Abstract
A highly efficient, rapid, and reliable PCR-based method for distinguishing Lactococcus lactis subspecies (L. lactis subsp. lactis and L. lactis subsp. cremoris) is described. Primers complementary to positions in the glutamate decarboxylase gene have been constructed. PCR analysis with extracted DNA or with cells of different L. lactis strains resulted in specific fragments. The length polymorphism of the PCR fragments allowed a clear distinction of the L. lactis subspecies. The amplified fragment length polymorphism with the primers and the restriction fragment length polymorphism of the amplified products agreed perfectly with the identification based on genotypic and phenotypic analyses, respectively. Isolates from cheese starters were investigated by this method, and amplified fragments of genetic variants were found to be approximately 40 bp shorter than the typical L. lactis subsp. cremoris fragments.
Lactococcal strains are essential to milk fermentation, especially in the cheese-making process, providing optimal conditions for curd formation and for the development of texture and flavor. It is important to the dairy industry to identify new strains of Lactococcus lactis for cheese manufacture. Dairy lactococcal strains are subdivided into L. lactis subsp. lactis and L. lactis subsp. cremoris based on a few phenotypic tests: growth in 4% NaCl, pH 9.2, at 40°C; the ability to hydrolyze arginine (7); and sensitivity to lithium chloride (1).
Recently, a novel criterion for distinguishing L. lactis subsp. lactis from L. lactis subsp. cremoris has been reported: glutamate decarboxylase (GAD; EC 4.1.1.15) activity, which has been observed in L. lactis subsp. lactis and not in L. lactis subsp. cremoris (8). GAD catalyzes the irreversible decarboxylation of glutamate to γ-aminobutyric acid (GABA). GAD constitutes a glutamate-dependent acid resistance mechanism with a glutamate-GABA antiporter (12). The gadB gene encoding L. lactis subsp. cremoris GAD was apparently inactivated by a frameshift mutation resulting from an adenine deletion or a thymine insertion and encoded a nonfunctional protein (10).
It has been discovered in recent years that L. lactis strains can be divided into two phylogenetic groups based on genotypic analysis (6, 11, 13), and it has been proposed that the subspecies diagnoses be redefined to reflect these natural relationships (6). The new taxonomic system requires the transfer of several strains across subspecies lines, from L. lactis subsp. lactis to L. lactis subsp. cremoris and vice versa.
As classification according to phenotypic criteria is complicated and requires skill, simple, fast, and reliable molecular methods have been developed (3, 5). Classification based on these methods, however, coincides with genotypic identification and does not always correlate with phenotypic characterization. The classification of L. lactis subspecies based on phenotypic characteristics is of primary importance in the dairy industry, as phenotypes directly reveal the abilities required in milk fermentation. It has been reported that PCR analysis with oligonucleotide primers designed for the rrnB-rrnC region correlates with phenotypic characterization (2).
In this paper, we describe useful PCR primers for distinguishing L. lactis subspecies. Genotypic and phenotypic characteristics of L. lactis can be determined by one PCR and subsequent nuclease digestion. Results of amplified fragment length polymorphism analysis with these primers agreed with the genotypic identification, and the restriction fragment length polymorphism (RFLP) of the amplified products concurred with the phenotypic identification.
In addition, 37 isolates of L. lactis were examined by the PCR-based method and by phenotypic differentiation, with strains being differentiated to the subspecies level. Four of 20 isolates of L. lactis subsp. cremoris were found to have shorter fragments than the other L. lactis subsp. cremoris isolates. The amplified fragments of these strains were sequenced.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
L. lactis subsp. lactis strains ATCC 9936 and ATCC 19435, L. lactis subsp. lactis biovar diacetylactis ATCC 13675, L. lactis subsp. cremoris ATCC 19257, Leuconostoc mesenteroides ATCC 8293, and Lactobacillus casei ATCC 393 were obtained from the American Type Culture Collection (Manassas, Va.). Enterococcus faecalis IFO 12964 was obtained from the Institute for Fermentation, Osaka (Osaka, Japan). Streptococcus thermophilus 9Y was from laboratory collections. Other L. lactis strains and their growth conditions have been described previously (8). Phenotypic characteristics were determined as described previously (8). Genotypic characterization of L. lactis was performed by PCR analysis with primers PALA-4 and PALA-14, as described by Garde et al. (5). Actively growing cultures were obtained by transferring a 1% inoculum to tryptone-yeast extract-glucose (10), M17 broth (14) containing 0.5% glucose (GM17), or MRS broth (4) and then incubating the cultures at 30°C for 16 h.
PCR and restriction endonuclease digestion.
Preparation of genomic DNA has been described previously (10). The sense primer (gadB21) was 5′-CGTTATGGATTTGATGGATATAAAGC-3′, located within the gadB gene, and the antisense primer (GAD7) was 5′-ACTCTTCTTAAGAACAAGTTTAACAGC-3′, which is located downstream from the gene. Each 50 μl of PCR mixture contained 200 ng of genomic DNA, 20 pmol of each primer, reagent mixture, and Ampli Taq gold DNA polymerase (Perkin-Elmer, Foster City, Calif.). PCR amplification was conducted with a GeneAmp PCR System 2400 (Perkin-Elmer). The PCR conditions were as follows: denaturation at 94°C for 9 min, followed by 45 cycles of denaturation at 94°C for 30 s, annealing at 50°C for 30 s, and extension at 72°C for 60 s, with an additional extension of 7 min at 72°C after the last cycle. The amplified fragments were digested with AseI restriction endonuclease (Toyobo, Tokyo, Japan) according to a supplier's instructions. The fragments were run on a 4% NuSieve GTG agarose gel (BioWhittaker Molecular Applications, Rockland, Maine) and were stained with ethidium bromide.
DNA sequence analysis.
Amplified double-stranded DNA was purified by electrophoresis on a 4% NuSieve GTG agarose gel for direct sequencing. Both strands of purified DNA were sequenced with a DYEnamic ET terminator cycle sequencing kit (Amersham Pharmacia Biotech, Piscataway, N.J.) and a 373A DNA sequencer (Applied Biosystems, Foster City, Calif.). The DNA sequences used in this study are listed in Table 1. The 16S rRNA gene was amplified and sequenced as described previously (15).
TABLE 1.
Sources of the L. lactis gadB gene sequences
| Strain | Accession no. | Reference or source |
|---|---|---|
| L. lactis subsp. lactis biovar diacetylactis NIAI 01-7 | AB010789 | 9 |
| L. lactis subsp. lactis ATCC 19435 | AB067750 | This study |
| L. lactis subsp. cremoris | ||
| MG1363 | AF005098 | 12 |
| ATCC 19257 | AB033218 | 10 |
| NIAI 01-1 | AB033220 | 10 |
| NIAI 53-2 | AB067751 | This study |
| 924 | AB033222 | 10 |
| F-16 | AB033224 | 10 |
| HP | AB033226 | 10 |
| ML | AB033228 | 10 |
| H-61 | AB033230 | 10 |
Nucleotide sequence accession numbers.
The sequences of the gadB genes of L. lactis subsp. lactis ATCC 19435 and L. lactis subsp. cremoris NIAI 53-2 have been assigned accession numbers AB067750 and AB067751, respectively.
RESULTS
The genotypes and phenotypes of L. lactis strains used in this study are summarized in Table 2. L. lactis strain MG1363 is classified as L. lactis subsp. cremoris based on its genotypic traits, although it shows the phenotypic abilities of L. lactis subsp. lactis. Strain MG1363 also exhibits GAD activity. Strain IL1403 showed the ability to produce GABA like the typical L. lactis subsp. lactis although it could not hydrolyze arginine.
TABLE 2.
Phenotypic and genotypic characteristics of L. lactis strains
| Strain | NH3 from arginine | GABA from glutamate | Amplification by PCR of:
|
|
|---|---|---|---|---|
| 1,131 bp | ∼700 bp | |||
| L. lactis subsp. lactis | ||||
| ATCC 19435 | + | + | + | + |
| NIAI 527 | + | + | + | + |
| ATCC 9936 | + | + | + | + |
| IL1403 | − | + | + | + |
| L. lactis subsp. lactis biovar diacetylactis | ||||
| ATCC 13675 | + | + | + | + |
| NIAI 01-7 | + | + | + | + |
| DRC1 | + | + | + | + |
| 17 isolatesa | + | + | NDb | ND |
| L. lactis subsp. cremoris | ||||
| MG1363 | + | + | + | − |
| ATCC 19257 | − | − | + | − |
| NIAI 01-1 | − | − | + | − |
| 924 | − | − | + | − |
| F-16 | − | − | + | − |
| HP | − | − | + | − |
| ML | − | − | + | − |
| H-61 | − | − | + | − |
| 20 isolatesa | − | − | ND | ND |
Isolated in previous work (8).
ND, not determined.
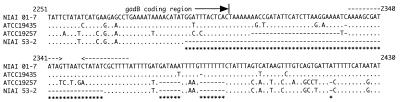
At first, the gadB sequences of L. lactis subsp. cremoris strains ATCC 19257 and MG1363 were compared with those of L. lactis subsp. lactis NIAI 01-7 and ATCC 19435 (Fig. 1). Two specific bases were observed only in the sequence of strain 01-7 (C2331 and T2382). At the 3′ untranslated region of L. lactis subsp. cremoris gadB (including strain MG1363), three fragmentary deletions were observed: a 24-bp deletion upstream of the stem-loop structure of the transcription terminator and two 6-bp deletions downstream of the stem-loop. Further, an additional 1-bp deletion in GAD-negative strains such as L. lactis subsp. cremoris ATCC 19257 was observed (Fig. 1). These deletions were also commonly observed in another six strains of GAD-negative L. lactis subsp. cremoris NIAI 01-1, 924, F-16, H-61, HP, and ML (data not shown). The PCR primers were designed to amplify the sequences comprising both the thymine insertion within the coding region (10) and the deletions at the 3′ untranslated region. The positions of these primers on the sequence determined by Nomura et al. (9) were 1901 to 1926 and 2502 to 2476, respectively. The lengths of the expected amplified fragments were 602 bp for the L. lactis subsp. lactis-type strain and 564 bp for L. lactis subsp. cremoris.
FIG. 1.
Multiple alignment of gadB sequences from L. lactis subsp. lactis NIAI 01-7, L. lactis subsp. lactis ATCC 19435, L. lactis subsp. cremoris MG1363, and L. lactis subsp. cremoris ATCC 19257. PCR primers are boxed. Dashed box, AseI restriction site; dashed arrows, inverted repeat, suggestive of a transcription terminator (not including the poly[T] stretch); asterisks, positions of insertions or deletions. The numbering is according to the sequence of L. lactis subsp. lactis NIAI 01-7 reported by Nomura et al. (9).
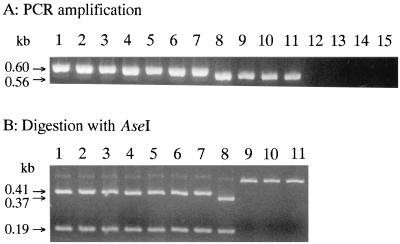
PCR amplification of seven L. lactis subsp. lactis strains and four L. lactis subsp. cremoris strains was performed. Specific DNA amplification was observed with all L. lactis strains tested, but not with other species such as S. thermophilus 9Y, Leuconostoc mesenteroides ATCC 8293, E. faecalis IFO 12964, and Lactobacillus casei ATCC 393 (Fig. 2A). The sizes of the amplified products were approximately 600 bp for L. lactis subsp. lactis and L. lactis subsp. lactis biovar diacetylactis (Fig. 2A, lanes 1 to 7) and approximately 560 bp for L. lactis subsp. cremoris (Fig. 2A, lanes 8 to 11). The product from strain MG1363, which is a GAD-positive L. lactis subsp. cremoris strain, revealed close to 560 bp that were like those of the GAD-negative strains, as predicted from the DNA sequence (12) (Fig. 2A, lane 8).
FIG. 2.
PCR amplification and digestion with AseI of L. lactis gadB. Conditions for PCR and electrophoresis are described in Materials and Methods. Lanes: 1, L. lactis subsp. lactis biovar diacetylactis ATCC 13675; 2, L. lactis subsp. lactis biovar diacetylactis NIAI 01-7; 3, L. lactis subsp. lactis biovar diacetylactis DRC1; 4, L. lactis subsp. lactis ATCC 19435; 5, L. lactis subsp. lactis NIAI 527; 6, L. lactis subsp. lactis ATCC 9936; 7, L. lactis subsp. lactis IL1403; 8, L. lactis subsp. cremoris MG1363; 9, L. lactis subsp. cremoris ATCC 19257; 10, L. lactis subsp. cremoris H-61; 11, L. lactis subsp. cremoris HP; 12, S. thermophilus 9Y; 13, Leuconostoc mesenteroides ATCC 8293; 14, E. faecalis IFO 12964; 15, Lactobacillus casei ATCC 393.
PCR fragments of L. lactis subsp. lactis were cut into portions of approximately 190 and 410 bp with endonuclease AseI, while those of L. lactis subsp. cremoris were not (Fig. 2B). The PCR product of L. lactis subsp. cremoris MG1363 was also digested into two fragments as were those of L. lactis subsp. lactis, although the sizes of the resulting fragments were different (approximately 190 and 370 bp) (Fig. 2B, lane 8). The results of the PCR-RFLP analyses are summarized in Table 3. For each strain, digestion with AseI appeared to correspond to the GAD phenotype.
TABLE 3.
PCR fragments and RFLP analyses for gadB gene in L. lactis
| Strain | PCR fragment of:
|
Digestion with AseI | |
|---|---|---|---|
| ∼600 bp | ∼560 bp | ||
| L. lactis subsp. lactis biovar diacetylactis | |||
| ATCC 13675 | + | − | + |
| NIAI 01-7 | + | − | + |
| DRC1 | + | − | + |
| L. lactis subsp. lactis | |||
| ATCC 19435 | + | − | + |
| NIAI 527 | + | − | + |
| ATCC 9936 | + | − | + |
| IL1403 | + | − | + |
| L. lactis subsp. cremoris | |||
| MG1363 | − | + | + |
| ATCC 19257 | − | + | − |
| H-61 | − | + | − |
| HP | − | + | − |
| S. thermophilus 9Y | − | − | NDa |
| Leuconostoc mesenteroides ATCC 8293 | − | − | ND |
| E. faecalis IFO 12964 | − | − | ND |
| Lactobacillus casei ATCC 393 | − | − | ND |
ND, not determined.
Bacterial cells were subsequently investigated with PCR templates instead of extracted DNA. A bacterial culture grown in tryptone-yeast extract-glucose, GM17, or MRS medium was sequentially diluted with sterile deionized water, and a 1-μl portion was used as a template. In addition, a colony on the agar plate was picked up with a sterile toothpick and then suspended directly into a PCR mixture. Amplification was observed with a culture broth diluted from 10- to 104-fold but not with undiluted broth. The reaction was also observed with a cell from the colony. The PCR fragments resulting from both cell preparations were the same as those obtained with the extracted DNA (data not shown). These results indicate that vegetative cells can be substituted for genomic DNA as a template for PCR.
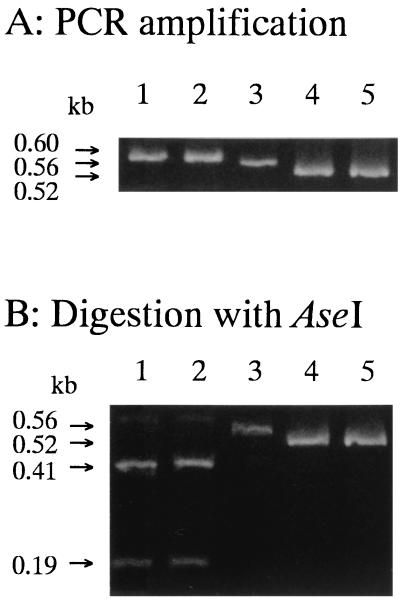
Thirty-seven isolates from cheese starters were investigated by this method. Based on some phenotypic characteristics, 17 of the 37 strains were identified as L. lactis subsp. lactis and 20 were identified as L. lactis subsp. cremoris (8). Fragments with the expected lengths were amplified from all 17 strains of L. lactis subsp. lactis and from 16 of 20 strains of L. lactis subsp. cremoris (data not shown). The PCR fragments of the remaining L. lactis subsp. cremoris strains, 53-2, 53-4, 53-6, and 53-8, were slightly shorter than those of the typical L. lactis subsp. cremoris strains (Fig. 3). The apparent sizes of the fragments were estimated to be approximately 520 bp. The fragments of strains 53-2 and 53-6 were sequenced, and it was found that the sequences were identical to each other. The sequences were compared with those of common L. lactis subsp. cremoris strains (Fig. 4). A further 43-bp deletion from the end of the coding region to the transcription terminator was observed. Two deletions downstream of the stem-loop (both 6 bp) were conserved. The expected length of each amplified fragment was 521 bp, which was the size estimated from gel electrophoresis.
FIG. 3.
PCR amplification and digestion with AseI of the gadB gene in isolates from cheese starters. Conditions for PCR and electrophoresis are described in Materials and Methods. Lanes: 1, L. lactis subsp. lactis biovar diacetylactis NIAI 01-7; 2, L. lactis subsp. lactis ATCC 19435; 3, L. lactis subsp. cremoris ATCC 19257; 4, L. lactis subsp. cremoris 53-2; 5, L. lactis subsp. cremoris 53-6.
FIG. 4.
Alignment of the nucleotide sequences of the 3′ region of L. lactis gadB. Dashed arrows, inverted repeat, suggestive of a transcription terminator (not including the poly[T] stretch); asterisks, positions of insertions or deletions. Base pair numbering is as in Fig. 1.
The 16S rRNA gene sequences of strains 53-2 and 53-6 were determined. The sizes of the amplified sequences were 300 bp. The two sequences were identical to each other and were found to have an identity to L. lactis subsp. cremoris (11). These results suggest that strains 53-2 and 53-6 should be classified as L. lactis subsp. cremoris, although gadB of these strains exhibited genetic polymorphism.
DISCUSSION
To improve starter cultures for the dairy industry, isolation and classification of new L. lactis strains from nature have been carried out. Dairy products are made by good use of phenotypic properties of starter organisms. Therefore, it is necessary to know the phenotypes of isolates for application to the dairy industry. However, the traditional microbiological characterization is usually time-consuming and requires skill. The simple and reliable methods elucidating phenotypic characteristics are extremely useful in obtaining new strains. A nucleic acid-based approach can provide technical simplicity and accuracy for differentiation of strains. For L. lactis subspecies identification, Southern hybridization (11) and PCR (3, 5) have been developed. These methods, however, are based on genetics. Genotypic identification does not always correlate with phenotypes. We, therefore, investigated a PCR-RFLP technique that specifically differentiates L. lactis subspecies.
In this study, some fragmentary deletions were observed at the 3′ untranslated region of L. lactis subsp. cremoris gadB. Since the PCR primers were designed to amplify the deletion region, the lengths of the products from L. lactis subsp. cremoris were 37 bp shorter than those of L. lactis subsp. lactis. The amplified fragment length polymorphism concurred with the genotypic classification.
A common thymine insertion within the coding region of L. lactis subsp. cremoris gadB has been observed (10). The insertion site is included in the amplified region with primers gadB21 and GAD7. An AseI site present in the amplified region of L. lactis subsp. lactis is absent in the counterpart of L. lactis subsp. cremoris due to this thymine insertion. Thus, the PCR products of L. lactis subsp. lactis can be cut with AseI into two fragments, while those of L. lactis subsp. cremoris cannot. Digestion with AseI appears to occur concurrently with GAD activity, making it a criterion for phenotypic classification (8).
Isolation of new strains is usually performed by picking a single colony grown on an agar plate. In this study, L. lactis could be detected by PCR using the cells of isolates as templates. After isolation from a plate, the isolates can be subsequently subjected to PCR without cultivation and DNA preparation.
The primers in this study identified all L. lactis strains tested and distinguished them to the subspecies level. The non-L. lactis strains showed no positive result. The assay is not affected by physiological cell parameters, and the identification procedure leads to reliable results in fast and easy steps. Unlike other available molecular techniques, this PCR assay can be used to determine genotypes and phenotypes of L. lactis subspecies.
Some mutations in the primer recognition sequence make it impossible to amplify a fragment. In this case, the fragment cannot be detected even if the unknown organism is L. lactis. Since there was a strain, 53-2, with a deletion greater than that found in a typical L. lactis subsp. cremoris strain, it is possible that there is a variant with a further deletion in the sequence complementary to the primers. It is also possible that the recognition sequence is replaced and becomes impossible to anneal with the primers.
The restriction enzyme site used for RFLP analysis is conserved in L. lactis subsp. lactis strains currently investigated. If there is a variant in which GAD is inactivated by mutations of other parts in gadB gene, even if the sequence of the restriction site is typical, the results of RFLP and phenotyping will not be in agreement.
This PCR-RFLP technique is useful for screening and grouping new lactococcal isolates. It is critical that the techniques used to differentiate between L. lactis subspecies allow for correlation between phenotypic and genotypic identification.
Acknowledgments
We are grateful to M. Takeuchi and K. Chikuni for their valuable discussions.
REFERENCES
- 1.Al-Zoreky, N., and W. E. Sandine. 1991. Lactococcus genus: a selective and differential agar medium. J. Food Sci. 56:1729-1734. [Google Scholar]
- 2.Basaran, P., N. Basaran, and I. Cakir. 2001. Molecular differentiation of Lactococcus lactis subspecies lactis and cremoris strains by ribotyping and site specific-PCR. Curr. Microbiol. 42:45-48. [DOI] [PubMed] [Google Scholar]
- 3.Beimfohr, C., W. Ludwig, and K. H. Schleifer. 1997. Rapid genotypic differentiation of Lactococcus lactis subspecies and biovar. Syst. Appl. Microbiol. 20:216-221. [Google Scholar]
- 4.de Man, J. C., M. Rogosa, and M. E. Sharpe. 1960. A medium for the cultivation of lactobacilli. J. Appl. Bacteriol. 23:130-135. [Google Scholar]
- 5.Garde, S., M. Babin, P. Gaya, M. Nunez, and M. Medina. 1999. PCR amplification of the gene acmA differentiates Lactococcus lactis subsp. lactis and L. lactis subsp. cremoris. Appl. Environ. Microbiol. 65:5151-5153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Godon, J.-J., C. Delorme, S. D. Ehrlich, and P. Renault. 1992. Divergence of genomic sequences between Lactococcus lactis subsp. lactis and Lactococcus lactis subsp. cremoris. Appl. Environ. Microbiol. 58:4045-4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mundt, J. O. 1986. Lactic acid streptococci, p. 1065-1066. In P. H. A. Sneath, N. S. Mair, M. E. Sharpe, and J. G,. Holt (ed.), Bergey's manual of systematic bacteriology, vol. 2. The Williams & Wilkins Co., Baltimore, Md. [Google Scholar]
- 8.Nomura, M., H. Kimoto, Y. Someya, and I. Suzuki. 1999. Novel characteristic for distinguishing Lactococcus lactis subsp. lactis from subsp. cremoris. Int. J. Syst. Bacteriol. 49:163-166. [DOI] [PubMed] [Google Scholar]
- 9.Nomura, M., I. Nakajima, Y. Fujita, M. Kobayashi, H. Kimoto, I. Suzuki, and H. Aso. 1999. Lactococcus lactis contains only one glutamate decarboxylase gene. Microbiology 145:1375-1380. [DOI] [PubMed] [Google Scholar]
- 10.Nomura, M., M. Kobayashi, S. Ohmomo, and T. Okamoto. 2000. Inactivation of the glutamate decarboxylase gene in Lactococcus lactis subsp. cremoris. Appl. Environ. Microbiol. 66:2235-2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salama, M., W. Sandine, and S. Giovannoni. 1991. Development and application of oligonucleotide probes for identification of Lactococcus lactis subsp. cremoris. Appl. Environ. Microbiol. 57:1313-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanders, J. W., K. Leenhouts, J. Burghoorn, J. R. Brands, G. Venema, and J. Kok. 1998. A chloride-inducible acid resistance mechanism in Lactococcus lactis and its regulation. Mol. Microbiol. 27:299-310. [DOI] [PubMed] [Google Scholar]
- 13.Swindell, S. R., H. G. Griffin, and M. J. Gasson. 1994. Cloning, sequencing and comparison of three lactococcal L-lactate dehydrogenase genes. Microbiology 140:1301-1305. [DOI] [PubMed] [Google Scholar]
- 14.Terzaghi, B. E., and W. E. Sandine. 1975. Improved medium for lactic streptococci and their bacteriophages. Appl. Microbiol. 29:807-813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ward, L. J. H., L. C. S. Brown, and G. P. Davey. 1998. Two methods for the genetic differentiation of Lactococcus lactis ssp. lactis and cremoris based on differences in the 16S rRNA gene sequence. FEMS Microbiol. Lett. 166:15-20. [DOI] [PubMed] [Google Scholar]