not contain carbohydrate moieties and was not heat sensitive. Alignment of amino acids of the adhering peptides with sequences deposited in the EMBL data library showed poor homology with previously published sequences. The protein represents an important molecule for development of probiotics.
Proteinaceous surface appendages or coverings, e.g., fimbriae, flagella, or surface (S)-layers with affinity for mammalian extracellular matrix (55) or mucosa (17, 28), have been extensively characterized for many pathogenic bacteria. It is thought that adhesion to mammalian extracellular matrix components is a prerequisite for bacterial colonization and invasion to subepithelial tissues (55) Cell surface-adhesive proteins from nonpathogenic bacteria and their role in colonization has not been as thoroughly characterized.
Lactobacillus, one of the common indigenous organisms of the gastrointestinal tracts of mammals (46, 48, 49) and a potential probiotic microbe that contributes to the health of the host (11, 16), has the capacity to adhere to epithelial cells (5, 6, 9, 10, 16, 21, 23, 24, 33, 34, 44, 49) and mucus gel (25, 32, 35, 38, 41, 51, 52) from the intestinal tracts of different species. Lactobacillus surface proteins have been proposed to be involved in colonization of gastrointestinal epithelial cells and mucosa of mammals (9, 12, 21, 41, 44, 53). Collagen binding by lactobacilli (1, 47) is known, and purification of collagen binding proteins from Lactobacillus reuteri has also been reported (2).
It is widely assumed that the mucus gel overlying the epithelial villi of the digestive tract has a large variety of potential functions, some of which protect the host against bacterial colonization by modifying or inhibiting bacterial association with the mucosal surface. Conversely, mucus may also provide a habitat for some bacteria (15). It is indeed quite possible that the mucus layer serves as an ecological niche for both commensal and potentially pathogenic microorganisms (36). Little is known about the function of intestinal mucus in the colonization of pathogenic and nonpathogenic bacteria. While characteristics of adhesion to purified mucin or crude mucus by pathogens have been described (14, 27, 28, 43, 45, 54, 56), there is little information about the mechanism of adhesion of nonpathogenic bacteria to gastrointestinal mucus or purified mucins. Wadström et al. (53) reported that none of the Lactobacillus strains isolated from pig intestine formed a capsule and that a great number of the isolates had high surface hydrophobicity. These workers speculated that surface proteins determine the hydrophobicity. Lindahl et al. (29) suggested that surface polymers other than highly negatively charged capsule material can also be produced by strains colonizing the small bowel of young piglets. Others have reported a protein-mediated binding of Lactobacillus to mucus-secreting cell lines (9). In a previous study it was shown that lactobacilli colonize the mucus layer of the small intestine of piglets and that proteinaceous compounds are involved in the binding of Lactobacillus fermentum 104R (41). We now report the purification and characterization of a cell surface protein on L. fermentum 104R that adheres to partially purified gastric mucin and crude small intestine mucus from the pig.
MATERIALS AND METHODS
Bacteria and culture conditions.
L. fermentum 104R was isolated from nonsecreting gastric tissue of 9-week-old pigs (21) and stored at −70°C in 30% glycerol. Individual glycerol ampoules of strain 104R were used to inoculate MRS broth (5 ml) for overnight incubation at 37°C under anaerobic conditions. An inoculum (1%) was transferred to semidefined medium (LDM) (12) containing glucose (2%) and similarly incubated. Cultures incubated for 14 or 24 h were used for extraction and purification of cell-associated mucin binding protein, whereas 8-h cultures were used for the adhesion studies using whole cells. Viability of the cells after the 1 M LiCl extraction step as described below was determined by plating serial dilutions on MRS agar and counting the CFU after overnight incubation at 37°C under anaerobic conditions.
Mucin and mucus preparations.
Partially purified mucin from the porcine stomach obtained from Sigma (hereafter referred to as gastric mucin) was purified by the method of Glenister et al. (18). Mucus was isolated from the small intestinal mucosa of 35-day-old pigs as previously described (13) and diluted in HEPES-Hanks buffer to yield 0.5 mg of protein per ml for the adhesion inhibition assay described below. Both mucus and gastric mucin were conjugated with horseradish peroxidase by the method of Hudson and Hay (22) as previously described by Rojas and Conway (40) Briefly, small intestinal mucus from 35-day-old pigs was supplemented with the low-molecular-weight proteinase inhibitors phenylmethylsulfonyl fluoride (in dry ethanol sprinkled on the frozen sample to allow this inhibitor to percolate through the gel during thawing) (0.01 mM), acetyl-leucyl-leucyl-arginal (leupeptin) (0.01 mM), and Na2EDTA (1 mM). After mixing, the protein concentration was adjusted to 4 mg ml−1 in 0.1 M sodium carbonate buffer, pH 9.5. The proteinase inhibitor-treated small intestinal mucus and the gastric mucin (4 mg/ml in carbonate buffer) were labeled with horseradish peroxidase (HRP) (Sigma). HRP (8 mg dissolved in 2 ml of distilled water) was added to 400 μl of freshly prepared sodium periodate (0.1 M) solution. The mixture was stirred gently for 20 min at room temperature and then dialyzed overnight at 4°C against 0.001 M acetate buffer, pH 4.4. Sodium carbonate buffer (0.1 M, pH 9.5; 20 μl) was added in order to raise the pH to approximately 9 to 9.5, and immediately 1 ml was mixed with 1 ml of the proteinase inhibitor-treated small intestinal mucus or 1 ml of gastric mucin. The mixtures were held at room temperature for 2 h with occasional stirring. Freshly prepared sodium borohydride solution (100 μl of a 4-mg/ml solution in distilled water) was added to reduce any free enzyme, and the mixture was dialyzed against borate buffer (0.1 M, pH 7.4). Labeled small intestinal mucus and gastric mucin were mixed with equal volumes of 80% glycerol and stored at −20°C. The labeled mucus and mucin were used for the dot blot adhesion assay and for Western blots.
Dot blot adhesion assay.
Samples from whole-cell extracts, dialyzed spent culture supernatant (SCS), or fractions from each 280-nm-absorbing peak eluted from the gel filtration and affinity chromatography columns were spotted on Immobilon polyvinylidene difluoride (PVDF) (Millipore) membranes, and the assay was performed by blotting with labeled mucus or mucin as described by Rojas and Conway (40). Binding was visualized by substrate localization of the HRP.
Extraction of a cell surface protein(s) with the capacity to bind small intestinal mucus and gastric mucin.
Cultures from 14- and 24-h growth of L. fermentum 104R were centrifuged at 3,000 × g for 10 min. Bacterial pellets were washed with HEPES-Hanks buffer, centrifuged as described above, and suspended in different extraction buffers, namely, HEPES-Hanks buffer, 1 M lithium chloride, lysozyme solution (0.1 M Tris, 0.015 M NaCl, 0.05 M MgCl2, 40 μg of lysozyme ml−1), and 0.2 M glycine (pH 3). Cell suspensions were incubated with gentle stirring at 4°C for 60 min, and the bacteria were removed by centrifugation (8,000 × g, 30 min). Viability of the bacterial cells in the pellet was determined by measuring CFU per milliliter. The supernatants were filtered through nitrocellulose membranes (0.45-μm pore size), dialyzed against 0.01 M ammonium bicarbonate, freeze-dried, and kept at 4°C until used. Activity was checked by the capacity to bind mucus and mucin by dot blot adhesion assays.
Purification of adhesion-promoting protein from L. fermentum 104R culture supernatant fluid.
SCS from 14- or 24-h cultures was collected by centrifuging the cultures at 6,000 × g for 20 min and dialyzing the supernatant at 4°C against MilliQ water. The retentate was concentrated by filtration through a 14,000-molecular-weight-cutoff membrane. The high-molecular-weight fraction was freeze-dried and stored at 4°C. SCS was also concentrated 10 times using a hollow-fiber ultrafilter, and ammonium sulfate was dissolved in the concentrate (40, 60, and 100% of saturation at 4°C). The precipitates were collected by centrifugation (18,000 × g for 30 min), dissolved in MilliQ water, and dialyzed against 0.01 M ammonium bicarbonate. The solutions were freeze-dried and kept at 4°C.
The freeze-dried preparation from 24-h SCS concentrated by filtration was dissolved in HEPES-Hanks buffer and filtered (0.22-μm-pore-size filter) to remove insoluble particles. A 4-ml sample of the solution (2.1 mg of protein) was applied to Sephadex G-200 in a XK-26 column (Pharmacia-LKB, Uppsala, Sweden) for gel filtration chromatography. HEPES-Hanks buffer was used to equilibrate the column and elute the sample. Fractions from each 280-nm-absorbing peak were assayed for the capacity to bind both HRP-labeled gastric mucin and HRP-labeled small intestinal mucus by the dot blot assay. In addition, fractions were tested for the capacity to inhibit lactobacillus binding to crude mucus in the microtiter plate adhesion assay. The active fractions in each 280-nm-absorbing peak were pooled, dialyzed, and freeze-dried for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot analysis.
Alternative purification.
Gastric mucin was covalently coupled to activated CH-Sepharose 4B according to the instructions of the manufacturer (Pharmacia-LKB Biotechnology). A C10/40 column (30-ml bed volume) was packed with this Sepharose and equilibrated with HEPES-Hanks buffer. L. fermentum LiCl cell extracts or active fractions from gel filtration chromatography were loaded onto the column in the amounts of 134 and 200 μg of protein, respectively. The column was washed with 2 bed volumes of equilibrating buffer and then successively washed with different solutions (0.1 M glycine [pH 3], 0.1 M Tris [pH 8], and a 0 to 2 M gradient of sodium chloride) at a flow rate of 6 ml h−1.
Electrophoresis and Western blotting.
SDS-PAGE and nondenaturing discontinuous PAGE were performed by the method of Laemmli (26) using a separating gel containing 12 and 9.5% (wt/vol) acrylamide, respectively, in a Mini-Protean II apparatus (Bio-Rad Laboratories). All minigels were stained with Coomassie blue according to the Mini-Protean II instruction manual. Freeze-dried samples of dialyzed SCS as well as 1 M lithium chloride, lysozyme, and glycine cell extracts and active fractions from gel filtration and affinity chromatography were rehydrated with distilled water or SDS-mercaptoethanol buffer. The separated proteins were electrophoretically transferred to an Immobilon PVDF membrane (Millipore) in a semidry electroblotter (LKB2117-005 Multiphor II NovaBlot Electrophoretic Transfer Unit). Electrophoresis buffers used were 25 mM Tris-40 mM 6-amino-n-hexanoic acid-20% methanol (cathodic side), 0.3 M Tris-20% methanol (anodic side 1), and 25 mM Tris-20% methanol (anodic side 2). Additional protein binding sites on the membrane were saturated by soaking the membrane in 3% (wt/vol) bovine serum albumin in phosphate-buffered saline (PBS) buffer for 20 min at room temperature, and the membranes were washed with PBS containing 0.05% Tween 20. The membranes were treated for 2 h at room temperature with 100 μl of HRP-labeled gastric mucin or HRP-labeled small intestinal mucus in 10 ml of PBS-Tween 20. The membranes were washed three times (20 min each time) with PBS-Tween 20 and then washed with 0.1 M sodium acetate (pH 5), and the reactive bands were visualized with 2.5 mg of diaminobenzidine and 2.5 μl of hydrogen peroxide (30%) dissolved in 10 ml of 0.1 M sodium acetate (pH 5).
Correlation between native and denatured adhesion-promoting protein.
Nondenaturing discontinuous 9.5% PAGE of LiCl extracts was performed. Protein bands from an excised segment of the gel were transferred to a membrane to be used in Western blotting with HRP-mucin. Another segment was stained with Coomassie blue. The rest of the gel was kept for 2 h in a wet chamber to cut the native protein band after comparison with the stained gel and with the mucin-bound proteins on the membrane. This excised band was homogenized in a syringe, treated with SDS sample buffer, heated, and analyzed by SDS-PAGE in a 1.5-mm gel with 12% (wt/vol) acrylamide run as described by Laemmli (26). The separated proteins from L. fermentum 104R were electrophoretically transferred to an Immobilon PVDF membrane and reacted with labeled gastric mucin and small intestinal mucus as described above.
Detection of carbohydrates in the adhesion-promoting protein.
A periodic acid-Schiff stain procedure (Sigma) was used for visualizing glycoproteins on polyacrylamide gels. A DIG Glycan Detection Kit for the detection of sugars and glycoconjugates by an enzyme immunoassay (Boehringer Mannheim Biochimica) was also used.
Relative molecular weight of the adhesion-promoting protein.
The relative molecular weight of the denatured bacterial adhesive protein was estimated using a calibration curve obtained with standard proteins separated by denaturing nongradient SDS-PAGE. The molecular weight of the native or nondenatured adhesive protein was estimated by gel filtration chromatography relative to the standard curve.
Heat treatment of the adhesion-promoting protein.
Samples (100 μl) of LiCl whole-cell extracts from strain 104R were heated for 5 min at 100°C and tested by dot blot adhesion assay for activity.
Internal amino acids sequence.
According to the results with the Western blot and stained gel, the better-defined band on native PAGE, which bound mucin (see Fig. 3), was excised from the gel and treated as described above. To visualize the protein, the gel was stained as described by Rosenfeld et al. (42) for in-gel digestion of proteins for determination of the internal sequence. Briefly, the staining procedure consisted of staining with 0.2% Coomassie blue in 20% methanol-0.5% acetic acid for 20 min without fixation and destaining in 30% methanol until protein bands became visible. Subunit protein excised from the gel was digested with modified porcine trypsin (SDS-Promega) at a trypsin/substrate ratio of 1:20 as described by Rosenfeld et al. (42). Reverse-phase high-pressure liquid chromatography (HPLC) was performed for peptide purification in a SMART system (Pharmacia). Peptides were separated using a μRPC C2/C18 SC 2.1/10 column (Pharmacia). The column was eluted with a linear gradient of 5 to 50% acetonitrile-0.1% trifluoroacetic acid at a flow rate of 0.1 ml/min. Elution was monitored at 215 nm. Peptides obtained from reverse-phase HPLC were tested for adhesion to mucin (Sigma) by a dot blot adhesion assay. Adhering peptides were sequenced on a protein sequencer (473A; Applied Biosystems) according to the manufacturer's specifications. All internal sequencing experiments were performed twice.
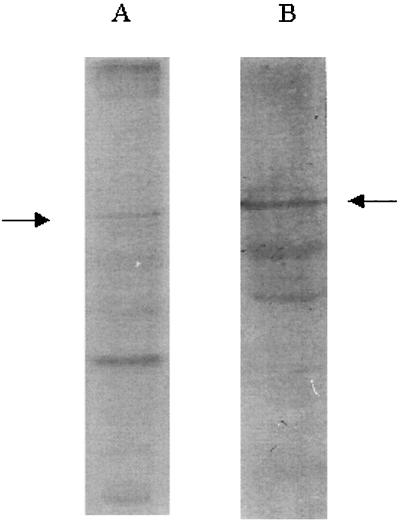
FIG. 3.
PAGE on minigels stained with Coomassie blue and Western blotting of strain 104R native MAPP. (A) PAGE (9.5%) of 1 M LiCl extract from 14-h culture; (B) Western blot of 1 M LiCl extract from 14-h culture.
N-terminal sequence.
Native protein from PAGE was excised from a nondenaturing gel as described above and was then denatured into subunits by SDS-PAGE and transferred to an Immobilon PVDF membrane as described above. It was visualized with Ponceau S solution (Boehringer Mannheim Biochemica), excised, and sequenced as described above. A computer-assisted search for related sequenced peptides was performed using the data library from GenBank of the European Molecular Biology Laboratory, Heidelberg, Germany.
RESULTS
Extraction of cell surface protein with capacity to bind to small intestinal mucus and gastric mucin.
SCS from 24-h cultures of L. fermentum strain 104R tested using the dot blot assay showed affinity for small intestinal mucus, while culture supernatant from exponential-phase cells (12 to 14 h of growth) did not. SCS from 24-h growth was used to purify the protein(s) involved in the adhesion. Extraction of cell-associated proteins with different solutions showed that the adhesion-promoting protein(s) with affinity for mucus could be extracted from the cells with 1 and 5 M LiCl and low concentrations of lysozyme but not with 0.2 M glycine (pH 3), as tested by dot blot assay. Lithium chloride-extracted cells retained viability, since 2.0 × 108 CFU per ml were detected.
Purification of the adhesion-promoting protein from L. fermentum strain 104R.
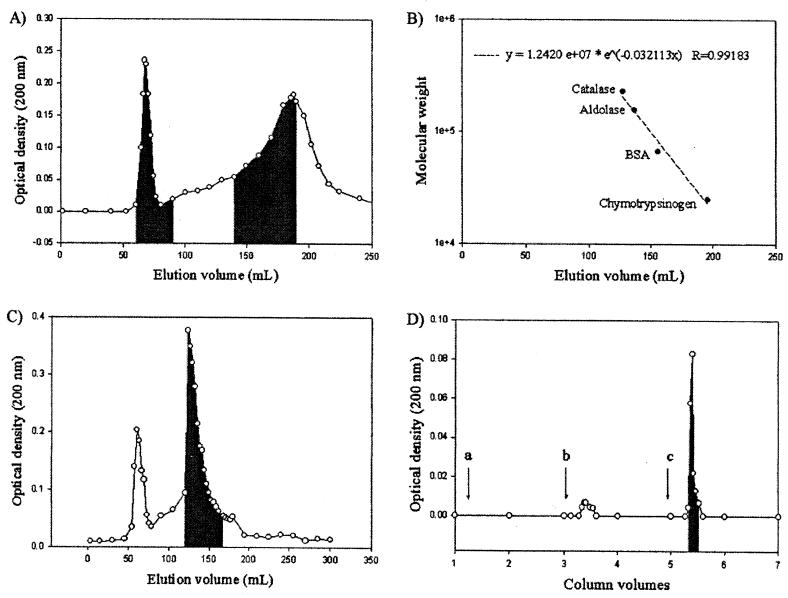
Fractionation of the culture supernatant fluid by filtration gave two fractions, with the fraction over 14 kDa containing all activity for small intestinal mucus and gastric mucin. Precipitation using either 40 or 60% ammonium sulfate gave precipitates with adhesive activity. Further purification was performed by gel filtration chromatography. When dialyzed SCS was fractionated by gel filtration, affinity for mucus and mucin by dot blot assay was found in the 60- to 90-ml and 140- to 190-ml elution volumes (Fig. 1A). According to the standard curve (Fig. 1B), the first elution volume corresponded to the void volume and the second one corresponded to a broad range of sizes; i.e., the 140-ml fraction corresponded to 145 kDa and the 190-ml fraction corresponded to 29 kDa. Samples of 1 M LiCl extracts were also fractionated under the same conditions. The strongest affinity for mucus and mucin was found in the 123- to 160-ml elution volume, a volume corresponding to 260 to 75 kDa (Fig. 1C). Some activity was found also in the 54- to 78-ml elution volume, which corresponded to the void volume (Fig. 1C). Affinity chromatography with gastric mucin-Sepharose of the pool of active fractions from gel filtration of both dialyzed culture supernatant and LiCl extract from whole cells showed a very sharp peak eluted with 0.1 M Tris (pH 8) with high activity as measured using the dot blot assay (Fig. 1D). Activity of the crude extracts at different purification stages of the protein was analyzed by dot blot assay.
FIG. 1.
Elution pattern of L. fermentum 104R protein extracts on Sephadex G-200 gel filtration chromatography. (A) SCS from strain 104R. Fractions which adhered to mucus and mucin were localized in the shaded fractions. (B) Standard curve with known proteins separated by Sephadex G-200 gel filtration chromatography in the same column. BSA, bovine serum albumin. (C) LiCl (1.0 M) extract from 104R whole cells. Fractions which adhered to mucus and mucin were localized in the shaded fractions. (D) Elution pattern after affinity chromatography using activated ch-Sepharose 4B covalently coupled to partially purified gastric mucin of a pool of adhering fractions from gel filtration chromatography of SCS eluted with different desorbing buffers, i.e., HEPES-Hanks buffer (a), 0.1 M glycine (pH 3) (b), and 0.1 M Tris (pH 8) (c). Adhesion to mucus and mucin was detected in the shaded peak.
Electrophoresis and Western blotting.
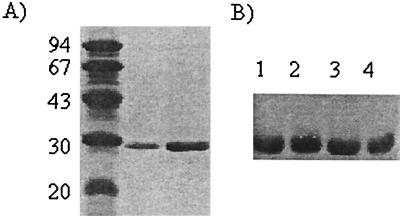
Freeze-dried samples from culture supernatants fractionated by gel filtration and affinity chromatography, cell wall extracts, and final purified mucus and mucin adhesion-promoting protein (MAPP) preparations (rehydrated in water or directly in SDS-mercaptoethanol buffer) were analyzed on a denaturing (SDS) gel (Fig. 2) and by nondenaturing discontinuous polyacrylamide gel electrophoresis and Western blotting with HRP-mucin. The results indicated that the adhesion-promoting protein was rather soluble in water as well as in the SDS-mercaptoethanol buffer when it was visualized by Coomassie blue staining after SDS-PAGE (results not shown) and that it had a molecular mass of 29 kDa under denaturing conditions (Fig. 2). Under nondenaturing conditions, where the separation is based on a combination of molecular properties, including size, shape, and charge, the native MAPP was visualized by Western blotting using HRP-mucin (Fig. 3). When the reacting bands that bound mucin were transferred from the gel to SDS-PAGE, the strongest-reacting band yielded only the 29-kDa subunit.
FIG. 2.
SDS-PAGE on minigels and Western blotting of the MAPP from L. fermentum 104R, using HRP-labeled mucus for blotting. (A) Twelve percent acrylamide gels were used and stained with Coomassie blue. Lane 1, low-molecular-weight standards; lane 2, MAPP after affinity chromatography (5 μl of protein was loaded); lane 3, MAPP from native PAGE. Numbers on the left are molecular masses in kilodaltons. (B) Western blotting of the MAPP from SDS-PAGE after gel filtration chromatography of LiCl extract (lane 1), affinity chromatography of LiCl extract (lane 2), 1 M LiCl extract from 14 h culture (lane 3), and the MAPP subunit from the native MAPP band in PAGE (lane 4).
Detection of carbohydrates in the adhesion-promoting protein.
Using the periodic acid-Schiff stain and the glycan enzymatic detection method, it was shown that there were no sugars in the denatured MAPP; however, when the protein was in the native state, sugar was detected (data not shown). The activity of MAPP was not destroyed by heating at 100°C for 5 min (results not shown).
Molecular mass of the adhesion-promoting protein.
Under denaturing conditions the relative molecular weight was 29 kDa, and in the native state it was variable. By gel filtration chromatography, and with reference to a standard curve of molecular mass markers, activity was found in the void volume and in a very broad range of molecular masses (29 to 261 kDa). When different pools from these fractions were analyzed by SDS-PAGE and Western blotting with HRP-mucus and HRP-mucin, the reacting 29-kDa protein was found in all of them. By use of 9.5% PAGE and Western blotting, activity was found in a band in the separating gels. Some activity was also seen between the stacking and separating gels and at the beginning of the stacking gel, suggesting that protein is arranged in different amounts of identical subunits.
Internal amino acid sequence.
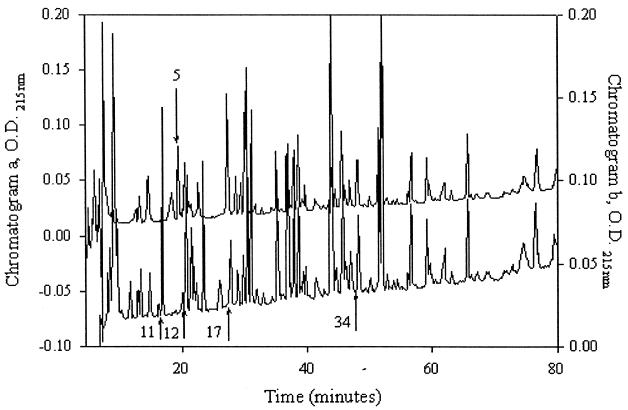
Two batches of MAPP were digested with modified porcine trypsin as described by Rosenfeld et al. (42), and peptides were purified by reverse-phase HPLC and tested for adhesion to HRP-mucin by dot blot adhesion assay. The peptide peaks which showed affinity for HRP-mucin are shown in Fig. 4. The numbers of the peaks and the sequences of adhering peptides in the chromatogram a are as follows: 7, IIAG(T)G(T)NNA; 11, DTAIQSSYNK; 12, ANFVPTK; 17, ISALFNK; and N terminal, AXXAVNXELV(V)K, where X probably corresponds to serine and parentheses indicate the most probable amino acid when identification was difficult. The sequences of the number 12, 34, and N-terminal adhering peptides in chromatogram a were reproducible in chromatogram b.
FIG. 4.
Chromatograms a (top) and b (bottom) from reverse-phase HPLC of trypsin-digested peptides fractionated in a SMART system using a μRPC C2/C18 SC 2.1/10 column. Sequenced adhering peptides are marked with their fraction numbers and with arrows. The N-terminal sequence was the same as peptide 34. The sequence of peptide 5 from chromatogram a was the same as that of peptide 12 from chromatogram b. Peptide 5 from chromatogram c (not shown) was also sequenced.
N-terminal sequence.
The 29-kDa MAPP with affinity for mucus and mucin had an N-terminal sequence of XX(S)AVNSELVVK. A computer-assisted search of the N-terminal and internal sequenced peptides showed that this sequence has yet to be published.
DISCUSSION
Association of Lactobacillus with an epithelial or mucosal surface is undoubtedly an important determinant in its colonization of the gastrointestinal tract. The data presented here show the purification and characterization of a 29-kDa cell surface protein from L. fermentum strain 104R that bound to both mucus isolated from the small intestine of 35-day-old piglets and partially purified gastric mucin from pig. This is consistent with the report of Henriksson (20), who showed that L. fermentum 104S binds to crude porcine gastric mucus. Conway and Kjelleberg (12) have reported that the adhesion of L. fermentum strain 737 to mouse nonsecreting stomach tissue was mediated by a 12- to 13-kDa protein and suggested that this tissue adhesion-promoting protein also aggregated with itself or other compounds. This 12- to 13-kDa tissue adhesion-promoting protein was found in the SCS of L. fermentum strain 737, which is consistent with the MAPP of L. fermentum 104R also being demonstrable in the culture supernatant fraction (12). The adhesion-promoting activity was found in the culture supernatant after 24 h of Lactobacillus growth but not after 12 to 14 h of growth. The MAPP, however, could be extracted from the whole cells with 1 M LiCl after 14 h of growth. One can postulate that the MAPP was attached to the cell wall and at the end of the growth some MAPP was released to the growth medium, perhaps due to conditions created in the growth medium. After extraction with 1 M LiCl, a high percentage of the cells (2 × 108 CFU ml−1) were viable, thus suggesting that the 29-kDa MAPP is on the cell surface and is noncovalently bound to the cell wall. This is consistent with the report of Lortal et al. (30), who found that 1 and 5 M LiCl were less lethal than guanidine hydrochloride during extraction of the S-layer protein from whole cells of L. helveticus.
The 29-kDa MAPP adhered to both the partially purified gastric mucin the small intestinal crude mucus from pig. Mucin is a large, heavily glycosylated molecule composed of multiple subunits which may range in size from 250 to 500 kDa and are responsible for the gel-like properties of the mucus layer (3). It has been proposed that mucins of various origins are similar in their macromolecular properties and polymeric structure (7). However, pig gastric glandular tissue comprises a number of large and oligomeric glycoproteins that differ from those from the surface epithelium in buoyant density, apoprotein structure, and carbohydrate substitution (37). The mucus layer represents a major structural feature of the small intestine, is complex in composition, and appears to consist of mucin molecules and a large amount of associated glycoproteins and glycolipids (3). From these results, one can suggest that the receptor of the L. fermentum strain 104R adhesion-promoting protein in the mucus layer of the small intestine and in the stomachs of piglets is on mucin. Recently a 29-kDa surface protein from L. fermentum RC-14 that inhibits adhesion of Enterococcus faecalis 1131 has been reported (19). Although more information is needed to compare the proteins, one can suggest that this mechanism of adhesion of Lactobacillus strains to mucus is relevant.
When the relative molecular weight of the native MAPP was estimated, a number of fractions from gel filtration chromatography with various molecular sizes had affinity for the mucus and mucin. It is likely that the native MAPP is built up of 29-kDa subunits, i.e., 261, 245, 232, 145, 87, and 29 kDa. When reacting bands from 9.5% PAGE were run in SDS-12% PAGE (Fig. 2A), there was always a yield of the 29-kDa subunit (Fig. 2B). Likewise, when a pool of active fractions from affinity chromatography was analyzed by SDS-PAGE, it also contained the 29-kDa subunit.
We suggest that the MAPP may be an oligomeric protein containing two or more polypeptide chains or subunits that are noncovalently bound to the cell surface of the bacteria. Furthermore, the MAPP could be released in different sizes because of the chaotropic properties of the lithium ion (i.e., Li+ ions disrupt the structure of water and reduce hydrophobic interactions). Alternately, the MAPP could be a protein which coaggregates. Reniero et al.(39) suggested that a 32-kDa protein from Lactobacillus plantarum 4B2 is an aggregation-promoting factor. S-layer proteins from lactobacilli have been also studied (4, 8, 31). Collagen binding proteins from Lactobacillus crispatus (43 kDa) (50) and from L. reuteri (29 and 32 kDa) (2) have been characterized.
Characterization of the MAPP showed that the protein is soluble in water. This characteristic was visualized by SDS-PAGE when the freeze-dried protein mixtures obtained during the purification stages were dissolved in water or in SDS-mercaptoethanol buffer. This characteristic helped to separate the MAPP from other proteins that are less soluble in water. While the 29-kDa subunit does not contain carbohydrates, the native form did contain sugar moieties. From these results, one can suggest that the native protein has affinity for carbohydrates that either are released during extraction or are present in the culture medium. During the denaturation with SDS, these sugar moieties were lost without affecting the adhesive activity. In addition, heat treatment at 100°C for 5 min did not affect the adhesive activity of the protein.
Trypsin digestion of the MAPP resulted in peptides that bound to HRP-mucin, indicating conserved affinity for mucin. One can postulate that these peptides are part of the active site of the adhesion-promoting protein. This is interesting from an ecological point of view, since trypsin is one of the pancreatic enzymes in the proximal small intestine. It can be hypothesized that this adhesion-promoting protein could be involved in colonization of the intestinal mucosa of piglets, since the adhesive capacity could be retained in the intestinal milieu.
The internal amino acid sequences of the trypsin-generated peptides as well as the N-terminal sequence of the MAPP were determined. These sequences of the adhesion-promoting protein were not in the database searched. The N-terminal sequence was very similar to that of a collagen binding protein of L. reuteri NCIB 11951 that has already been published (2). Further homology studies need to be carried out to find the relationship between the two proteins.
In conclusion, an adhesion-promoting protein that mediated binding of L. fermentum 104R to both small intestinal porcine mucus and porcine gastric mucin has been purified and characterized. The protein is probably noncovalently bound to the cell wall and is released to the growth medium when the bacterial cells reach stationary phase. From the amino acid sequence of the N terminus, similarity with a collagen binding protein of L. reuteri was noted.
Acknowledgments
This work was supported by Stiftelsen Lantbruksforskningen and the National Council for Science and Technology, Mexico.
We gratefully acknowledge the technical assistance of Anders Blomberg and Joakim Norbeck in the sequencing of peptides.
REFERENCES
- 1.Alejung, P., M. Paulsson, L. Emödy, M. Andersson, A. S. Naidu, and T. Wadström. 1991. Collagen binding by lactobacilli. Curr. Microbiol. 23:33-38. [Google Scholar]
- 2.Alejung, P., W. Shen, B. Rozalska, U. Hellman, A. Ljungh, and T. Wadström. 1994. Purification of collagen-binding proteins of Lactobacillus reuteri NCIB 11951. Curr. Microbiol. 28:231-236. [DOI] [PubMed] [Google Scholar]
- 3.Allen, A., and D. Snary. 1972. The structure and function of gastric mucus. Gut 13:666-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boot, H. J., C. P. Kolen, J. M. Van Noort, and P. H. Pouwels. 1993. S-layer protein of Lactobacillus acidophilus ATCC 4356: purification, expression in Escherichia coli, and nucleotide sequence of the corresponding gene. J. Bacteriol. 175:6089-6096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boris, S., J. E. Suarez, F. Vazquez, and C. Barbes. 1998. Adherence of human vaginal lactobacilli to vaginal epithelial cells and interaction with uropathogens. Infect. Immun. 66:1985-1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brooker, B. E., and R. Fuller. 1975. Adhesion of lactobacilli to the chicken crop epithelium. J. Ultrastruct. Res. 52:21-31. [DOI] [PubMed] [Google Scholar]
- 7.Carlstedt, I., and J.-K. Sheehan. 1984. Macromolecular properties and polymeric structure of mucus glycoproteins. Ciba Found. Symp. 109:157-172. [DOI] [PubMed] [Google Scholar]
- 8.Chagnaud, P., H. F. Jenkinson, and G. W. Tannock. 1992. Cell surface associated proteins of gastrointestinal strains of lactobacilli. Microb. Ecol. Health Dis. 5:121-131. [Google Scholar]
- 9.Coconnier, M. H., T. R. Klaenhammer, S. Kerrnéis, M. F. Bernet, and A. L. Servin. 1992. Protein-mediated adhesion of Lactobacillus acidophilus BG2F04 on human enterocyte and mucus-secreting cell lines in culture. Appl. Environ. Microbiol. 58:2034-2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conway, P. L., S. L. Gorbach, and B. R. Goldin. 1986. Survival of lactic acid bacteria in the human stomach and adhesion to intestinal cells. J. Dairy Sci. 70:1-12. [DOI] [PubMed] [Google Scholar]
- 11.Conway, P. L., and A. Henriksson. 1994. Strategies for the isolation and characterisation of functional probiotics, p. 75-93. In S. A. W. Gibson (ed.), Human health: the contribution of microorganisms. Springer-Verlag, London, United Kingdom.
- 12.Conway, P. L., and S. Kjelleberg. 1989. Protein-mediated adhesion of Lactobacillus fermentum strain 737 to mouse stomach squamous epithelium. J. Gen. Microbiol. 135:1175-1186. [DOI] [PubMed] [Google Scholar]
- 13.Conway, P. L., A. Welin, and P. S. Cohen. 1990. Presence of K88-specific receptors in porcine ileal mucus is age dependent. Infect. Immun. 58:3178-3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drumm, B., A. M. Roberton, and P. M. Sherman. 1988. Inhibition of attachment of Escherichia coli RDEC-1 to intestinal microvillus membranes by rabbit ileal mucus and mucin in vitro. Infect. Immun. 56:2437-2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freter, R. 1984. Factors involved in the penetration of the mucus layer in experimental cholera, p. 44-49. In E. C. Boedeker (ed.), Attachment of organisms to the gut mucosa, vol. II. CRC Press, Inc., Boca Raton, Fla. [Google Scholar]
- 16.Fuller, R. 1994. Probiotics: an overview, p. 63-73. In S. A. W. Gibson (ed.), Human health: the contribution of microorganisms. Springer-Verlag, London, United Kingdom.
- 17.Girardeau, J. P., M. D. Vartanian, J. L. Ollier, and M. Contrepois. 1988. CS31A, a new K88-related fimbrial antigen on bovine enterotoxigenic and septicemic Escherichia coli strains. Infect. Immun. 56:2180-2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glenister, A., K. E. Salamon, K. Smith, D. Beighton, and C. W. Keevil. 1988. Enhanced growth of complex communities of dental plaque bacteria in mucin-limited continuous culture. Microb. Ecol. Health Dis. 1:31-38. [Google Scholar]
- 19.Heinemann, C., J. E. Van Hylckama Vlieg, D. B. Janssen, H. J. Busscher, H. C. van der Mei, and G. Reid. 2000. Purification and characterization of a surface-binding protein from Lactobacillus fermentum RC-14 that inhibits adhesion of Enterococcus faecalis 1131. FEMS Microbiol. Lett. 190:177-180. [DOI] [PubMed] [Google Scholar]
- 20.Henriksson, A. 1993. Lactobacillus colonisation of the porcine gastrointestinal tract. Ph.D. thesis. Göteborg University, Göteborg, Sweden.
- 21.Henriksson, A., R. Szewzyk, and P. L. Conway. 1991. Characteristics of the adhesive determinants of Lactobacillus fermentum 104. Appl. Environ. Microbiol. 57:499-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hudson, L., and F. C. Hay. 1989. Antibody as a probe, p. 44-46. In K. H. Elaine and K. W. Frances (ed.), Practical Immunology. Blackwell Scientific Publications, London, United Kingdom.
- 23.Kapczynski, D. R., R. J. Meinersmann, and M. D. Lee. 2000. Adherence of Lactobacillus to intestinal 407 cells in culture correlates with fibronectin binding. Curr. Microbiol. 41:136-141. [DOI] [PubMed] [Google Scholar]
- 24.Kawai, Y., Y. N. Suegara, and H. Shimohashi. 1982. Colonization of lactic acid bacteria isolated from rats and humans in the gastrointestinal tract of rats. Microbiol. Immunol. 26:363-373. [DOI] [PubMed] [Google Scholar]
- 25.Kirjavainen, P. V., A. C. Ouwehand, E. Isolauri, and S. J. Salminen. 1998. The ability of probiotic bacteria to bind to human intestinal mucus. FEMS Microbiol. Lett. 167:185-189. [DOI] [PubMed] [Google Scholar]
- 26.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 27.Laux, D. C., E. F. McSweegan, T. J. Williams, E. A. Wadolkowski, and P. S. Cohen. 1986. Identification and characterization of mouse small intestine mucosal receptors for Escherichia coli K-12(K88ab). Infect. Immun. 52:18-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lindahl, M., and I. Carlstedt. 1990. Binding of K99 fimbriae of enterotoxigenic Escherichia coli to pig small intestine mucin glycopeptides. J. Gen. Microbiol. 136:1609-1614. [DOI] [PubMed] [Google Scholar]
- 29.Lindahl, M., A. Faris, T. Wadström, and S. Hjerten. 1981. A new test based on salting out to measure relative surface hydrophobicity of bacterial cells. Biochim. Biophys. Acta 677:471-476. [DOI] [PubMed] [Google Scholar]
- 30.Lortal, S., J. Heijenoort, K. Gruber, and U. B. Sleytr. 1992. S-layer of Lactobacillus helveticus ATCC 12046: isolation, chemical characterization and re-formation after extraction with lithium chloride. J. Gen. Microbiol. 138:611-618. [Google Scholar]
- 31.Masuda, K., and T. Kawata. 1983. Distribution and chemical characterization of regular arrays in the cell walls of strains of the genus Lactobacillus. FEMS Microbiol. Lett. 20:145-150. [Google Scholar]
- 32.Matsumura, A., T. Saito, M. Arakuni, H. Kitazawa, Y. Kawai, and T. Itoh. 1999. New binding assay and preparative trial of cell-surface lectin from Lactobacillus acidophilus group lactic acid bacteria. J. Dairy Sci. 82:2525-2529. [DOI] [PubMed] [Google Scholar]
- 33.Mäyrä-Mäkinen, A., M. Manninen, and H. Gyllenberg. 1983. The adherence of lactic acid bacteria to the columnar epithelial cells of pigs and calves. J. Appl. Bacteriol. 55:241-245. [DOI] [PubMed] [Google Scholar]
- 34.Morata de Ambrosini, V. I., S. N. González, and G. Oliver. 1999. Study of adhesion of Lactobacillus casei CRL 431 to ileal intestinal cells of mice. J. Food Prot. 62:1430-1434. [DOI] [PubMed] [Google Scholar]
- 35.Mukai, T., S. Kaneko, and H. Ohori. 1998. Haemagglutination and glycolipid-binding activities of Lactobacillus reuteri. Lett. Appl. Microbiol. 27:130-134. [DOI] [PubMed] [Google Scholar]
- 36.Neutra, M. R., and J. F. Forstner. 1988. Gastrointestinal mucus: synthesis, secretion and function, p. 975-1009. In L. R. Johnson (ed.), Physiology of the gastrointestinal tract. Raven Press, New York, N.Y.
- 37.Nordman, H., J. R., Davies, and I. Carlstedt. 1998. Mucus glycoproteins from pig gastric mucosa: different mucins are produced by the surface epithelium and the glands. Biochem. J. 331:687-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ouwehand, A. C., S. Tolkko, J. Kulmala, S. Salminen, and E. Salminen. 2000. Adhesion of inactivated probiotic strains to intestinal mucus. Lett. Appl. Microbiol. 31:82-86. [DOI] [PubMed] [Google Scholar]
- 39.Reniero, R., P. Cocconcelli, V. Bottazzi, and L. Morelli. 1992. High frequency of conjugation in Lactobacillus mediated by an aggregation promoting factor. J. Gen. Microbiol. 138:763-768. [Google Scholar]
- 40.Rojas, M., and P. L. Conway. 2001. A dot blot assay for adhesive components relative to probiotics in microbial growth in biofilms. Methods Enzymol. 336:389-402. [DOI] [PubMed] [Google Scholar]
- 41.Rojas, M., and P. L. Conway. 1996. Colonisation by Lactobacillus of piglet small intestinal mucus. J. Appl. Bacteriol. 81:474-480. [DOI] [PubMed] [Google Scholar]
- 42.Rosenfeld, J., J. Capdevielle, J. C. Guillemot, and P. Ferrara. 1992. In-gel digestion of proteins for internal sequence analysis after one- or two-dimensional gel electrophoresis. Anal. Biochem. 203:173-179. [DOI] [PubMed] [Google Scholar]
- 43.Sajjan, S. U., and J. F. Forstner. 1990. Characteristics of the binding of Escherichia coli serotype 0157:H7 strain CL-49 to purified intestinal mucin. Infect. Immun. 58:860-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schneitz, C., L. Nuotio, and K. Lounatma. 1993. Adhesion of Lactobacillus acidophilus to avian intestinal epithelial cells mediated by the crystalline bacterial cell surface layer (S-layer). J. Appl. Bacteriol. 74:290-294. [DOI] [PubMed] [Google Scholar]
- 45.Simpson, D. A., R. Ramphal, and S. Lory. 1992. Genetic analysis of Pseudomonas aeruginosa adherence: distinct genetic loci control attachment to epithelial cells and mucins. Infect. Immun. 60:3771-3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith, H. W. 1965. Observations on the flora of the alimentary tract of animals and factors affecting its composition. J. Pathol. Bacteriol. 89:95-122. [PubMed] [Google Scholar]
- 47.Styriak, I., V. Demeèková, and R. Nemcová. 1999. Collagen (Cn-I) binding by gut lactobacilli. Berl. Munch. Tierarztl Wochenschr. 112:301-304. [PubMed] [Google Scholar]
- 48.Tannock, G. W. 1999. Analysis of the intestinal microflora: a renaissance. Antonie Leeuwenhoek 76:265-278. [PubMed] [Google Scholar]
- 49.Tannock, G. W., R. Blumershine, and R. Archibald. 1987. Demonstration of epithelium-associated microbes in the oesophagus of pigs, cattle, rats and deer. FEMS Microbiol. Ecol. 45:199-203. [Google Scholar]
- 50.Toba, T., R. Virkola, B. Westerlund, Y. Björkman, J. Sillanpää, T. Vartio, N. Kalkkinen, and T. K. Korhonen. 1995. A collagen binding S-layer protein in Lactobacillus crispatus. Appl. Environ. Microbiol. 61:2467-2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tuomola, E. M., A. C. Ouwehand, and S. J. Salminen. 2000. Chemical, physical and enzymatic pre-treatments of probiotic lactobacilli alter their adhesion to human intestinal mucus glycoproteins. Int. J. Food Microbiol. 15:75-81. [DOI] [PubMed] [Google Scholar]
- 52.Tuomola, E. M., A. C. Ouwehand, and S. J. Salminen. 1999. Human ileostomy glycoproteins as a model for small intestinal mucus to investigate adhesion of probiotics. Lett. Appl. Microbiol. 28:159-163. [DOI] [PubMed] [Google Scholar]
- 53.Wadström, T., K. Andersson, M. Sydow, L. Axelsson, S. Lindgren, and B. Gullmar. 1987. Surface properties of lactobacilli isolated from the small intestine of pigs. J. Appl. Bacteriol. 62:513-520. [DOI] [PubMed] [Google Scholar]
- 54.Wanke, C. A., S. Ronan, C. Goss, K. Chadee, and R. L. Guerrant. 1990. Characterization of binding of Escherichia coli strains which are enteropathogens to small-bowel mucin. Infect. Immun. 58:794-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Westerlund, B., and T. K. Korhonen. 1993. Bacterial proteins binding to the mammalian extracellular matrix. Mol. Microbiol. 9:687-694. [DOI] [PubMed] [Google Scholar]
- 56.Yamamoto, T., S. Endo, T. Yokota, and P. Echeverria. 1991. Characteristics of adherence of enteroaggregative Escherichia coli to human and animal mucosa. Infect. Immun. 59:3722-3739. [DOI] [PMC free article] [PubMed] [Google Scholar]